Abstract
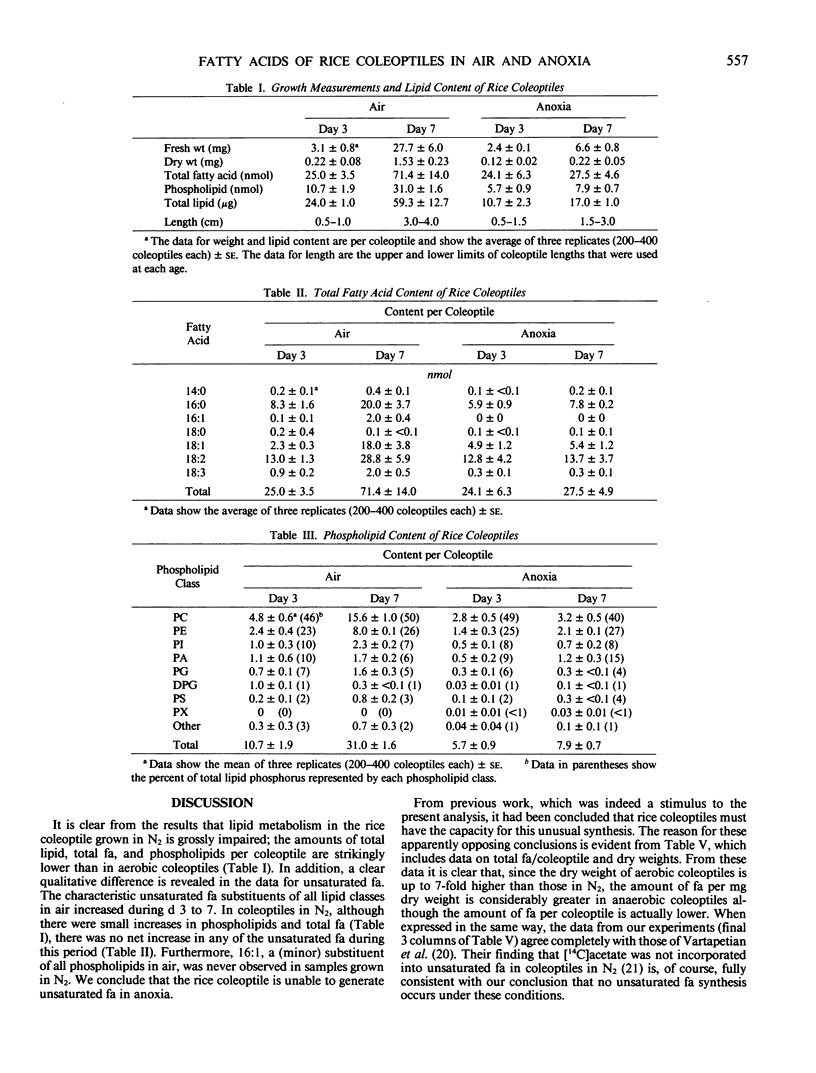
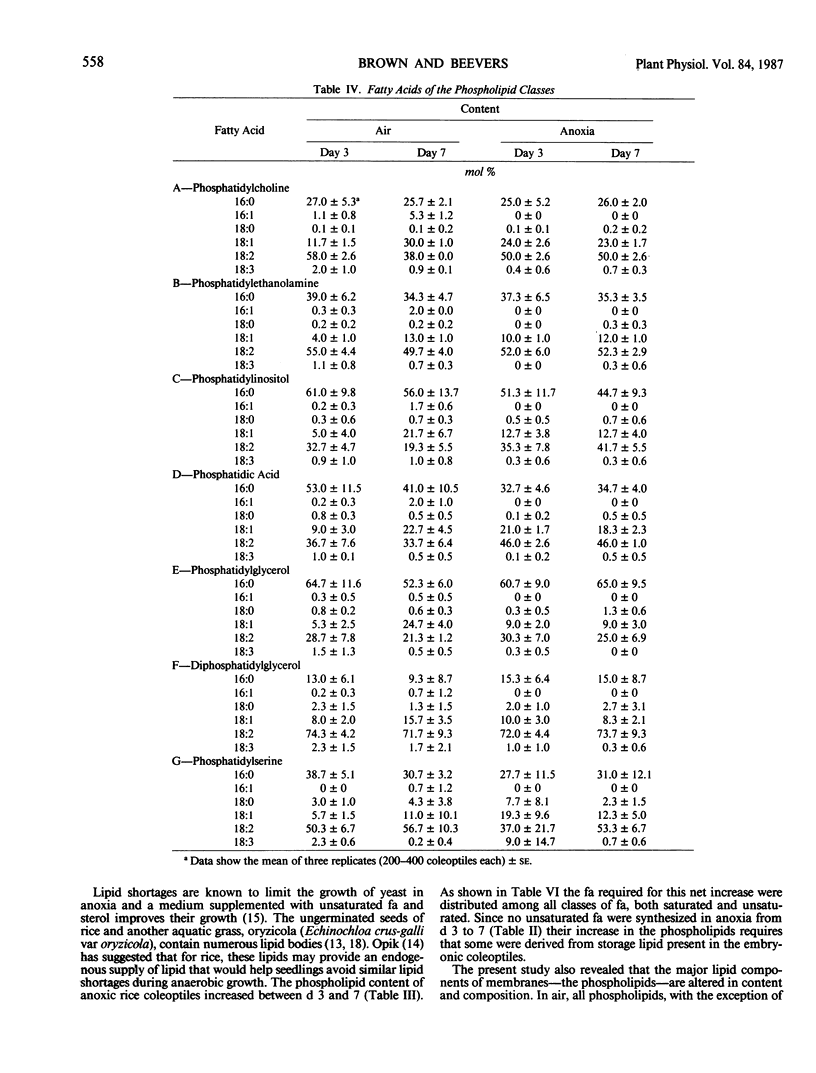
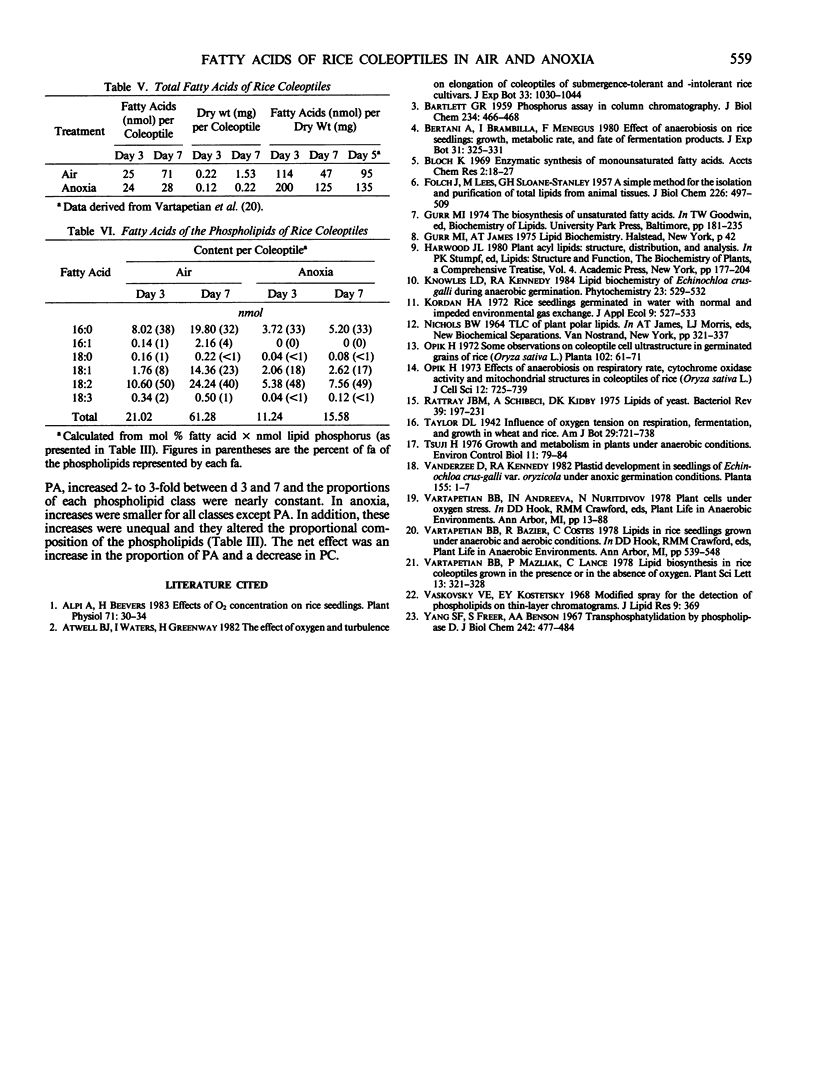
The metabolism of lipids, like that of other components, was adversely and strongly affected when rice (Oryza sativa L.) coleoptiles were grown anaerobically. In aerobic coleoptiles, the amounts of total fatty acid, phospholipid, and total lipid per coleoptile increased by 2.5- to 3-fold between days three and seven, whereas under anoxia, the increases were all less than 60%. The total amount of lipid at day seven in anoxia was less than 30% of that in air. In air, the total fatty acid content at day three was 25 nanomoles per coleoptile and this increased to over 71 nanomoles per coleoptile at day seven. All acids except 18:0 showed substantial increases. In anoxia, the corresponding values for total fatty acids were 24 nanomoles and 27 nanomoles. The small increases were confined to the saturated fatty acids; no significant increase occurred in unsaturated fatty acids. A minor fatty acid constituent (16:1) increased from 0.09 to 1.99 nanomoles per coleoptile between days three and seven in air. This component was never observed in any fatty acid preparation from anaerobic coleoptiles. The major phospholipids under all conditions were phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, and phosphatidic acid. A small amount of unidentified phosphoester, not present on thin layer chromatography plates from aerobic coleoptiles, was seen in extracts of anaerobic coleoptiles. The fatty acyl substituents of each of the phospholipids were analyzed at days three and seven in coleoptiles grown aerobically and in anoxia. Each phospholipid had its own distinctive fatty acid composition which remained fairly constant under all treatments; 16:0 and 18:2 were the most abundant fatty acids in every phospholipid class. In air, the percentages of total fatty acids that were in the phospholipids were 86% on day three and 87% on day seven. In anoxia, the values at the corresponding ages were 47 and 57%. Since no net synthesis of unsaturated fatty acids occurred in anaerobic conditions, the small increase in total unsaturated acids in the phospholipids between days three and seven must have occurred at the expense of fatty acids preexisting in the neutral lipid. No unusual pathways of biosynthesis or unusual precursors are required to explain the presence of unsaturated fatty acids in the rice coleoptile. The present study and results of experiments where coleoptiles were fed [14C]acetate (BB Vartapetian et al. 1978 Plant Sci Lett 13:321-328) clearly show that unsaturated fatty acid synthesis in rice coleoptiles requires O2, as it does in other plants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpi A., Beevers H. Effects of o(2) concentration on rice seedlings. Plant Physiol. 1983 Jan;71(1):30–34. doi: 10.1104/pp.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Opik H. Effect of anaerobiosis on respiratory rate, cytochrome oxidase activity and mitochondrial structures in coleoptiles of rice (Oryza sativa L.). J Cell Sci. 1973 May;12(3):725–739. doi: 10.1242/jcs.12.3.725. [DOI] [PubMed] [Google Scholar]
- Rattray J. B., Schibeci A., Kidby D. K. Lipids of yeasts. Bacteriol Rev. 1975 Sep;39(3):197–231. doi: 10.1128/br.39.3.197-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]


