Abstract
Diabetic foot complications represent a substantial health burden and are the foremost cause of hospitalization in patients with diabetes. Diabetes mellitus (DM) is known to cause several other problems. Diabetes is rapidly becoming the leading cause of illness and death worldwide. Diabetic foot ulcers (DFU) are one of the most painful complications of diabetes. These complications cause problems in blood vessels, nerves, and other organs throughout the body. DFU pathophysiology is attributed to a triad of neuropathies, trauma with secondary infection, and arterial occlusive disease. This review aims to identify the types of wounds that diabetics can develop. Owing to the complexity of their disease pathology, diabetics are susceptible to a variety of wounds, such as diabetic ulcers due to trauma (DUDT); neuropathic, ischemic, neuroischemic, arterial, venous, and mixed wounds; and diabetic bullae, furuncles, cellulitis, and carbuncles. Therefore, it is essential for healthcare providers to recognize the specific classification of a diabetic wound based on its distinctive attributes to provide appropriate wound care and therapeutic interventions. In the context of individuals with diabetes, it is of paramount significance to precisely identify the types of wounds during the initial evaluation to provide appropriate care and treatment, thereby enhancing the probability of favorable outcomes.
Keywords: Diabetic wounds, neuropathic, neuroischemic, ischemic, DUDT, venous, arterial
Introduction
Diabetic foot ulcer (DFU), a complication associated with diabetes, is estimated to have a prevalence of 6.3% worldwide. The prevalence rates of DFU have been documented to be 13% in North America, 5.5% in Asia, 5.1% in Europe, 7.2% in Africa, and 3.0% in Oceania. 1 The potential complications associated with DFU encompass the necessity of amputation due to gangrene and infection. 1 Patients with diabetes have a 25% higher likelihood of developing foot and chronic ulcers. 2 They commonly develop ulcers because of peripheral arterial disease (PAD), high plantar pressure, inadequate foot care, trauma, neuropathy, and foot pathologies such as fissures and callosities.2,3
Diabetic foot ulcers are considered neuropathic if the patient has peripheral neuropathy; ischemic if the patient has PAD without peripheral neuropathy; and neuroischemic if the patient has both neuropathy and ischemia. 4 Additionally, patients with diabetes may develop arterial, venous, or mixed ulcers (a combination of both arterial and venous ulcers), as well as furuncles, carbuncles, cellulitis, and diabetic bullae.5 -10 Several studies have examined the different types of ischemic and neuropathic ulcers. However, only a few have studied the other types of diabetic ulcers or wounds.
Diabetic Ulcers due to Trauma (DUDT)
A DUDT is a traumatic wound caused by external forces such as accidents, surgeries, physical contact, burns, radiation, thermal injuries, and mechanical trauma.7,8,11 Trauma is the primary cause of diabetic ulcers. 8 Diabetic wounds due to trauma or injury may also be caused by various factors like being pricked by thorns, nails, or glass shards. The ankle–brachial index (ABI) value is normal or >0.9, and its tendency can be used to determine the presence of nervous and vascular disorders.
The characteristics of DUDT wounds (Figure 1A) differ from those of ischemic ulcers (pale, yellow, and cold with weak or absent pulse), neuropathic ulcers (preceded by callus formation), arterial ulcers (characterized by intense pain, punched-out appearance, shininess, decreased hair growth, pallor on leg elevation, weak or absent pulse, and delayed capillary refill), neuroischemic ulcers (with an abnormal ABI), and peripheral neuropathic ulcers.10,11 DUDT can result from various types of trauma and appears distinct from other diabetic ulcers.9,12 Depending on the pathological effects of PAD and/or neuropathy, DUDT may present as a neuropathic or an ischemic ulcer. Consequently, patients with DUDT often have a poor prognosis. However, DUDT wounds may heal faster if the ABI is ⩾0.8,9,10 and if there are no comorbidities, such as neuropathy, PAD, or infection; older age, smoking, poor glycemic control, prior foot ulceration or amputations, or ischemia of the small and large blood vessels are also factors influencing healing.9,13 -15 Although rare, the presence of DUDT indicates that it arises from multiple traumatic factors and does not involve callus formation or hyperkeratosis, ischemia, an ABI ⩾ 0.8, or a location that can affect both extremities and/or other body parts. 9 Wound care management in DUDT typically employs the moist principle. Dressings suitable for wound care include various options serving different purposes. These dressings absorb exudates, possess antimicrobial properties, and maintain a moist wound bed to promote rapid healing.
Figure 1.
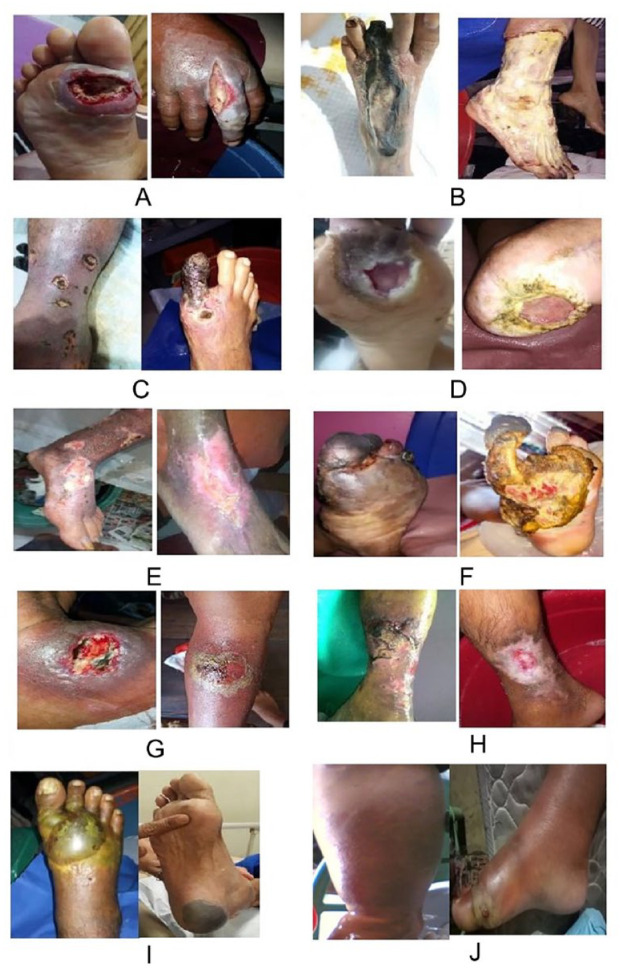
(A) DUDT, (B) ishemic ulcer, (C) arterial ulcer, (D) neuropathic ulcer, (E) mix ulcer, (F) neuro-ischemic, (G) furuncle/carbuncle, (H) venous ulcer, (I) diabetic bullae, and (J) cellulitis.
Source: Data adapted from Suriadi, 8 Cellulitis contributed by Suriadi.
Ischemic Ulcers
Ischemic ulcers result from inadequate blood flow, which causes local ischemia in the skin and the underlying tissues. These ulcers can initially manifest as blue discoloration, necrosis, or gangrene, often starting at the fingertips, and pose an increased risk of amputation if left untreated. PAD is the most frequent underlying cause, leading to symptoms including localized ulceration, gangrene, intermittent claudication, and pain. Neuroischemic ulcers are caused by the combination of nerve damage and PAD.
Differentiating between types of diabetic ulcers is crucial for accurately identifying the wound type, thereby enabling the selection of appropriate treatments for improved prognosis and infection prevention. Although a moist wound environment is generally recommended, it may not necessarily apply to all wounds. Based on the patient’s condition, the treatment plan should encompass careful regulation of moisture in diabetic feet. Hydration, for instance, may not be appropriate for an ischemic or neuroischemic foot with dry gangrene (dry necrotic tissue) and the absence of infection because the gangrenous regions may become wet, leading to infection. Furthermore, with adequate care and attention, toes, feet, and ulcers can naturally dry and mummify, thereby facilitating autoamputation.4,16
Ischemic ulcers frequently develop distally on the dorsum of feet or toes. 17 Initially, these ulcers usually have irregular edges; however, as they progress, they become more clearly defined. Unlike the other types of ulcers, ischemic ulcers tend to bleed slightly or not at all during manipulations such as debridement. 18 In contrast, PAD, which typically affects the tibial and peroneal arteries but spares the dorsalis pedis artery, results in ischemic ulcers. Such ulcers develop independently, are often painful, and usually manifest as gangrene on the toes, heels, and edges of the feet. 19 Additionally, patients may experience characteristic pain, which is alleviated by dependency on the extremities, particularly at night while in a supine position. Upon examination, the patient is noted to have chronic ischemia characterized by hair loss, pale skin, and an absent pulse. Calcification is frequently observed in the digital and metatarsal arteries 18 ; the feet are cold, but the pulse may be felt. Although neuroischemic ulcers may resemble ischemic ulcers, they are usually painless because of the accompanying neuropathy. 8 An ischemic ulcer is diagnosed when a diabetic foot ulcer displays features consistent with PAD. PAD is defined as an ABI < 0.9. Approximately 0.7% of patients with diabetes and ischemic ulcers have an ABI of 0.5, indicating an ischemic condition. 9 The patients exhibit typical clinical manifestations of ischemic ulcers, such as paleness, coldness, and the absence of a pulse 5 (Figure 1B). Distinguishing between arterial and ischemic ulcers can be challenging for clinicians. These may differ considerably,9,19 and the ABI can be used for differentiation. Understanding the clinical presentation and ABI results is crucial for the accurate identification and management of ulcers. Several risk factors have been associated with severe tissue loss and major amputation in patients with lower limb ischemic ulcers. 20
Arterial Ulcers
Arterial ulcers occur specifically in patients with diabetes because of insufficient blood flow into the peripheral blood vessels, resulting in severe pain. Insufficient blood circulation leads to a reduction in the delivery of oxygen and essential nutrients to the tissues, resulting in detrimental effects eventually leading to cellular demise, which is commonly referred to as necrosis. 21 Consecutively, the aforementioned process may result in distressing ulcers characterized by delayed or nonexistent healing. Such ulcers are characterized by pale wound areas, slightly cold skin, and a shiny and puffy appearance. The wounds are necrotic or gangrenous, with a weak pulse. Arterial ulcers are frequently associated with a history of hypertension, hyperlipidemia, diabetes, and smoking. 21 Approximately 2.1% of patients with diabetes have arterial ulcers, which are caused by a decrease in arterial blood supply to the lower limbs with atherosclerotic disease affecting the medium and large arteries. 17 Furthermore, ischemic ulcers are occasionally referred to as arterial ulcers.10,22 Patients with diabetes and arterial ulcers have an ABI of <0.9,23,24 whereas the ABI of those with painful arterial ulcers ranges from 0.5 to 0.8. 23 Although the ABI indicates the presence of ischemia, the characteristics of the ulcer, including its features, skin temperature, color, pain sensation, pulse, and location, pertain to arterial ulcers. 13 Arterial ulcers predominantly affect toes, heels, and bony protrusions of the foot. These ulcers are characterized by well-defined edges and a pale and nongranulated necrotic base, with the surrounding skin showing dusky erythema and being cool to the touch. The skin may appear hairless, thin, brittle, or shiny. The common features of the toenails include a thickening, “punched-out” appearance with well-demarcated edges and a pale, non-granulating necrotic base, and the necrotic base often turns opaque and falls off. Gangrene may also be present in the extremities. The dorsalis pedis and posterior tibial arteries may exhibit diminished or absent pulses, and the presence of bruits in the proximal leg arteries may indicate atherosclerosis. 23 Patients with diabetes and an ABI ⩾ 0.8 are generally not at a significant risk of developing ischemic ulcers, although appropriate treatment is necessary to prevent their occurrence. Depending on the severity of the PAD-related conditions, arterial ulcers can cause lesion expansion, extensive ischemia, infection, and, ultimately, amputation, 24 (Figure 1C). Management of arterial ulcers may involve surgical procedures such as angioplasty, stenting, bypass grafting, and amputation in severe cases. An effective pain control approach is a crucial component in the management of arterial ulcers. Management of severe ischemic pain, often associated with arterial ulcers, requires adequate analgesia. Treatment management involves addressing risk factors such as smoking cessation, diabetes control, blood pressure, and hyperlipidemia. Compression should not be applied to wounds. The medical management of arterial ulcers typically involves the prescription of antiplatelets, a class of medications used in the management of certain conditions. Among these, aspirin, is a common antiplatelet drug which involves the inhibition of platelet aggregation to effectively restrict the advancement of peripheral vascular disease; clopidogrel can be considered as a potentially superior alternative; and cilostazol is a vasodilator used in the management of peripheral vascular disease. 25 Unlike the management of other chronic wounds, it is necessary to keep arterial ulcers dry as moist wound healing is not recommended for these types of ulcers. Though absorbent dressings should be used to remove the excess moisture, it is important to ensure that the wound is not excessively dry, as dressing changes may cause trauma to the wound bed.
Neuropathic Ulcers
Neuropathic ulcers have a distinct appearance and are most frequently observed in the plantar region (Figure 1D). These ulcers arise due to neuropathy, a physiological process that leads to decreased sensory function, motor weakness, and loss of autonomic function. The loss of sensory perception prevents individuals from withdrawing the affected area in response to painful stimuli, such as friction, shear forces, or traumatic events, leading to skin breakdown and ulceration. 25 Neuropathic ulcers often contain calluses, fibrotic tissue, and hyperkeratotic tissues. 26 Approximately 8.2% of diabetic patients are affected by neuropathic ulcers 9 and commonly experience a loss of peripheral sensation. Ulceration typically arises from increased pressure on the metatarsal, toes, interdigital surfaces, or bony structures of the foot due to deformities and trauma.13,26 The severity of neuropathy and other contributing factors likely influence the characteristics of neuropathic ulcers. 23 Based on the findings from clinical management of neuropathic ulcers, the utilization of dry dressings, regular debridement of the callus during each visit, and the implementation of off-loading techniques to potentially expedite the treatment process have been noted.
Mixed Ulcers
Mixed arterial venous disease, a condition characterized by the coexistence of arterial and venous pathology, has been reported to have a prevalence of approximately 26% among individuals presenting with lower-extremity ulcerations. 26 Chronic venous disease, characterized by venous reflux and/or obstruction, has been widely recognized as the underlying cause of ulcerations in the lower extremities. Skin ulcerations in the foot or leg may arise because of arterial insufficiency, characterized by insufficient perfusion and oxygenation of the dermis.
Mixed and arterial ulcers are primarily associated with venous conditions accompanied by detectable arterial impairment (ABI = 0.7-0.9) and generally minor superficial venous insufficiency (ABP index [ABPI] ⩽0.7). 11 Individuals with diabetes have a 0.7% higher likelihood of developing mixed ulcers. 8 Mixed ulcers are more frequently observed at ABI values ranging from 0.6 to 0.8,9,13,15 whereas ABI values ⩽0.6 are more commonly associated with ischemic ulcers and a poorer prognosis. 22 The correlation between improvement in ischemic conditions and ABI values ⩾.5, which can vary depending on the patient’s condition and the chosen therapy, should be considered.9,15,27,28 The lateral malleolus, anterior tibia, toes, heels, and other bony prominences are the common sites for arterial ulcers 21 (Figure 1E). Clinicians may face a specific challenge when patients present with lower-extremity ulcers caused by mixed arterial venous disease. Although the optimal treatment algorithm for mixed arterial venous lower extremity ulcers is not yet clear, the primary goal of therapy should be to achieve wound healing and preserve the limb. The objectives of management are to address the fundamental causes of ulceration and promote wound healing by following the latest guidelines and utilizing the available wound care therapies. The first step in patient management is to obtain a comprehensive clinical history, with particular emphasis on the duration and size of the ulcer as well as any accompanying lower extremity symptoms. Wound care management involves assessing the condition of the wound bed, ensuring optimal moisture levels, promoting circulation, managing exudates, minimizing edema, and preventing infections. Compression therapy can also be performed with minimal pressure on mixed ulcers.
Neuroischemic Ulcers
Neuroischemic foot ulcers occur in individuals with peripheral neuropathy and ischemia resulting from PAD.19,29 Owing to nerve damage and PAD, neuropathic ulcers, which frequently occur in the plantar region, are characterized by calluses or thick hyperkeratosis at the wound edges. Such patients may experience typical pain, particularly at night when they are in the supine position, which is relieved by elevating the affected extremity. 8 Compared with patients with ischemic ulcers, more patients with neuroischemic ulcers reported external triggers for their ulcers and were less likely to experience pain. Minor trauma in the presence of neuropathy can lead to the formation of a shallow ulcer over the ischemic area where local edema blocks the superficial branch of a small artery. Patients with chronic ischemia experience hair loss, pale skin, and the absence of a pulse. As per a study, neuroischemic ulcers are observed in patients with neuropathy and PAD, typically with an ABI < 0.9, 30 while another reported patients to have an ABI ⩾ 0.6. 9 These ulcers can be painless and self-limiting, but at times can become infected, develop cellulitis, and lead to pain. 19 Approximately 0.7% to 29.9% of diabetic patients have neuroischemic ulcers.9,31 Neuroischemic ulcers typically present with other signs of ischemia and changes in peripheral sensation 18 (Figure 1F). The management of neuroischemic wounds is multidisciplinary, involving both medical and surgical procedures. The crucial components of a physical examination include evaluation of the vascular condition, assessment of neuropathy status, examination of the integumentary system, and evaluation of the musculoskeletal system. Diagnostic tests that may be conducted include the use of a vascular doppler to assess the ABI at the beginning of the examination. Additional tests include doppler ultrasound, laser doppler velocimetry, and vascular imaging. Wound care is conditioned by the state of the wound bed. The moist concept treatment considers circulatory conditions in the management of neuroischemic diabetic ulcers. In cases of insufficient circulation, it is advisable to refrain from performing debridement and choose dry dressings instead. The condition of the wound should be properly managed using an antimicrobial dressing to prevent infections. In individuals presenting with arterial and ischemic–neuroischemic injuries, prompt identification of arterial blockage is crucial for expediting wound healing. Among the various available interventions, angioplasty is the most effective choice. 32
Furuncles and Carbuncles
A furuncle is an infectious gangrene of the skin and subcutaneous tissue around the roots of hair follicles, primarily caused by Staphylococcus aureus. 28 In contrast, a carbuncle is a bacterial infection that arises from a cluster of furuncles and is frequently seen in patients with diabetes and commonly occurs on the nape of the neck and back. 32 Furuncles can develop on any body part with hair follicles and commonly occur on the face, neck, arms, buttocks, anogenital region, and areas prone to friction, such as the nose and earlobes. The furuncles are tender and acute, and larger lesions can cause throbbing pain. Additional symptoms include lymphangitis, painful lymphadenitis, fever, malaise, prostration, and other mild constitutional symptoms. Complications include metastatic abscesses of the kidneys, lungs, bones, and other organs. 33 A carbuncle appears as a hard and painful red lump that rapidly grows to a diameter of 3 to 10 cm within a few days (Figure 1G). After 5 to 7 days, suppuration occurs, with pus discharge from multiple follicular orifices and inflammatory infiltration extending into the subcutaneous and fascial tissues. In severe cases, extensive necrosis can occur. Toxemia or metastatic infections can potentially lead to death in frail or ill patients. 33 Diabetes is associated with a high prevalence of developing furuncles and carbuncles, and approximately 9.2% of diabetics 8 develop these predominantly in the gluteal area. 32 Furuncles and carbuncles, being complications associated with diabetes, must be treated seriously. 28 Managing wounds on the furuncles/carbuncles does not appear to be overly challenging. According to management guidelines, it is deemed necessary to perform an incision for drainage. The use of antibiotics in clinical settings remains inconsistent, with some opting to use them, whereas others refrain from their use. The severity and extent of the wound determine the course of action. The dressings used are those with maximum exudate absorption properties, capable of lysing necrotic tissue or slough and effectively preventing the spread of infection.
Venous Ulcers
Venous ulcers can result from chronic venous insufficiency or hypertension. In a healthy venous system, exercise aids in reducing pressure through calf muscle pump activity. When muscles relax, the valves connecting the superficial veins to the deep venous circulation prevent backward flow (reflux) and maintain low pressure. However, in systems with incompetent valves, venous pressure remains high. Among patients with diabetes with venous ulcers, approximately 0.7% have an ABI of ⩾0.8. 26 Additionally, 13.97% of patients with diabetes experience venous insufficiency. 34 Patients with diabetes may develop venous ulcers as a complication because there is an increased risk of developing and progressing chronic venous disease. This heightened risk is likely due to a shared pathophysiology involving hemodynamic abnormalities in the lower limbs, such as vascular wall remodeling, increased vascular permeability, reduced blood flow, elevated oxidative stress, vascular inflammation, and endothelial dysfunction. 22 Individuals with diabetes and chronic venous ulcers exhibit reduced arterial perfusion and pathological venous insufficiency, leading to extensive edema that further impairs circulation in the affected extremities.30,35 The majority (approximately 95%) of venous ulcers occur in the gaiter region of the lower leg, typically around the malleoli, and can either be localized or circumferential. Venous ulcers have irregular and gently sloping edges, with a fibrous layer of granulated tissue covering the ulcer bed. They commonly present with edema, venous dermatitis, varicosity, and lipodermatosclerosis (Figure 1H). Ulcers above the midcalf or on the foot are most likely to have a different etiology. 23 The treatment of venous ulcers involves compression therapy, dressings to keep the wound bed moist, and, in some cases, surgical interventions.
Diabetic Bullae
Diabetic bullae are an intriguing component of diabetic ulcers. Multiple studies have investigated diabetic bullae, and it is widely recognized that diabetic skin disorders have multifactorial causes. In approximately 39.7% of patients with diabetes, the underlying cause of diabetic bullae or blister formation remains unidentified (Figure 1I). 9 Blisters often develop following minor trauma or exposure to ultraviolet light. 36 The etiology of diabetic bullae may involve microangiopathy; immune-mediated vasculitis; impaired calcium, magnesium, or carbohydrate metabolism; tissue hypoxia; or microcirculation ischemia.29,30 Microangiopathy may be responsible for diabetic bullae in patients with an ABI < 0.8 (1.07%).9,24 However, various factors contributing to bullae formation in patients with an ABI of >0.8 are not yet fully understood. 9 For the effective management of diabetic ulcers, debridement should be prioritized, and a dressing that can absorb exudate and potentially have antimicrobial properties should be developed, especially for larger ulcers. Bullae that are small and mild typically heal naturally without requiring debridement. However, if the wound is infected, debridement is also needed.
Cellulitis
Cellulitis, an acute bacterial infection of the deep dermis and subcutaneous tissues, can occur in various parts of the body but is particularly common in the foot and poses a significant risk for patients with diabetes. The symptoms of cellulitis include a warm, poorly defined area of redness beneath the skin, accompanied by edema and tenderness upon touch 37 (Figure 1J). Acute bacterial infections of the deep dermis and subcutaneous tissues lead to cellulitis. Normal skin bacteria and other pathogens typically cannot enter the tissues or the lymphatic system because of the natural protective barrier of the skin. However, when the skin is breached, these pathogens can invade the dermis and subcutaneous tissue. 38 Wound cleaning, debridement of necrotic or gangrenous debris, and testing for foreign bodies are the necessary initial steps. Offloading, that is, relieving pressure on ulcers, is crucial for wound healing. Wound dressings keep wounds moist, aiding in healing. Healthcare providers use various commercial wound dressings and clinical experience to treat foot ulcers. Clinicians generally agree that most patients with mild (and sometimes moderate) infections can be treated with narrow-spectrum antibiotics against Staphylococcus and Streptococcus species, with subsequent therapy based on the clinical response. 39 Culture results and recent antibiotic exposures should also be taken into account. Oral antibiotics for 7 to 14 days are recommended for mild infections; however, this may not be practical depending on the patient’s reaction and infection severity.
Conclusion
Gaining a comprehensive understanding of the various types of wounds that develop in individuals with diabetes is of paramount importance. This review may offer healthcare providers a better understanding of specific diabetic ulcer-based clinical characteristics to enable them to manage the wound appropriately. The current approach for managing the wound environment focuses on wound bed preparation interventions. These interventions encompass various aspects such as debridement, bacterial balance, exudate management, and the condition of local tissue within the wound environment. A wide range of conditions can cause diabetic ulcers, each of which results in distinct ulcers. More research on diabetic ulcers is needed to develop wound identification systems that are accurate and practical for the early identification of wound etiology.
Footnotes
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SJ contributed to the review, drafting, manuscript preparation, revision, and final approval of the manuscript.
Ethics Statement: It was an examination of aggregate online data. Ethics approval was not required.
Data Availability Statement: No datasets were generated or analyzed during the course of this study, so data sharing is not applicable.
References
- 1. Zhang J, Guan M, Xie C, Luo X, Zhang Q, Xue Y. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 2014;2014:273475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haji Zaine N, Burns J, Vicaretti M, Fletcher JP, Begg L, Hitos K. Characteristics of diabetic foot ulcers in Western Sydney, Australia. J Foot Ankle Res. 2014;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosyid FN. Etiology, pathophysiology, diagnosis and management of diabetics’ foot ulcer. Int J Res Med Sci. 2017;5:4206-4213. [Google Scholar]
- 4. Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agale SV. Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. Ulcers. 2013;2013:4136041-4136049. [Google Scholar]
- 6. Jain AKC, Nisha ST, Vishwanath S. Carbuncle in diabetics-our experience. Scholars J Appl Med Sci. 2019;1:493-495. [Google Scholar]
- 7. Bhutani R, Walton S. Diabetic bullae. Br J Diabetes Vasc Dis. 2015;15:8-10. [Google Scholar]
- 8. Suriadi. An appearance characteristic ulcer in diabetic patients at Kitamura Clinic in Pontianak, Indonesia. Eur J Mol Clin Med. 2020;7:3436-3444. [Google Scholar]
- 9. Spiliopoulos S, Festas G, Paraskevopoulos I, Mariappan M, Brountzos E. Overcoming ischemia in the diabetic foot: minimally invasive treatment options. World J Diabetes. 2021;12:2011-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tasci I, Saglam K, Basgoz BB. Ankle brachial index and foot ulcer etiology. Adv Skin Wound Care. 2016;29:104. [DOI] [PubMed] [Google Scholar]
- 11. Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and management of lower-extremity ulcers. N Engl J Med. 2018;378:302-303. [DOI] [PubMed] [Google Scholar]
- 12. Forsythe RO, Apelqvist J, Boyko EJ, et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3278. [DOI] [PubMed] [Google Scholar]
- 13. Czerniecki JM, Turner AP, Williams RM, et al. The development and validation of the AMPREDICT model for predicting mobility outcome after dysvascular lower extremity amputation. J Vasc Surg. 2017;65:162-171.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azuma N. The diagnostic classification of critical limb ischemia. Ann Vasc Dis. 2018;11:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arisandi D, Oe M, Roselyne Yotsu R, et al. Evaluation of validity of the new diabetic foot ulcer assessment scale in Indonesia. Wound Repair Regen. 2016;24:876-884. [DOI] [PubMed] [Google Scholar]
- 16. Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Interv Radiol. 2009;26:286-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eleftheriadou I, Kokkinos A, Liatis S, et al. Ischemic and neuro-ischemic ulcers and gangrene. In: Liatis S, Tsapogas P. eds. Atlas of the Diabetic Foot. 3rd ed. Wiley-Blackwell; 2019;107-134. [Google Scholar]
- 18. Singh CG, Sil A, Sanyal D, Mandal A. Characteristics of neuropathic, ischaemic and neuroischaemic diabetic foot ulcers - a prospective cohort study. J Clin Diagn Res. 2023;17:5-8. [Google Scholar]
- 19. Liao X, Li SH, El Akkawi MM, Fu XB, Liu HW, Huang YS. Surgical amputation for patients with diabetic foot ulcers: a Chinese expert panel consensus treatment guide. Front Surg. 2022;9:10033391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. BMJ. 2006;332:347-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aydin A, Shenbagamurthi S, Brem H. Lower extremity ulcers: venous, arterial, or diabetic? Emerg Med. 2009;41:18-24. [Google Scholar]
- 22. Alexandrescu VA, Deleeuw P, Kovanda J-S. Ischemic and venous wound identification : what we look for. Endovasc Today. 2017;16:1-6. [Google Scholar]
- 23. Rosas-saucedo J, Lukanova D, Glauser F, et al. Treatment patterns for chronic venous disease and diabetes mellitus: lessons from an international market-research survey. Diabetes Manag. 2021;S1:1-10. [Google Scholar]
- 24. Katsilambros N, Dounis E, Makrilakis K, Tentolouris NTP. Neuropathic Ulcer. 2nd ed. Wiley-Blackwell; 2010. [Google Scholar]
- 25. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367-2375. [DOI] [PubMed] [Google Scholar]
- 26. Hedayati N, Carson JG, Chi YW, Link D. Management of mixed arterial venous lower extremity ulceration: a review. Vasc Med. 2015;20:479-486. [DOI] [PubMed] [Google Scholar]
- 27. Cueto J, Ochoa R, Bert ET. Chronic venous ulceration in obese patients with diabetes mellitus. A therapeutic challenge. J Diabetol. 2019;3:1-6. [Google Scholar]
- 28. De Caridi G, Massara M, Stilo F, et al. Effectiveness of prostaglandin E1 in patients with mixed arterial and venous ulcers of the lower limbs. Int Wound J. 2016;13:625-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yotsu RR, Pham NM, Oe M, et al. Comparison of characteristics and healing course of diabetic foot ulcers by etiological classification: neuropathic, ischemic, and neuro-ischemic type. J Diabetes Complications. 2014;28:528-535. [DOI] [PubMed] [Google Scholar]
- 30. Demetriou M, Papanas N, Panopoulou M, Papatheodorou K, Bounovas A, Maltezos E. Tissue and swab culture in diabetic foot infections: neuropathic versus neuroischemic ulcers. Int J Low Extrem Wounds. 2013;12:87-93. [DOI] [PubMed] [Google Scholar]
- 31. Pemayun TGD, Naibaho RM. Clinical profile and outcome of diabetic foot ulcer, a view from tertiary care hospital in Semarang, Indonesia. Diabetic Foot Ankle. 2017;8:1312974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pamungkas W, Darwis P. Effectiveness of balloon angioplasty and stent angioplasty: Wound healing in critically limb ischemic. New Ropanasuri J Surg. 2019;4:4-8. [Google Scholar]
- 33. Bodman M, Friedman S, Clifford LB. Bullosis diabeticorum. A report of two cases with a review of the literature. J Am Podiatr Med Assoc. 1991;81:561-563. [DOI] [PubMed] [Google Scholar]
- 34. Venkatesan R, Baskaran R, Asirvatham AR, Mahadevan S. ‘Carbuncle in diabetes’: a problem even today! BMJ Case Rep. 2017;2017:bcr-2017-220628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ellis S, Patel M, Koshchak E, Lantis J, II. Location of lower-extremity diabetic foot ulcers with concomitant arterial or venous disease. Wounds Int. 2020;11:20-23. [Google Scholar]
- 36. Newton H. Leg ulcers: differences between venous and arterial. Wound Essent. 2011;6:20-28. [Google Scholar]
- 37. Chen Y, Ma Y, Li N, et al. Efficacy and long-term longitudinal follow-up of bone marrow mesenchymal cell transplantation therapy in a diabetic patient with recurrent lower limb bullosis diabeticorum. Stem Cell Res Ther. 2018;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nurzaman YA, Setiawan PR, Kasnadi MS. Cellulitis with ulcer on diabetes mellitus : a case report. Med Clin Updat J. 2023;2:8-11. [Google Scholar]
- 39. Khan MJ. Complications of cellulitis in diabetic foot infections. US Pharm. 2011;36:63-66. [Google Scholar]


