Abstract
Twelve healthy volunteers participated in this randomized crossover study to compare the concentrations and recovery levels of fleroxacin and pefloxacin in urine and to assess their bactericidal activities against 12 strains of urinary pathogens with different susceptibilities over a wide range of MICs. The volunteers received a single oral dose of 400 mg of fleroxacin or 800 mg of pefloxacin. The mean cumulative renal excretion of unchanged fleroxacin, N-demethyl-fleroxacin, and N-oxide-fleroxacin accounted for 67, 7, and 6% of the total dose, respectively. The total urinary recovery of pefloxacin and the active metabolite norfloxacin was 34%. In the time-kill and the urinary bactericidal titer (UBT) studies, only the subjects’ urine not supplemented with broth was used. With most tested organisms and both quinolones it took more than 8 h to achieve a reduction in CFU of 99.9% (3 log units). Overall, there was a good correlation between UBTs and MICs for the strains. Against Escherichia coli ATCC 25922 the median UBTs were similar for both antibiotics and at least 1:8 for 96 h; against the E. coli strain for which the MIC was 0.5 μg/ml the UBT was at least 1:4 for 48 h. The UBTs of both drugs against Klebsiella pneumoniae were at least 1:16 for 72 h. The UBTs for Staphylococcus aureus (the MIC for which was 16 μg/ml) of both antibiotics were low, and in some of the samples, no bactericidal titers were observed. UBTs for Proteus mirabilis of pefloxacin are significantly higher than those of fleroxacin. For Pseudomonas aeruginosa the median UBTs were present for the 24-to-48-h interval. The same is true for Enterococcus faecalis. Against Staphylococcus saprophyticus, UBTs were present for at least 48 h with both quinolones. Overall, a single oral dose of 400 mg of fleroxacin exhibits UBTs comparable to those of 800 mg of pefloxacin. Therefore, it may be expected that half of the dose of fleroxacin gives comparable results in the treatment of urinary tract infections; this should be substantiated in comparative clinical trials.
Fleroxacin (FLX) and pefloxacin (PFL) belong to the class of fluoroquinolones. They are highly effective against most gram-negative and some gram-positive bacteria. Data from comparative and noncomparative studies have demonstrated their efficacy against a wide range of infections, especially in complicated and uncomplicated urinary tract infections. Both antibiotics have long serum half-lives of about 10 to 12 h and sustain high urinary levels following a single oral dose. Whereas PFL is excreted into the urine as only about 10% parent drug and about 20% active metabolite, FLX is mainly excreted unchanged into the urine. Their pharmacokinetics and antibacterial activities make them the best drugs for once-a-day dosing for the treatment of urinary tract infections.
Usually, in vitro susceptibility testing in conjunction with urinary concentration measurements is used to guide the treatment, assuming that this information is sufficient to predict the clinical effectiveness of the chosen chemotherapy. In contrast to conventional standard media, urine is a complex and constantly changing medium. Hydrogen ion concentration and osmolality can vary over a wide range within a short period of time. Changes in the composition of urine can profoundly alter antimicrobial activity. Thus, when bacterial strains have been tested in human urine, MICs have been found to be higher than those in conventional media (18, 21).
This study was carried out to determine the concentrations and recovery levels of FLX and PFL in the urine of volunteers after single doses of 400 and 800 mg, respectively, and to compare the bactericidal urinary activity of FLX with that of PFL against urinary pathogens by measuring the bactericidal killing rate in urine and urinary bactericidal titers (UBTs). In contrast to other studies, only undiluted urine was used as the growth medium.
(This work was presented in part at the 19th International Congress of Chemotherapy, Montreal, Canada, 16 to 21 July 1995 [19].)
MATERIALS AND METHODS
Study design and subjects.
Twelve healthy volunteers (six male and six female) participated in this randomized crossover study. The protocol of the study was approved by an independent ethical committee (Freiburger Ethikkommission). Persons from 18 to 44 years of age (median, 32 years) with body weights ranging from 53 to 100 kg (median, 74 kg) were included in the study. Good general health was determined by medical history and by physical and laboratory examinations. Female volunteers were neither pregnant nor breast-feeding, and sufficient contraception was indicated. Exclusion criteria consisted of clinically significant abnormal baseline laboratory parameters, antibiotic treatment 4 weeks prior to and during the study period (controlled by a microbiological assay), allergy to the study drugs, bacteriuria of ≥104 CFU/ml of urine, need for a special diet, use of alcohol or coffee, and nicotine abuse. No medication besides hormonal contraception was allowed. Urine samples were collected during the 24 h before the drug administration, assayed for antibiotic concentrations, and stored.
Drug administration and sample collection.
After written informed consent was obtained, each volunteer received in random order and in a crossover design one oral dose of 400 mg of FLX (two tablets, 200 mg each; Hoffmann-La Roche Ltd., Basel, Switzerland) or 800 mg of PFL (two tablets, 400 mg each; Rhone Poulence Rorer GmbH, Cologne, Germany) while in an overnight fasting state. Alcohol- and xanthine-containing beverages and meals were not allowed 12 h before and 24 h after drug administration. Urine was collected at the following intervals during the 7 days: 0 to 6, 6 to 12, 12 to 24, 24 to 48, 48 to 72, 72 to 96, 96 to 120, 120 to 144, and 144 to 168 h. The volumes of urine were recorded, and a sample was retained for assay. Urine samples were centrifuged, sterile filtered, and stored at −20°C until the assay. The washout period between the two oral administrations was 14 days.
Drug analyses.
The urinary concentrations of FLX and its metabolites, FLX-N-oxide (FNO) and N-demethyl-FLX (NDF), and that of PFL and its metabolites, N-demethyl-PFL (norfloxacin [NOR]) and PFL-N-oxide (PNO), were determined by a high-performance liquid chromatography (HPLC) method with a high degree of sensitivity and specificity. Urine samples were defrosted and shaken for 1 min and diluted 1:50 with buffer, and 5 or 10 μl of the FLX samples and 10 or 50 μl of the PFL samples were used for HPLC analysis. If necessary the samples were further diluted with urine sampled before the drug administration to bring the expected concentration within the range of the standards. The chromatographic system included a Spectroflow 400 pump (Kratos Analytical Instruments, Ramsay, N.J.) operating at a flow rate of 1.2 ml/min. An Autosampler 460 (Kontron, Eching, Germany) automatic sampler was used for automatic sample injection. The column used was a Spherisorb ODS II (pore size, 5μm; 250 by 4.6 mm). The solvent used for the FLX test was a mixture of 0.1 M citric acid, 22 nM ammonium perchlorate, 5 mM tetrabutylammonium hydroxide, and acetonitrile (87:13). For the PFL test, a mixture of 0.11 M citric acid, 22 nM ammonium perchlorate, 5 mM tetrabutylammonium hydroxide, and acetonitrile (88:12) was used. A wavelength fluorescence detector was set at an excitation of 290 nm and an emission wavelength of 460 nm to determine FLX levels; the corresponding settings were 275 and 450 nm to determine PFL levels. The retention times for FLX, FNO, NDF, PFL, NOR, and PNO were 7.7, 9.2, 6.3, 9.8, 8.3, and 11.7 min, respectively. The limits for detection were 0.454, 0.926, 0.690, 0.0884, 0.0876, and 0.0897 μg/ml for FLX, FNO, NDF, PFL, NOR, and PNO, respectively. In quality control tests 94.7 to 98.0% of FLX, 97.1 to 99.9% of FNO, 95.7 to 98.2% of NDF, 97.6 to 101.0% of PFL, 93.6 to 100.5% of NOR, and 92.9 to 101.1% of PNO were detected. The between-day precision was found to be not higher than 6.2% for FLX and its metabolites and 10.1% for PFL and its metabolites.
Bacteria.
Eleven pathogens were cultured from the urine of patients with urinary tract infections. For each pathogen, the MIC of FLX was the same as that of PFL, as determined by the agar dilution method. The pathogens included Klebsiella pneumoniae, Escherichia coli, Proteus mirabilis, two strains of Pseudomonas aeruginosa (I and II), two strains of Enterococcus faecalis (I and II), two strains of Staphylococcus aureus (I and II), Staphylococcus saprophyticus, and a Streptococcus group B sp. The reference strain, E. coli ATCC 25922, which was susceptible to nalidixic acid, was also tested. The MICs and minimal bactericidal concentrations (MBCs) for the strains are given in Table 1.
TABLE 1.
MICsa and MBCs of FLX and PFL for the 12 bacterial strains used in the study
| Species or strain | FLX
|
PFL
|
||||
|---|---|---|---|---|---|---|
| MIC1 | MIC2 | MBC | MIC1 | MIC2 | MBC | |
| E. coli ATCC 25922 | 0.03 | ≤0.06 | ≤0.06 | 0.03 | ≤0.06 | ≤0.06 |
| K. pneumoniae | 0.125 | ≤0.06 | 0.125 | 0.125 | 0.125 | 0.25 |
| E. coli | 0.5 | 0.5 | 1 | 0.5 | 1 | 1 |
| S. aureus I | 0.5 | ≤0.06 | ≤0.06 | 0.5 | 0.125 | 0.125 |
| P. mirabilis | 1 | ≤0.06 | 0.25 | 1 | 0.125 | 0.25 |
| P. aeruginosa I | 2 | 2 | 4 | 2 | 2 | 4 |
| P. aeruginosa II | 4 | 2 | 4 | 4 | 2 | 4 |
| S. saprophyticus | 4 | 2 | 4 | 4 | 2 | 4 |
| E. faecalis I | 4 | 4 | 4 | 4 | 2 | 4 |
| E. faecalis II | 8 | 4 | 4 | 8 | 4 | 4 |
| S. aureus II | 16 | 8 | 32 | 16 | 8 | 16 |
| Streptococcus group B sp. | 16 | 8 | 16 | 16 | 8 | 16 |
MIC1, MIC (μg/ml) by agar dilution method; MIC2, MIC (μg/ml) by broth microdilution method.
Inhibitory and bactericidal activity.
The MICs were determined by a microdilution method (Mueller-Hinton broth) using an inoculum of 105 CFU/ml as well as by an agar dilution method (Iso-Sensitest agar) using a multipointer delivery with an inoculum of 104 CFU per point. The MIC was defined as the lowest concentration inhibiting visible growth after incubation at 37°C for 18 h. Minimal bactericidal concentrations (MBC) were determined in a two-step procedure by counting the CFUs on antibiotic-free media. Bactericidal activity was defined as a reduction of CFUs by more than 99.9% (more than 3 log units) from an inoculum of 105 CFU/ml after incubation at 37°C for 18 to 24 h.
For time-kill studies the bactericidal activities of FLX and PFL in pooled urine taken during the 0-to-6-h urine sample-collecting period were determined. The FLX and PFL-plus-NOR concentrations in the pooled urine sample were determined by HPLC and were 216.7 and 167.8 μg/ml, respectively. After inoculation with 106 CFU/ml the urine samples were incubated at 37°C, and samples were taken at 0, 1, 2, 3, 4, 6, 8, and 16 to 24 h and subcultured for determination of viable cells. CFUs were counted after 18 h of incubation at 37°C.
UBTs.
Urinary bactericidal titer (UBT) determinations were based on the principles of a modified and extended Schlichter test with the patient urine instead of serum as the antimicrobial milieu (6). UBTs were determined by assaying microtiter plates. The urine from each collecting period was diluted in a twofold manner with the undiluted antibiotic-free urine of the corresponding individual, collected 24 h before drug administration. The final inoculum was 105 CFU/ml. The inoculated plates were incubated at 37°C for 18 h and subcultivated on antibiotic-free Iso-Sensitest agar supplemented with 5% blood for counting the CFUs. The UBT was defined as the greatest urinary dilution bactericidal for the pathogen tested. The measured UBTs ranged from 1:1 (undiluted urine) to 1:1,024. The inoculum preparation, the incubation, and the subculturing techniques were identical to the methods used for the MIC and MBC determinations. For quality control reference strain E. coli ATCC 25922 was used. The methodological variability of the UBT assay was determined earlier as follows. By using three different fluoroquinolones and six different bacterial strains a total of 18 UBTs (eight replicates each) were determined. A standard deviation of ±0.37 dilution steps (variant coefficient, 0.14) was found (data not published).
The area under the UBT-versus-time curve (AUBTC) was calculated by the trapezoidal rule by using transformed UBTs (see below) of the corresponding collecting periods.
Statistical analysis.
Statistical analysis was primarily based on an analysis of variance on the transformed data, where the actual transformation used was y = log2 (x + 1), where x denotes the titer corresponding to no bactericidal effect for the undiluted urine. The effects related to error and volunteer are considered to be random effects; all other effects are considered fixed. Three analyses were run; all analyses were based on the assumption of complete independence between subjects. In the simplest analysis, measurements for a subject were considered independent. The second analysis assumed an autoregressive model of first order between patients, and the third analysis assumed a Toeplitz structure. Calculations were made with SAS version 6.09 software. Assumption of normality was roughly checked by using a normal probability plot on the residuals.
In the second analysis simple paired t tests were performed with transformed data for the UBTs and AUBTCs. A P value of <0.05 was set as statistically significant. For indicating the size of the effects, it was decided that medians rather than geometric means would be used. The artificial value of 0 used to indicate no bactericidal effect of undiluted urine creates in our opinion problems for interpreting geometric means.
RESULTS
Urinary excretion.
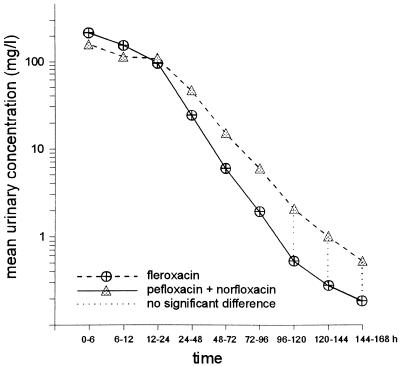
The mean cumulative renal excretion of unchanged FLX accounts for 67.1% ± 4.5% of the total dose (range, 59 to 72%). The urinary excretion of NDF and FNO accounted for 6.9% ± 2.2% and 5.8% ± 1.6% of the dose, respectively. Due to extensive metabolism of PFL only 13.2% ± 4.2% of unchanged PFL was detected in urine. The principal metabolites NOR and PNO accounted for 20.8% ± 3.8% and 19.2% ± 2.5% of the administered dose, respectively. The total urinary recovery of the parent drug and the active metabolite NOR was 34%. Thus, the mean percentage of the total amount of FLX recovered in the urine over the study period of 7 days was about twice as much as that of PFL plus NOR. With both drugs, FLX and PFL plus NOR, the renal excretion was almost complete after 48 h. The mean urinary concentrations of FLX and PFL plus NOR are shown in Fig. 1. During the 0-to-6-h, the 6-to-12-h, and the 12-to-24-h periods the mean concentrations of the metabolite NDF were 16.3, 16.8, and 12.2 μg/ml, respectively (Table 2). The interindividual variations were considerable. The urinary concentrations of unchanged FLX and those of PFL plus NOR were in the same range during the total time of observation after single oral doses of 400 and 800 mg, respectively. There were no significant differences (P > 0.05) in the concentrations (in micrograms per milliliter) of FLX and PFL plus NOR during the first 24 h. Between the third and the fifth day the PFL-plus-NOR concentrations were higher but were similar on days 6 and 7.
FIG. 1.
Mean urinary concentrations of FLX and PFL plus NOR. For significant differences P < 0.05.
TABLE 2.
Concentrations of FLX, PFL, and their metabolites in urine
| Collection interval (h) | Mean (range) concn (μg/ml) and na for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FLX
|
FNO
|
NDF
|
PFL
|
NOR
|
PNO
|
|||||||
| n | Concn (range) | n | Concn (range) | n | Concn (range) | n | Concn (range) | n | Concn (range) | n | Concn (range) | |
| preb | 0 | -c | 0 | - | 0 | - | 0 | - | 0 | - | 0 | - |
| 0–6 | 12 | 217 (98–427) | 12 | 21.4 (4–70) | 12 | 16.3 (5–40) | 12 | 84.0 (37–219) | 12 | 80.3 (20–144) | 12 | 115.4 (29–233) |
| 6–12 | 12 | 160 (63–355) | 12 | 17.6 (4.6–62) | 12 | 16.8 (4–49) | 12 | 50.1 (22–97) | 12 | 76.3 (46.1–133) | 12 | 93.8 (19–192) |
| 12–24 | 12 | 102 (43–178) | 12 | 10.2 (5–19) | 12 | 12.2 (5.5–24.5) | 11d | 46.0 (8–104) | 11d | 72.9 (31–109) | 11d | 70.5 (31–115) |
| 24–48 | 12 | 26.8 (12–49) | 12 | 2.4 (1.2–5.8) | 12 | 3.1 (0.8–9) | 12 | 19.0 (2.3–53) | 12 | 29.1 (14–68) | 12 | 27.3 (10–70) |
| 48–72 | 12 | 6.3 (2.4–11.8) | 1 | 1.5e | 8 | 0.7 (0.7–1.7) | 12 | 5.8 (0.2–13.8) | 12 | 10.2 (1.7–23) | 12 | 8.7 (1.2–19) |
| 72–96 | 12 | 2.2 (0.8–3.9) | 0 | - | 2 | 0.2 (0.9–1.3) | 10 | 2.4 (0.3–8.9) | 12 | 3.9 (0.4–7) | 12 | 3.4 (0.2–12) |
| 96–120 | 8 | 0.6 (0.5–1.4) | 0 | - | 0 | - | 9 | 0.9 (0.3–3.4) | 12 | 1.5 (0.1–4.4) | 10 | 1.3 (0.1–4.2) |
| 120–144 | 4 | 0.3 (0.7–1) | 0 | - | 2 | 0.1 (0.6–0.8) | 8 | 0.5 (0.2–3.2) | 10 | 0.5 (0.1–2.4) | 8 | 0.5 (0.2–3.7) |
| 144–168 | 4 | 0.2 (0.5–0.8) | 0 | - | 1 | 0.8e | 5 | 0.2 (0.1–2) | 8 | 0.3 (0.1–2) | 6 | 0.3 (0.1–2.8) |
n, number of individuals with concentrations above the quantification limit.
pre, sample taken prior to drug administration.
-, below quantification limit.
One sample not available.
Since n = 1, the concepts of mean and range are not applicable.
Bactericidal kinetics in urine.
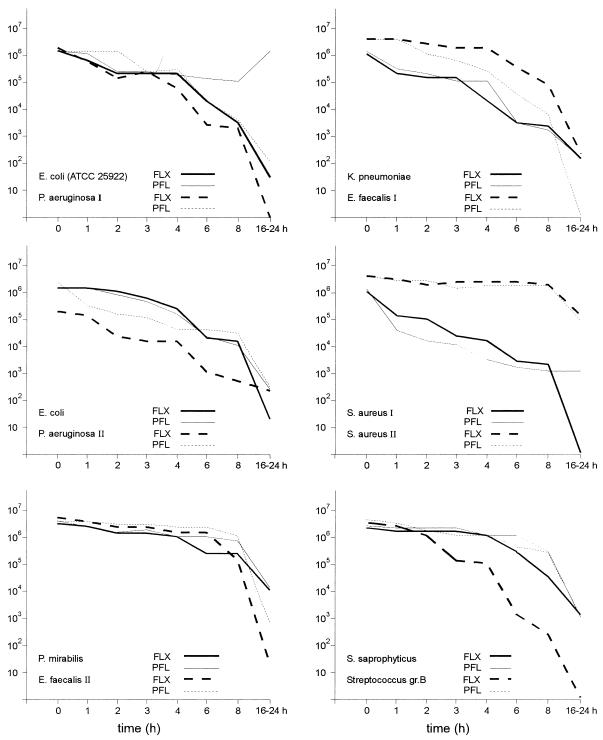
The pathogens used for this study reflect the clinical situation where fluoroquinolones are still the most active drugs against Enterobacteriaceae, according to a recently published susceptibility study (20). The bactericidal kinetics of the tested strains are shown in Fig. 2. FLX reduced the initial number of bacteria by at least 99% (2 log units) at 6 h for E. coli ATCC 25922, S. aureus I, P. aeruginosa I and II, and the Streptococcus group B sp. However, bactericidal activity, defined as a reduction in the number of CFU by at least 99.9% (3 log units) was not achieved during the 16-to-24-h period for P. mirabilis, P. aeruginosa II, and S. aureus II. PFL reduced the initial number of bacteria by at least 99% at 6 h for K. pneumoniae, S. aureus I, and E. faecalis I. Bactericidal activity during the 16-to-24-h period was not achieved for P. mirabilis and S. aureus II, as with FLX. The results of bacterial killing do not always exactly correlate with the MIC. With PFL there is more rapid killing of K. pneumoniae than of E. coli ATCC 25922. P. mirabilis was more resistant to killing by both substances than the other Enterobacteriaceae. In spite of high urinary concentrations there was only a slight reduction in the number of S. aureus II organisms.
FIG. 2.
Bactericidal kinetics in urine (collecting period, 0 to 6 h) after a single oral dose of 400 mg of FLX or 800 mg of PFL. gr.B, group B.
UBTs.
The antibacterial activity of quinolones including FLX in urine is reduced: the MIC of FLX against the tested E. coli, K. pneumoniae, and P. aeruginosa organisms was reduced by 3 dilution steps in the pooled urine of 12 volunteers compared to the FLX MIC in Mueller-Hinton broth (data not shown). The mean pH of the urine samples was 6 (±0.8) with a range of 5 to 8. Median UBTs and associated ranges versus the collecting period for each organism and antibiotic are shown in Table 3. Overall, there is a good correlation between the UBTs and MICs for the strains. In general, for the strains for which the MICs are relatively low the UBTs are higher than for those for strains for which the MICs are higher. For P. mirabilis and S. saprophyticus, however, the UBTs were 1 to 2 dilution steps higher than expected from the MICs (agar dilution) of both antibiotics. All individuals showed UBTs for FLX in the 12-to-24-h collecting period for each strain except the less-susceptible strain of S. aureus. The same is true for PFL except for E. faecalis, S. aureus, and the Streptococcus group B sp. Against E. coli ATCC 25922 the median UBTs were similar and at least 1:8 for 96 h; against the other E. coli strain the median UBTs were at least 1:4 for 48 h. The UBTs of both drugs against K. pneumoniae were at least 1:16 for 72 h. Different UBTs for the staphylococci strains reflect their different susceptibilities. For the strain for which the MIC was relatively high, the median UBTs of both antibiotics were low, and in some of the samples there were no bactericidal titers. UBTs for P. mirabilis were significantly higher with PFL than with FLX. For P. aeruginosa the UBTs were higher for the strain with the lower MIC. Similarly, UBTs for E. faecalis were higher in the more susceptible strain. Against S. saprophyticus, all individuals showed UBTs for at least 48 h with both quinolones.
TABLE 3.
Median (range) urinary bactericidal titers (reciprocal values)a
| Collecting period (h) | Median (range) UBTb (reciprocal value) of indicated drug for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli ATCC 25922
|
K. pneumoniae
|
E. coli
|
S. aureus I
|
P. mirabilis
|
P. aeruginosa I
|
|||||||
| FLX | PFL | FLX | PFL | FLX | PFL | FLX | PFL | FLX | PFL | FLX | PFL | |
| 0–6 | 512 (128–1,024) | 256 (128–1,024) | 512 (64–1,024) | 128 (64–512) | 32 (8–256) | 16 (4–128) | 128 (16–512) | 128 (64–512) | 256 (128–1,024) | 512 (256–1024) | 32 (16–128) | 16 (4–64) |
| 6–12 | 512 (64–1,024) | 128 (64–512) | 256 (64–1,024) | 128 (32–512) | 24 (4–256) | 16 (4–64) | 128 (16–512) | 128 (64–256) | 256 (64–1,024) | 512 (128–1,024) | 32 (8–128) | 16 (8–32) |
| 12–24 | 256 (32–1,024) | 128 (32–1,024) | 256 (16–512) | 128 (16–512) | 16 (4–64) | 8 (2–64) | 64 (4–256) | 128 (32–256) | 128 (64–1,024) | 512 (128–1,024) | 16 (4–32) | 8 (4–32) |
| 24–48 | 64 (32–512) | 64 (16–512) | 64 (16–256) | 32 (8–512) | 4 (0–32) | 4 (0–64) | 32 (2–128) | 64 (16–128) | 32 (32–256) | 128 (64–512) | 4 (2–16) | 2 (0–32) |
| 48–72 | 24 (4–128) | 24 (2–256) | 16 (2–64) | 16 (0–128) | 0 (0–8) | 1 (0–16) | 8 (0–16) | 16 (4–32) | 8 (4–32) | 64 (16–128) | 0 (0–4) | 0 0–8) |
| 72–96 | 8 (0–32) | 8 (0–128) | 3 (0–16) | 4 (0–32) | 0 | 0 (0–8) | 1 (0–4) | 4 (1–16) | 2 (0–8) | 16 (4–128) | 0 | 0 (0–4) |
| 96–120 | 0 (0–8) | 2 (0–32) | 0 (0–2) | 1 (0–32) | 0 | 0 (0–2) | 0 (0–8) | 1 (0–4) | 0 (0–2) | 4 (1–32) | 0 | 0 (0–1) |
| 120–144 | 0 (0–4) | 0 (0–64) | 0 (0–2) | 0 (0–32) | 0 | 0 | 0 | 1 (0–8) | 0 | 2 (0–32) | 0 | 0 |
| 144–168 | 0 (0–4) | 0 (0–32) | 0 (0–4) | 0 (0–16) | 0 | 0 | 0 | 0 (0–4) | 0 | 1 (0–32) | 0 | 0 |
| Median (range) UBT (reciprocal value) of indicated drug for:
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
P. aeruginosa II
|
E. faecalis I
|
S. saprophyticus
|
E. faecalis II
|
S. aureus II
|
Streptococcus group B sp.
|
||||||
| FLX | PFL | FLX | PFL | FLX | PFL | FLX | PFL | FLX | PFL | FLX | PFL |
| 16 (4–64) | 8 (4–32) | 16 (4–32) | 16 (0–32) | 32 (16–64) | 32 (8–64) | 8 (4–32) | 16 (4–32) | 4 (1–16) | 1 (0–8) | 8 (4–32) | 8 (4–16) |
| 8 (2–64) | 8 (4–32) | 16 (2–32) | 8 (0–16) | 24 (8–64) | 16 (8–64) | 8 (2–16) | 8 (4–16) | 2 (1–8) | 1 (0–4) | 8 (4–32) | 8 (2–16) |
| 8 (2–16) | 4 (2–16) | 8 (2–16) | 8 (0–16) | 16 (4–32) | 32 (8–32) | 4 (2–8) | 4 (2–16) | 2 (0–2) | 1 (0–2) | 6 (2–16) | 8 (0–16) |
| 2 (0–16) | 2 (0–16) | 2 (0–4) | 2 (0–8) | 4 (2–8) | 8 (4–16) | 1 (0–4) | 2 (0–8) | 0 (0–2) | 1 (0–1) | 2 (0–4) | 2 (0–4) |
| 0 (0–2) | 0 (0–4) | 0 (0–1) | 1 (0–2) | 0 (0–2) | 2 (8–8) | 0 (0–1) | 0 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–2) | 0 (0–2) |
| 0 | 0 (0–4) | 0 | 0 (0–1) | 0 | 1 (0–2) | 0 | 0 (0–1) | 0 | 0 (0–1) | 0 (0–2) | 0 (0–2) |
| 0 | 0 (0–1) | 0 | 0 | 0 | 0 (0–1) | 0 | 0 | 0 | 0 | 0 (0–1) | 0 (0–2) |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0–1) | 0 (0–1) |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0–1) | 0 |
Dilution with antibiotic-free urine
Values in boldface are those that are significantly (P < 0.05) different for the two treatment groups. Ranges with endpoints of 1,024 include values greater than 1,024.
The UBTs described above represent median values. Within a species, however, there were occasionally large ranges in UBTs (Table 3). This was most clearly seen in the first collection period (0 to 6 h), in which there were differences of more than 4 dilution steps between maximal and minimal UBTs for both quinolones in most cases.
Concerning statistics the three analyses based on an analysis of variance lead to the same interpretation. The main effects and the second-degree interactions between fixed effects are significant, with P values that are ≤0.0001. The third-degree interaction between fixed factors was never in a range indicating an effect (P > 0.4). For estimating the effect of gender all analyses according to this model resulted in P values that were >0.25. Descriptively, values for male subjects were 17% lower than those for female subjects. This difference may well be explained by the higher body weights of the male subjects. As only differences between treatments for specific species and specific points in time appeared to be of interest, only the results of the paired T tests are presented in Table 2, especially since a rough check has indicated normal probability. Because of the highly significant results in the overall analysis, significant differences in these comparisons cannot be considered artifacts created through numerous comparisons.
In comparing AUBTCs, there was no statistical significance for the differences for 8 of the 12 strains. For four strains, E. coli (uropathogen), P. mirabilis, S. saprophyticus, and E. faecalis II, a statistically significant (P < 0.05) difference could be found in favor of PFL.
DISCUSSION
Successful antibiotic treatment is based on pharmacokinetic and pharmacodynamic principles. For lower urinary tract infections it is not difficult to get access to the site of infection and the concentrations of drugs are measured in the urine. The elimination of FLX occurs by both renal and nonrenal pathways. The main metabolic pathways include N demethylation to antimicrobially active NDF and N oxidation to inactive FNO (24, 26). The N-demethyl metabolite has antimicrobial activity against Enterobacteriaceae comparable to that of the parent drug (2). In plasma, Griggs et al. could not detect any concentrations of the active metabolite (11). In contrast to those in plasma, the concentrations of the active metabolite in the urine are high enough to contribute to the antimicrobial activity during the first 3 days. For calculating the concentration of the active drug in the urine, it would be reasonable to add the concentration of the metabolite to that of the parent compound; this would yield up to 74% recovery of the administered dose.
In contrast to FLX, PFL is eliminated predominantly by nonrenal mechanisms (16). PFL metabolism is extensive, mainly by oxidation, to form the principal metabolites NOR and PNO. The latter has little antibacterial activity (8). The small contribution of renal clearance to the elimination of PFL is also confirmed by our results of 12% urinary recovery of unchanged substance. This result, together with that for NOR, indicates that 34% of the dose administered was excreted in the urine as antimicrobially active substance. The pharmacokinetic comparison with FLX reveals considerable differences in the percentages of the total amounts recovered in the urine. Because of the double dose, however, there are no major differences in the concentrations found in urine.
Both antibiotics have good pharmacokinetic and antimicrobial properties and are useful for the treatment of urinary tract infections. The MIC is usually determined under standardized in vitro conditions in defined artificial media at a pH of 7.2. As pointed out in a recent review broth macrodilution methods sometimes yield slightly higher MICs than microdilution methods (7). The differences in our study were mainly within 1 dilution step. Since the antibacterial activities of newer fluoroquinolones are, in general, reduced in urine, the urinary concentrations measured after drug administration cannot be correlated directly with the MICs of the antibiotic obtained in a nutrient broth or agar (10). All quinolones, including FLX and PFL, are up to 60-fold less active in urine at pH 5 than in broth at pH 7. This is partly due to the acid pH and the relatively high magnesium content found in urine (4). Hohl et al. found that the activities of FLX and NOR in urine at pH 7 against a standard inoculum of 2 × 105 CFU/ml were 2- to 4-fold lower than in broth, whereas in urine at pH 5 the activities were as much as 32-fold lower against P. aeruginosa, 16-fold lower against staphylococci, and as much as 64-fold lower against E. coli tested in broth at pH 7 (14a). The decrease in activity was less for FLX than for NOR and smaller against the more resistant strains (P. aeruginosa and S. aureus) than against the very susceptible strains (E. coli). Zhanel et al. studied the effect of human urine on the MIC of ciprofloxacin against E. coli. In urine at pH 5.5 MICs increased 64-fold compared to those measured in Mueller-Hinton broth at pH 7.3 (28). Similar results with newer quinolones have been obtained by other investigators (5, 9, 13, 17, 22, 25). From the data reported in the literature it appears that the discrepancy between in vitro conditions and the physiological environment of urine could be too great to be neglected. However, since the concentration in urine is very high, this could be considered a problem, perhaps, only for pathogens for which the MICs are high, such as enterococci, staphylococci, and Pseudomonas.
MBCs of the fluoroquinolones are usually within two dilutions of the MICs when tested at standard inocula. This is also true for our results. The MBCs of FLX and PFL yielded comparable results and differed only slightly (1 dilution) in three cases. These minor discrepancies do not reflect the differences in the UBTs. Killing of bacteria versus drug concentration is one relevant pharmacodynamic end point. Studies that examine the bactericidal activities of fluoroquinolones versus time confirm their good activities against most gram-negative isolates and have been reviewed recently (7). Exposure of enterobacteria and P. aeruginosa to various fluoroquinolones at concentrations two to four times the MIC typically reduces viable cell counts by 3 log10 units or more by 2 h of incubation. Most of these studies have been performed in Mueller-Hinton agar or similar media; some have been performed in serum. Factors that affect inhibitory activities also alter bactericidal activities. In our in vitro model we used constant concentrations for the entire duration of the experiment. In contrast to those in serum, the kinetics in urine depend on the amount of excreted urine and frequency of micturition. Our results show that the bactericidal kinetics in urine are slower than those in media. The results of an early study, which used simulated serum concentrations in Mueller-Hinton agar, showed the reduction of the initial number of E. coli cells (MIC for E. coli, 0.25 to 0.5 μg/ml) by at least 99% in 1.5 to 4.5 h versus 8 h in our study (3).
The assessment of UBTs integrates the pharmacodynamic aspects of antibacterial activity in urine as medium and obtainable urinary concentrations. This allows for the direct comparison of pharmacodynamic properties of different substances of the fluoroquinolone group with the comparable antimicrobial activities. Since interindividual variations in urinary excretion and in content of urine (pH and cations) can be expected, not only the median but especially also the minimal obtainable UBTs may be of clinical relevance. Aguilar et al. measured the UBTs of rufloxacin and NOR and found NOR UBTs against E. coli in a range of 1:64 to 1:1,024 and somewhat lower rufloxacin UBTs, 1:4 to 1:512, in the first 4 h (1). However the investigators used urine which was supplemented with broth for dilution. The same is true for another study where supplemented urine was used for determining the antibacterial activity of PFL in urine (12). Results from the present study are consistent with findings of previous work on the UBTs of PFL compared to those of NOR, where median UBTs of PFL against E. coli (PFL MIC, 0.125 μg/ml) were measured over 5 days and those against a nalidixic acid-resistant E. coli (PFL MIC, 1.0 μg/ml), K. pneumoniae (PFL MIC, 0.5 μg/ml), and S. saprophyticus (PFL MIC, 2.0 μg/ml) were measured over 3 days (14). Zeiler et al. determined the UBTs of ciprofloxacin, NOR, and ofloxacin under various conditions. They demonstrated that at a neutral pH the UBTs for all three quinolones increased compared to those at pH 5.6 (27).
Calculation of AUBTC from the serial measurement of UBTs could be a rational method to compare antibacterial drugs, since UBTs integrate pharmacokinetics and antibacterial activity, measured in urine. This concept has been evaluated in animal and human clinical studies using measurements of the AUC/MIC ratio in serum and correlating the individual results with clinical outcome for infections other than those of the urinary tract (15, 23). There were no significant differences in the AUBTCs between FLX and PFL, with the exception of those for four strains (S. aureus I, P. mirabilis, S. saprophyticus, and E. faecalis II), in favor of PFL. These differences cannot be explained by different antimicrobial activities but rather reflect longer-lasting UBTs of PFL for these strains. Measured UBTs and AUBTCs for E. coli (uropathogen) of both drugs were smaller than predicted by the MIC. Measured and predicted values for the other bacteria showed close agreement. The clinical significance of the differences in UBTs and AUBTCs is unknown, since specific UBTs and AUBTCs have not been correlated to clinical outcome in prospective human trials. The greater variability of results obtained by using urine, which cannot be standardized as well as defined artificial nutrient media, as the medium may be regarded as a disadvantage of this method. On the other hand two possible advantages should be considered: results obtained for an individual patient may reflect much better the specific clinical situation and results obtained for a collection of individuals may reflect much better the variability in “real life” that one has to consider when giving dosage recommendations. Thus, measurement of UBTs and AUBTCs could be a suitable investigational tool for the comparison of drugs of the same class and for the development and evaluation of new drugs. This method should be included, therefore, in the clinical studies to evaluate its predictive value.
In conclusion, the study has shown that after a single oral doses of 400 mg of FLX and 800 mg of PFL, comparable urinary concentrations of antibacterial active substances can be found, resulting in comparable killing rates and UBTs against uropathogens with different susceptibilities over a wide range of MICs. For two strains (E. faecalis I and S. aureus II) UBTs of FLX, not those of PFL, were present in all individuals at any time, whereas for four strains (S. aureus I, P. mirabilis, S. saprophyticus, and E. faecalis II) the AUBTC for PFL was significantly larger than that for FLX. The remaining strains showed no difference. The data of this study are based on strains with comparable MICs and MBCs. Overall, a single oral dose of 400 mg of FLX exhibits UBTs comparable to those of 800 mg of PFL. Therefore, it may be expected that for FLX half of the dose of PFL gives comparable results in the treatment of urinary tract infections. This hypothesis should be substantiated in comparative clinical trials.
ACKNOWLEDGMENTS
We thank B. Birner, K. Hollauer, and D. Kirchbauer for excellent technical assistance and P. Reimnitz and G. Boudnitzki for statistical analysis.
This work was supported by Hoffmann-La Roche Ltd., Basel, Switzerland.
REFERENCES
- 1.Aguilar L, Balcabao I P, Salvá P, Martín M, Costa J, Prieto J, Dal-Ré R. Ex vivo antibacterial properties of rufloxacin in a study with healthy volunteers. Antimicrob Agents Chemother. 1996;40:17–21. doi: 10.1128/aac.40.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anghern P, Gmünder H. Metabolites of fleroxacin: antibacterial properties and inhibition of DNA gyrase. In: Adam D, Lode H, Rubinstein E, editors. Recent advances in chemotherapy. Munich, Germany: Futuramed Publishers; 1992. pp. 2370–2371. [Google Scholar]
- 3.Bauernfeind, A., E. Eberlein, and G. Hörl. 1988. Bactericidal kinetics of various dosages of fleroxacin simulated in bacterial cultures. J. Antimicrob. Chemother. 22(Suppl. D):81–89. [DOI] [PubMed]
- 4.Chin N X, Brittain D C, Neu H C. In vitro activity of Ro 23-6240, a new fluorinated 4-quinolone. Antimicrob Agents Chemother. 1986;29:675–680. doi: 10.1128/aac.29.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen M A, Huband M D, Mailloux G B, Yoder S L, Roland G E, Heifetz C L. In vitro antibacterial activities of the fluoroquinolones PD 117596, PD 124816, and PD 127391. Diagn Microbiol Infect Dis. 1991;14:245–258. doi: 10.1016/0732-8893(91)90039-i. [DOI] [PubMed] [Google Scholar]
- 6.Edberg S C. The measurement of antibiotics in human body fluids: techniques and significance. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 466–467. [Google Scholar]
- 7.Eliopoulos G M, Eliopoulos C T. Activity in vitro of the quinolones. In: Hooper D C, Wolfson J S, editors. Quinolone antimicrobial agents. Washington, D.C: American Society for Microbiology; 1993. pp. 161–193. [Google Scholar]
- 8.Frydman, A. M., Y. le Roux, M. A. Lefebvre, F. Djebbar, J. B., Fourtillan, and J. Gaillot. 1986. Pharmacokinetics of pefloxacin after repeated intravenous and oral administration (400mg bid) on young healthy volunteers. J. Antimicrob. Chemother. 17(Suppl. B):65–79. [DOI] [PubMed]
- 9.Gargallo-Viola D, Esteve R, Llovera S, Roca X, Guinea J. In vitro and in vivo antibacterial activities of E-4497, a new 3-amine-3-methyl-azetidinyl tricyclic fluoroquinolone. Antimicrob Agents Chemother. 1991;35:442–447. doi: 10.1128/aac.35.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gesu G P, Eftimiadi C, Debbia E, Schito G C. Effects of changes in pH, medium and inoculum size on the in vitro activity of different quinolone and fluoroquinolone antibiotics against urinary pathogens. Drugs Exp Clin Res. 1987;13:79–84. [PubMed] [Google Scholar]
- 11.Griggs, D. J., R. Wise, B. Kirkpatrick, and J. P. Ashby. 1988. The metabolism and pharmacokinetics of fleroxacin in healthy subjects. J. Antimicrob. Chemother. 22(Suppl. D):191–194. [DOI] [PubMed]
- 12.Guibert J, Kitzis M D, Brumpt I, Acar J F. Antibacterial activity of pefloxacin in the urine during the 7 days following a single 800 mg oral dose. Pathol Biol. 1989;37:406–410. [PubMed] [Google Scholar]
- 13.Guinea J, Robert M, Gargallo V D, Xicota M A, Garcia J, Tudela E, Esteve M, Coll R, Pares M, Roser R. In vitro and in vivo antibacterial activities of E-4868, a new fluorquinolone with a 7-azetidin ring substituent. Antimicrob Agents Chemother. 1993;37:868–874. doi: 10.1128/aac.37.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofbauer H, Kinzig M, Kresken M, Naber K G, Reiz A, Sörgel F, Rustige-Wiedemann C, Wiedemann B. Urine bactericidal titres of pefloxacin versus norfloxacin in volunteers receiving a single oral dose of 800mg. Infection. 1997;25:121–125. doi: 10.1007/BF02113592. [DOI] [PubMed] [Google Scholar]
- 14a.Hohl, P., and A. M. Felber.. 1988. Effect of method, medium, pH and inoculum on the in-vitro antibacterial activities of fleroxacin and norfloxacin. J. Antimicrob. Chemother. 22(Suppl. D):71–80. [DOI] [PubMed]
- 15.Israel D, Gillum J G, Turik M, Harvey K, Ford J, Dalton H, Towle M, Echols R, Heller A H, Polk R. Pharmacokinetics and serum bactericidal titers of ciprofloxacin and ofloxacin following multiple oral doses in healthy volunteers. Antimicrob Agents Chemother. 1993;37:2193–2199. doi: 10.1128/aac.37.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karabalut N, Drusano G L. Pharmacokinetics of the quinolone antimicrobial agents. In: Hooper D C, Wolfson J S, editors. Quinolone antimicrobial agents. Washington, D.C: American Society for Microbiology; 1993. pp. 195–244. [Google Scholar]
- 17.Leigh D A, Tait S, Walsh B. Antibacterial activity of lomefloxacin. J Antimicrob Chemother. 1991;27:589–598. doi: 10.1093/jac/27.5.589. . (Erratum, 28:481, 1991.) [DOI] [PubMed] [Google Scholar]
- 18.Machka K, Braveny I. Inhibitorische Wirkung verschiedener Faktoren auf die Aktivität von Norfloxacin. In: Stille W, Adam D, Eickenberg H U, Knothe H, Ruckdeschl G, Simon C, editors. Gyrase-Hemmer I. Fortschritte der Antimikrobiellen und Antineoplastischen Chemotherapie. IAC. 3-5. Munich, Germany: Futuramed; 1984. pp. 557–562. [Google Scholar]
- 19.Naber, K. G. 1995. Urinary excretion and bactericidal titers following a single oral dose of 400mg fleroxacin versus 800mg pefloxacin in healthy volunteers. Can. J. Infect. Dis. 6(Suppl. C):268.
- 20.Naber K G, Witte W, Bauernfeind A, Wiedemann B, Grimm H. Resistenzentwicklung gegenüber Fluorquinolonen. Chemother J. 1995;2:106–111. [Google Scholar]
- 21.Norrby S R. Treatment of urinary tract infections with quinolone antimicrobial agents. In: Hooper D C, Wolfson J S, editors. Quinolone antimicrobial agents. Washington, D.C: American Society for Microbiology; 1993. pp. 273–283. [Google Scholar]
- 22.Sato K, Hoshino K, Tanaka M, Hayakawa I, Osada Y. Antimicrobial activity of DU-6859, a new potent fluoroquinolone, against clinical isolates. Antimicrob Agents Chemother. 1992;36:1491–1498. doi: 10.1128/aac.36.7.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schentag J J, Nix D E, Forrest A. Pharmacodynamics of fluorquinolones. In: Hooper D C, Wolfson J S, editors. Quinolone antimicrobial agents. Washington, D.C: American Society for Microbiology; 1993. pp. 259–271. [Google Scholar]
- 24.Sörgel, F., R. Seelmann, K. Naber, R. Metz, and P. Muth. 1988. Metabolism of fleroxacin in man. J. Antimicrob. Chemother. 22(Suppl. D):169–170. [DOI] [PubMed]
- 25.Tanaka M, Hoshino K, Ishida H, Sato K, Hayakawa I, Osada Y. Antimicrobial activity of DV-7751a, a new fluoroquinolone. Antimicrob Agents Chemother. 1993;37:2112–2118. doi: 10.1128/aac.37.10.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weidekamm E, Portmann R, Partos C, Dell D, Lucker P W. Single and multiple-dose pharmacokinetics of fleroxacin, a trifluorinated quinolone, in humans. Antimicrob Agents Chemother. 1987;31:1909–1914. doi: 10.1128/aac.31.12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeiler, M. J., D. Beerman, W. Wingender, D. Föster, and P. Schaht. 1988. Bactericidal activity of ciprofloxacin, norfloxacin, and ofloxacin in serum and urine after oral administration to healthy volunteers. Infection 16(Suppl. 1):19–23. [DOI] [PubMed]
- 28.Zhanel G G, Karlowsky J A, Davidson R J, Hoban D J. Influence of human urine on the in vitro activity and postantibiotic effect of ciprofloxacin against Escherichia coli. Chemotherapy. 1991;37:218–223. doi: 10.1159/000238857. [DOI] [PubMed] [Google Scholar]