Abstract
Background:
Tunneled hemodialysis catheter-related bloodstream infection is a major cause of morbidity and mortality in end-stage renal disease patients. Side holes positioned near the tip of catheters have been linked to formation of thrombi, which, in turn, have been implicated in predisposition to infection. In addition, side holes allow spillage of catheter locking solution, including antibiotics, thereby minimizing the lock solution’s effect on the catheter tip. This study assessed the infection events that occurred in a series of hemodialysis patients using a non-side-hole catheter.
Methods:
Over a period of 2 years, a novel symmetric-tip non-side-hole catheter was placed in 60 patients. Hemodialysis was performed thrice weekly. Prescribed dialyzer flows were 300–350 mL/min. Catheters were routinely locked with heparin 5000 units/mL between treatments. Patients were followed up for any catheter related complications, specifically infection events.
Results:
Seven events of catheter-related bloodstream infection occurred for a rate of 0.76 events per 1000 catheter-days, with the first event occurring 9 weeks after insertion. These events were treated by locking the affected catheter with 2 g of clindamycin in 2 mL of heparin 1000 units/mL and administration of intravenous antibiotics, in most cases, for 7–14 days. Two catheters were removed due to infection.
Conclusions:
Catheter-related bloodstream infections with non-side-hole hemodialysis catheters do occur at a relatively low rate and in this initial preliminary study it seems that most of these infections can be successfully treated without removal of the affected catheters.
Keywords: Pristine, hemodialysis, catheter, tunneled, symmetric
Introduction
Tunneled dialysis catheters (TDCs) have firmly established themselves as a valuable tool for providing vascular access in hemodialysis patients. 1 Unlike the arteriovenous fistula (AVF) or graft (AVG), TDCs do not require maturation. However, they are more prone to infectious complications than AVF or AVG. 2 due to bacterial colonization secondary, mostly, to inadequate aseptic technique of dialysis unit operators, as particularly evident from the reduction in catheter-related bloodstream infection (CRBSI) rates during the infection-conscious COVID-19 era. 3 The higher rate of CRBSI experienced with TDCs translates to higher morbidity and mortality rates 2 and to a considerable healthcare expenditure. 4 Despite advances in the material design 5 and introduction of antibiotic coatings, 6 this challenge remains unresolved. 7
Side holes at the tip of catheters, intended to support inflow in case of obstruction of the end hole of a catheter, 8 may exacerbate the infectious risk of TDCs. Presence of side holes favors clot formation by creating a low flow zone at the catheter tip, 9 allowing rapid removal of anticoagulant lock solutions, 10 and by presenting imperfections of the cut surfaces to which thrombi have been shown to attach firmly.8,11 Since, clinically, complicated catheter-related bacteremia is linked to infected mural thrombi, 12 a catheter devoid of side holes may be associated with less bacterial growth, thereby reducing occurrence of complicated CRBSI and their associated morbidity. Salvage of the already infected catheters is yet another hurdle that must be considered in hemodialysis patients with TDCs. While side-hole catheters allow an almost instantaneous wash-out of antibiotic locking solution from the catheter tip through the side holes, 8 mainly the most proximal ones, 13 in side-hole–free TDCs the antibiotic locking solution reaches the catheter tip and is likely to linger longer. This may increase the time of exposure of intraluminal bacteria to antibiotics in the locking solution, leading to resolution of infection and salvage of the affected catheter.
To test this hypothesis, we retrospectively assessed CRBSI associated with use of a side-hole–free catheter in a series of hemodialysis patients. The Pristine hemodialysis catheter (Pristine Access Technologies Ltd., Israel) has a split, symmetrical, side-hole–free tip designed to be placed in the upper right atrium and oriented in the anterior posterior position (Figure 1). In an animal model, the Pristine hemodialysis catheter was largely superior to a symmetric tip catheter, which features multiple side holes at its tip, in impeding clot formation. 14 In addition, the Pristine hemodialysis catheter also allowed complete aspiration of the intraluminal thrombus which accumulated in the catheter tips. In this paper we aimed to assess how the reduction of clot accumulations with side-hole–free catheter tip design translates to clinical practice, specifically with regard to CRBSIs.
Figure 1.
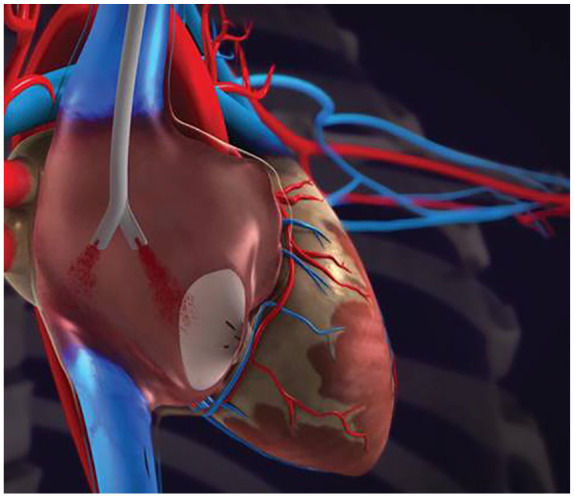
Illustration of the Pristine hemodialysis catheter tip orientation in the right atrium.
Materials and methods
Patients
Between November 2016 and January 2019, the Pristine hemodialysis catheter was inserted de novo in 60 adult men and women, 18 years of age or older, diagnosed with end-stage kidney disease (ESKD), and prescribed chronic hemodialysis treatments three times per week for a minimum of 90 days. All patients had a patent right internal or external or left internal jugular vein (RIJJV, REJV, and LIJV, respectively). None of the patients suffered from central venous stenosis, active infection, or bacteremia within 7 days prior to catheter insertion. None of the female patients were pregnant or breastfeeding. The study was approved by the local ethics committee and informed consent was obtained from all participants.
Catheter insertion procedure
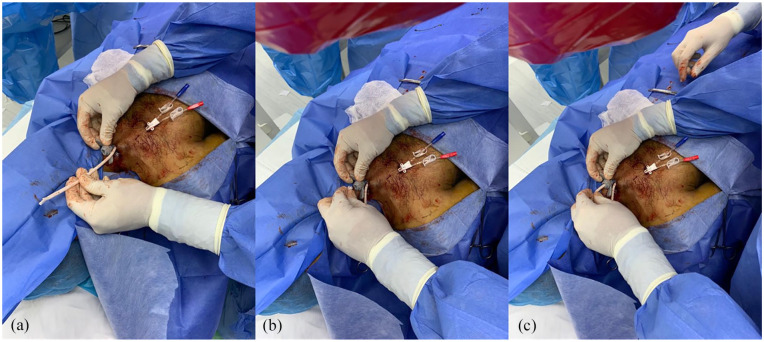
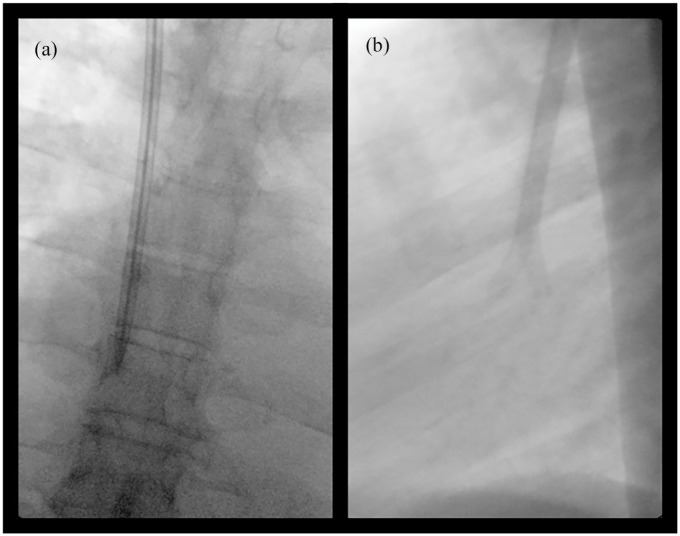
Catheters were implanted by six operators (one surgeon, one radiologist, and four nephrologists). Following local anesthesia of entry site, an introducer needle was inserted into the vein under ultrasound visualization. A 0.038″ J/Straight stainless-steel guidewire was then inserted, and the introducer needle was removed. The Pristine hemodialysis catheter was attached to the proximal end of its tunneler which was then inserted under the skin, leading the catheter through the created tunnel. The tunneler was then separated from the catheter, and the latter was positioned with its polyester felt cuff under the skin. The access hole to the vein was widened with 10-14–Fr dilators threaded one after the other over the guidewire. A peel-away sheath was inserted into the vein over the guidewire and the catheter was introduced into the vein through the peel-away sheath (Figure 2). Anterior posterior orientation of the tip was maintained to allow for positioning of the arterial lumen in the mid-right atrium and prevent contact of the tip with tissue (Figure 3). The peel-away sheath was then extracted and both lumens of the Pristine hemodialysis catheter were forcefully aspirated with blood several times, using a 20-mL syringe. X-ray imaging was performed to confirm tip placement in the mid- to upper-right atrium. If there was difficulty aspirating, the catheter was pulled back 2 cm until free flow was achieved. The catheter was then flushed with normal saline. Heparin lock was added to each lumen according to the amount indicated on the lumen, and the extension legs of the catheter were clamped. A sterile sealing cap was attached to the Luer adapters of the lumens. Finally, the insertion site was sutured. Catheter position was again confirmed by X-ray examination.
Figure 2.
Images of the Pristine Hemodialysis catheter (a) pulled through the tunnel and proximally to the peel-away sheath, (b) catheter tip squeezed together, and (c) inserted into the peel-away sheath while maintaining the AP orientation.
Figure 3.
X-ray images in the anterior-posterior (a) and lateral projection (b) of the Pristine hemodialysis catheter preferred orientation in the right atrium.
Treatment and follow-up
Dialysis treatments were delivered, and follow-up visits were performed, according to the standard dialysis treatment common practice, at four different locations in the Dominican Republic (Manzano, La Romana; Cerdial, La Romana; MUSA/Hospital Dr. Jaime Oliver Pino, San Pedro de Macoris; Hospital El Buen Samaritano, La Romana). Four catheters were inserted in the left and the other 56 catheters – in the right internal jugular vein (LIJV and RIJV, respectively). Forty-one catheters were 19 cm in length, 15–23 cm, and 4–28 cm. All but one were inserted in patients in whom the previously inserted acute catheters had failed. Contact with one of the 60 patients (19-cm–long catheter inserted in the RIJV) was lost after catheter implantation and before dialysis.
Patients had dialysis three times per week for 6 months after insertion of the Pristine hemodialysis catheter. Treatments were administered at a dialyzer-delivered blood flow rate of 300–350 mL/min and follow-up visits were performed three times per week for 6 months. Inter-treatment catheter locking was routinely performed with sterile saline solution containing 5000 units/mL of unfractionated heparin. No measures were taken to prevent CRBSI and thrombolytic agents were not administered in any of the patients. CRBSI was defined as occurrence of one positive blood culture without obvious source.
Results
The mean age of the patients was 53.38 (SD = 14.15). Demographic and baseline descriptors of the patients are summarized in Table 1. A total of 9152 catheter days were observed in 59 patients (mean and median follow-up length of 155 and 183 days, respectively).
Table 1.
Demographic and baseline characteristics.
| N | % | |
|---|---|---|
| Age distribution | ||
| Under 65 years | 46 | 76.67 |
| 65–79 years | 13 | 21.67 |
| 80 + years | 1 | 1.67 |
| Male gender | 37 | 61.67 |
| Body mass index (mean kg/m2) | 24.41 | |
| Cause of ESKD | ||
| Diabetes | 12 | 20.00 |
| Hypertension | 38 | 63.33 |
| Diabetes and hypertension | 3 | 5.00 |
| Glomerulonephritis | 5 | 8.33 |
| Medical comorbidities | ||
| Diabetes | 26 | 43.33 |
| Hypertension | 55 | 91.67 |
| Tobacco smoker, past | 17 | 28.33 |
In only two cases CRBSI eventually resulted in catheter removal. In all other cases, the infection was resolved by locking the affected catheter with an antibiotic solution (clindamycin admixed with heparin) and systemic antibiotic therapy with imipenem or meropenem. Administration of the systemic antibiotics was limited to 7–14 days in all cases but one. In the remaining patient, with gram-negative infection, 1 g of imipenem was administered in every dialysis treatment for a month. One patient experienced two infectious events. Both responded well to treatment, the catheter was retained, and the patient completed the planned follow-up.
Discussion
Historically, CRBSI rates in hemodialysis patients using a TDC range from 0.6 to 6.5 episodes per 1000 catheter days,15,16 with 1.6/1000 catheter days being the point incidence rate calculated from data of 16 prospective studies conducted in adults. 17 Consequently, the rate of 0.76 events per 1000 catheter days observed with the side-hole–free TDCs in the series reported here falls below the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NK KDOQI) guideline of low CRBSI rate. 2 This statistic is of an even greater value when reported in a study conducted in a limited-resource country, where the rate of central-line associated blood stream infections, in general, is inherently higher 18 than, for instance, in the United States. 19 Equally interesting, in this regard, is the lack of CRBSI events in the first 9 weeks post implantation.
Of note, the CRBSI definition in this study differed from that recommended by the 2019 KDOQI guideline, which were published after the end of this study. However, it is aligned with the definition of “probable” CRBSI in the previous edition of the guideline, that is, defervescence of symptoms after antibiotic therapy with or without removal of the catheter, in the setting in which blood culture confirms infection, but catheter tip does not, in a symptomatic patient with no other apparent source of infection. 2 Consequently, the results reported here represent the worst-case scenario in terms of the rate of CRBSI events observed with the side-hole–free TDCs.
Figure 4.
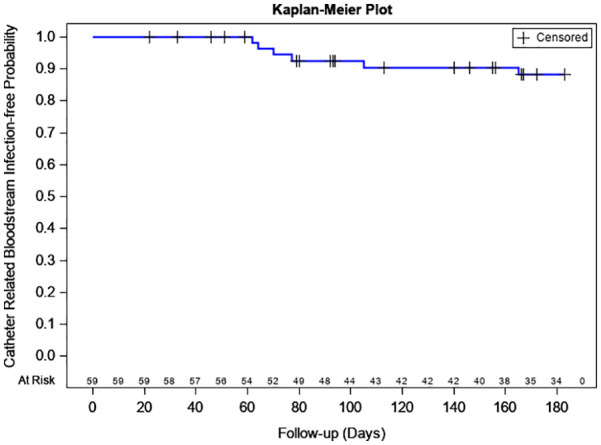
Kaplan–Meier curve of estimated probabilities of freedom from catheter-related bloodstream infection.
The apparent ability to resolve catheter-related infection in this study is promising, as the reported salvage rates for antibiotic locks used in conjunction with systemic antibiotics, usually fall below 70%. 20 Of note, contrary to these reports, treatment in our study did not include vancomycin-based antibiotic locks, which may promote the emergence of vancomycin-resistant pathogens. 21 Also promising is the ability to salvage the catheter once a catheter-related infection has occurred. Consequently, aside of the obvious immediate benefit to the patients, the results seen in the current study may also add the considerations of reducing the possible spread of antibiotic resistance. 2
Table 2.
Kaplan–Maier estimated probabilities of freedom from catheter-related bloodstream infection.
| Time (days) | Kaplan–Meier estimated probability of no catheter-related bloodstream infection (%) | Lower 95% confidence limit (%) | Upper 95% confidence limit (%) |
|---|---|---|---|
| 30 | 100.0 | 100.0 | 100.0 |
| 60 | 100.0 | 100.0 | 100.0 |
| 90 | 92.59 | 81.46 | 97.15 |
| 120 | 90.49 | 78.60 | 95.94 |
| 180 | 88.11 | 75.28 | 94.51 |
Randomized multi-center studies directly comparing side-hole to side-hole–free catheters are needed to better assess our observations. In addition, rigorous investigation of the role of side holes in TDC outcomes is warranted. As CRBSI-related mortality rate in hemodialysis patients with TDCs is high and, unlike the all-cause mortality, does not show signs of declining, 22 future studies of TDCs should probably add assessment of infection-related mortality to their outcomes.
Acknowledgments
We thank Dr. Igor Ruvinsky for his help in drafting the manuscript.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have equity in Pristine Access Technologies, the developer of the Pristine Catheter.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided by Pristine Access Technologies Ltd.
ORCID iD: Michael G Tal  https://orcid.org/0000-0002-2642-4296
https://orcid.org/0000-0002-2642-4296
References
- 1. United States Renal Data System. 2019 annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2019. [Google Scholar]
- 2. Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 2020; 75: S1–S164. [DOI] [PubMed] [Google Scholar]
- 3. Heidempergher M, Sabiu G, Orani MA, et al. Targeting COVID-19 prevention in hemodialysis facilities is associated with a drastic reduction in central venous catheter-related infections. J Nephrol 2021; 34(2): 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramanathan V, Chiu EJ, Thomas JT, et al. Healthcare costs associated with hemodialysis catheter-related infections: a single-center experience. Infect Control Hosp Epidemiol 2007; 28: 606–609. [DOI] [PubMed] [Google Scholar]
- 5. Tal MG, Ni N. Selecting optimal hemodialysis catheters: material, design, advanced features, and preferences. Tech Vasc Interv Radiol 2008; 11: 186–191. [DOI] [PubMed] [Google Scholar]
- 6. Moist L, Vachharajani T. Chapter 3 - Central venous access for hemodialysis A2 - Nissenson, Allen R. In: Fine RN. (ed.) Handbook of dialysis therapy. 5th ed. Elsevier, UK, 2017, pp.40–9.e1. [Google Scholar]
- 7. Gilbert RE, Harden M. Effectiveness of impregnated central venous catheters for catheter related blood stream infection: a systematic review. Curr Opin Infect Dis 2008; 21: 235–245. [DOI] [PubMed] [Google Scholar]
- 8. Ash SR. Advances in tunneled central venous catheters for dialysis: design and performance. Semin Dial 2008; 21: 504–515. [DOI] [PubMed] [Google Scholar]
- 9. Mareels G, De Wachter DS, Verdonck PR. Computational fluid dynamics-analysis of the Niagara hemodialysis catheter in a right heart model. Artif Organs 2004; 28: 639–648. [DOI] [PubMed] [Google Scholar]
- 10. Barbour MC, Gow KW, Aliseda A. Convectively dominated heparin leakage from multiple catheter designs: an in vitro experimental study. ASAIO J 2018; 64: e94–e104. [DOI] [PubMed] [Google Scholar]
- 11. Moore HL. Side holes at the tip of chronic hemodialysis catheters are harmful. J Vasc Access 2001; 2: 8–16. [DOI] [PubMed] [Google Scholar]
- 12. Strinden WD, Helgerson RB, Maki DG. Candida septic thrombosis of the great central veins associated with central catheters. Clinical features and management. Ann Surg 1985; 202: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sungur M, Eryuksel E, Yavas S, et al. Exit of catheter lock solutions from double lumen acute haemodialysis catheters—an in vitro study. Nephrol Dial Transplant 2007; 22: 3533–3537. [DOI] [PubMed] [Google Scholar]
- 14. Tal MG, Livne R, Neeman R. Clot accumulation at the tip of hemodialysis catheters in a large animal model. J Vasc Access. Epub ahead of print 25 December 2020. DOI: 10.1177/1129729820983617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 2011; 79: 587–598. [DOI] [PubMed] [Google Scholar]
- 16. Ravani P, Gillespie BW, Quinn RR, et al. Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol 2013; 24: 1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006; 81: 1159–1171. [DOI] [PubMed] [Google Scholar]
- 18. The Joint Commission. Preventing central line-associated bloodstream infections: a global challenge, a global perspective. Oak Brook, IL: Joint Commission Resources, 2012, p.136. [Google Scholar]
- 19. Centers for Disease Control and Prevention. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 2011; 60: 243–248. [PubMed] [Google Scholar]
- 20. Manierski C, Besarab A. Antimicrobial locks: putting the lock on catheter infections. Adv Chronic Kidney Dis 2006; 13: 245–258. [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep 2002; 51: 565–567. [PubMed] [Google Scholar]
- 22. Moore CL, Besarab A, Ajluni M, et al. Comparative effectiveness of two catheter locking solutions to reduce catheter-related bloodstream infection in hemodialysis patients. Clin J Am Soc Nephrol 2014; 9: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]