Abstract
Background
Dietary management plays a crucial role in the treatment of patients with ulcerative colitis (UC). While various e-services provide dietary advice, the long-term dietary management requires continuous monitoring and dynamic adjustment to accommodate the evolving nature of the disease and meet the patients’ nutritional needs. Consequently, the development of a novel dietary management tool that incorporates diet tracking, personalized nutritional feedback, and evidence-based advice becomes imperative. This study aims to address this need by developing a WeChat applet called “HealthyGut” specifically designed for the dietary management of UC patients, and evaluate its feasibility, acceptability, and preliminary efficacy.
Methods
A total of 134 UC patients were equally allocated into the intervention group (receiving a 12-week mobile-based dietary management via HealthyGut) and control group (receiving a paper-based food diary and routine advice). The feasibility outcomes were recruitment, retention, engagement, satisfaction, and acceptability in the intervention group. Dietary intakes were effective outcomes.
Results
Both groups had satisfactory retention rates (89.6% and 77.6%, respectively). The System Usability Scale in the intervention group yielded “good usability” with a mean score of 79.63 (SD 7.39), and all participants reported good user experiences and perceived benefits after using HealthyGut. At week 12, intervention responders reported significantly higher daily energy intake than control group (Z = −3.089, p = 0.002).
Conclusions and Implications
The results display that HealthyGut as a dietary management tool is feasible and accepted by UC patients, and it may help them make healthier food choices. Larger sample studies should be considered in the future.
Keywords: Ulcerative colitis, dietary management, mobile health, telemedicine
Introduction
Ulcerative colitis (UC) is a non-specific gastrointestinal inflammatory disorder characterized by periods of remission and a flare-up of gastrointestinal symptoms affecting millions of people worldwide. 1 Its incidence is relatively high in European and American countries, but recent epidemiological studies have revealed a rising disease burden for UC in Asian countries, including China. 2 Given that UC is a lifelong condition with a high nutritional risk,3,4 long-term dietary management is required to ensure improved health and quality of life in patients with UC.5–7
Despite new evidence linking diet to inflammatory bowel disease (IBD),8–10 its long-term dietary management with continuous monitoring and dynamic adjustment remains a significant challenge. It is a complex multi-factor dynamic adjustment process in which the type, size, and frequency of food intake, food pairings, and cooking methods must be adjusted based on disease stages, nutritional needs, dietary contexts, and patient preferences.11,12 Dietitians and gastroenterologists provide dietary tracking, nutritional support, and recipes during hospitalization. 13 Patients with UC suffer improper dietary intake or excessive dietary restriction due to uncertainty of the association between diet and health and dietary cognitive bias, resulting in aggravating intestinal symptoms, malnutrition, disease relapse, or serious complications such as intestinal obstruction or bowel perforation.14–18 Consequently, in-home dietary management remains a crucial yet weak link.
Previous studies have demonstrated that evidence-based dietary interventions incorporating real-time monitoring, dynamic diet evaluation, and tailored feedback are essential for ensuring consistent access to affordable and sustainable health benefits.19,20 However, it is difficult for conventional interventions to provide real-time monitoring, tailored feedback, and user-centered care. A food diary is a dietary self-monitoring method used to record diets for a dietician or clinician to review at the next in-person meeting that can be incorporated into ongoing treatment. 21 Despite their limitations, self-report methods remain the only validated methods for measuring dietary intake in free-living situations, 22 which can help raise awareness and improve food choices. Despite the importance of self-monitoring in recovery, nonadherence is commonly observed among patients who are asked to maintain a paper-based dietary diary because of feedback lags or lack of reflection.
The utilization of eHealth has the potential to enhance and streamline data collection and assessment, thereby benefiting a wider demographic. 23 The prevalence of smartphone applications designed to manage personal diet has surged in recent times, owing to their cost-effectiveness, ubiquitous availability of health-related information, and the added advantages of interactivity and customization. Nevertheless, a significant proportion of commercially available apps are not scientifically developed, based on behavioral theory or evidence, and have yet to undergo rigorous evaluation.24,25 Additionally, some dietary management applications, which are grounded in sound scientific principles, are solely employed for the collection of nutritional data without any intention to evoke behavior change.21,26 Consequently, there is a pressing need for a novel dietary management app that encompasses both real-time dietary tracking and customized dietary feedback, tailored to the user's unique attributes and nutritional needs, including age, gender, nutritional status, disease condition, and eating preferences.
HealthyGut is a WeChat applet developed to address these issues by providing dietary tracking, tailored feedback, and user-centered dietary advice for patients with UC in China. This study aims to investigate the feasibility and acceptability of HealthyGut and compare the effects of mobile-based and traditional paper dietary records on dietary intakes in patients with UC through a pilot study.
Method
Design and setting
This study was a 12-week, multicenter, prospective randomized controlled trial with parallel groups and equal randomization (1:1). Written informed consent was obtained from all participants. This study was approved by the Ethics Committee of the Affiliated Hospital (Project approval number: KY2021081). The trial was registered in Chinese trial register (trial-ID: ChiCTR2100051539).
Participants and recruitment
The study was advertised via text messages, posters, and social media at gastroenterology clinics in four tertiary hospitals in Nanjing. Patients aged 18–65 years diagnosed with UC at least 6 months before the study were recruited. Patients had to have daily access to a smartphone and be willing and able to perform dietary self-management to be eligible. The exclusion criteria were as follows: (1) pregnant or lactating women; (2) severe psychiatric illness and physical disabilities; (3) concurrent diagnosis of diabetes, kidney failure, or other chronic diseases; (4) following any special diet or dietary pattern (vegetarian, vegan, or related to particular religious or social traditions); and (5) participation in other dietary education or research programs.
HealthyGut applet
HealthyGut is a mobile-based tool designed to assist patients with UC in making healthier food choices through real-time dietary tracking, tailored feedback, and user-centered dietary advice. HealthyGut was developed using an online platform based on WeChat, currently regarded as the most popular social media platform for the general public in China. 27 The specific content and features of HealthyGut were developed by a multidisciplinary team with expertise in nutrition, gastroenterology, nursing, behavioral science, and psychology, ensuring that the solution was underpinned by strong science.
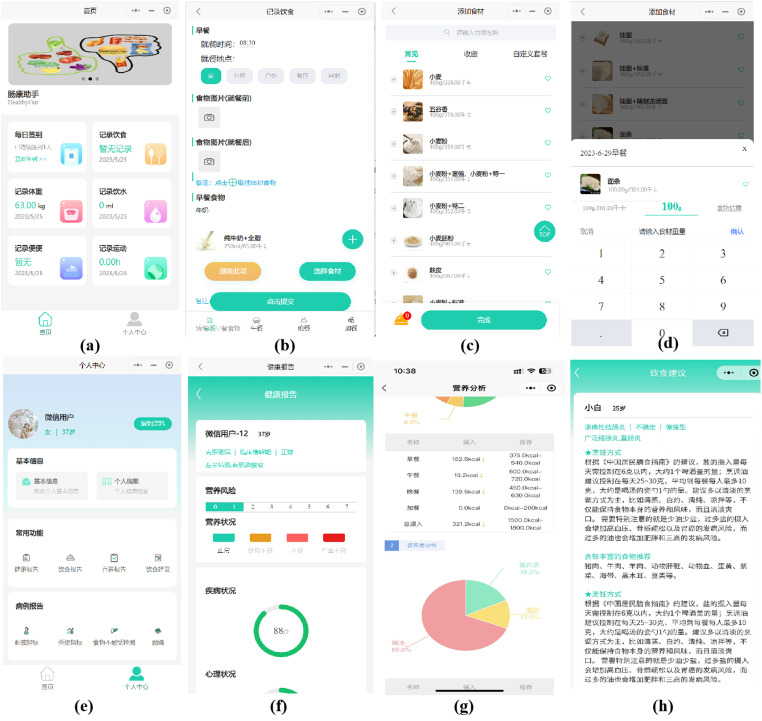
The design process followed a user-centered approach, incorporating the perspectives and feedback of patient representatives (n = 5) who actively participated in group discussions at various stages of design, implementation, and system refinement. This comprehensive approach ensured continuous user involvement throughout the entirety of the design and development process. Finally, HealthyGut consists of two functional components: a patient applet for patients with UC and a care provider platform. This project required four functional modules: user management, diet recording, nutritional feedback, and dietary advice. The detailed contents of the applet are presented in Figure 1.
Figure 1.
Screenshots of the used healthyGut applet. (a) Home page (daily Check-in, diet record, weight record, drinking record, stool record, exercise record); (b) Diet record (meal times, meal locations, pre- and post-meal photographs of food and beverages); (c) Food selection for each meal (breakfast, lunch, dinner, and snacks); (d) Corresponding portion sizes selection; (e) User center (health information, nutritional report, dietary advice, and laboratory tests uploaded); (f) Health report (disease condition, nutritional risk score, nutritional status, anxiety conditions); (g) Nutritional evaluation report (actual intakes of energy and nutrients, personalized goals); (h) Dietary advice.
Participants were asked to register and complete a comprehensive assessment of the user management module, including demographic information (age, sex, education, income, country of birth, and marital status), disease condition (diagnosis date, disease location, disease stage, extraintestinal manifestations, and current medications), nutritional status (height, weight, and physical activity level), and dietary conditions (food preference, drinking history, and dietary intolerance) upon downloading the app. Once all assessments were completed, all data were integrated to set personalized goals for energy and nutrient intake according to recommendations from nutrition and diet guidelines of patients with UC.7,28 Participants recorded their daily diet in the diet recording module by selecting dishes and foods consumed from the applet menu, and the corresponding portion sizes for each meal (breakfast, lunch, dinner, and snacks). We designed the nutritional evaluation module that incorporated a food analysis program utilizing the Chinese Food Composition Database 29 to automatically calculate the daily intake of energy and nutrients. Upon completion of a full day of dietary records, participants were able to access a nutritional feedback module containing a daily report of nutritional evaluation. Participants were instructed to upload pre- and post-meal photographs of food and beverages consumed when it was inconvenient to record the weighed food. Food was photographed using a phone camera positioned at a distance of 20 cm away from the center of the meal plate. Prior to the photography session, participants were instructed to arrange the food items in a flat manner on the meal trays, if feasible, to enhance visibility. Additionally, they were directed to position the trays on a measuring place mat, measuring 40 cm in length and 30 cm in width, which was provided by the researchers. This aided in accurately estimating portion size and color. In the event that the food items were difficult to identify in the photograph uploaded, participants were encouraged to input the corresponding food name into the designated text box in the dietary recording module. Two trained analysts who were registered dietitians then received notifications on the care provider platform and could browse the uploaded food photographs and independently enter diet data based on their professional judgment. Discrepancies in dietary intake were resolved through discussion. If participants did not submit any entries for a month, a reminder message was sent to them for three days, followed by a phone call from the research staff. Participants who failed to submit any entries after being reminded were considered dropouts. Upon completion of the diet entry via the administrator port by the dietitian, the participant is able to promptly access the daily nutritional evaluation report in the nutritional evaluation module. In light of the 3-day food records, personalized dietary recommendations were formulated in accordance with the balanced dietary principles of the food pyramid for individuals with UC. 9 Simple suggestions such as advising decreased intakes of red meat or increased intakes of vegetables were included. Dietitians provided monthly suggestions for recipes that closely resembled the participant's original diet. The dietary advice module enabled participants to review all of the aforementioned information.
Procedures
After completing the surveys and anthropometrics, all participants were trained by trained research staff to complete food diaries. Verbal and written instructions were given to participants for estimating portion sizes and selecting food items from the applet to obtain an accurate estimate of dietary intakes. Participants in the intervention group were assisted by a trained research assistant to download HealthyGut app on their smartphones and were instructed to record their daily diet for at least 3 days a month. Participants in the control group were asked to submit their paper food diaries monthly and received no targeted dietary advice but routine diet education. Both groups received the same culturally appropriate educational materials to encourage healthier eating behaviors. The pilot trial duration was 12 weeks.
Measures
The assessment of the program feasibility included recruitment rates and retention of enrolled participants, defined as at least 60% adherence to completing three or more full days of food diaries per month during the 12 weeks. HealthyGut acceptability was assessed using quantitative and qualitative data. Participants were asked to complete the 10-item System Usability Scale (SUS), 30 rated on a 5-point Likert scale from strongly disagree to strongly agree, to assess the usability of the system at week 12. Scores ≥68 indicated above-average usability. 31 Moreover, semi-structured interviews were conducted by the first author following the 12-week program with those who agreed to participate to gain additional insights into their perceptions of using HealthyGut and suggestions for improvement. The first author is a PhD candidate who has studied qualitative research knowledge and interview skills systematically and detail. Two pilot interviews were carried out before the interviews began, but were not included in analysis. Saturation was reached after 19 interviews. Participants’ characteristics are illustrated in Table S1. Every interview lasted 30–60 min in a quiet room that was acceptable to the interviewees. Repeat interviews were not carried out in this study, and transcripts were not returned to informants for comments.
Preliminary exploratory outcomes were assessed using dietary intakes of patients with UC. The daily intake of each energy and macronutrient was estimated using the average of two or three consecutive days from a dietary diary at baseline and week 12.
Data analysis
Quantitative data were analyzed using SPSS (version 22.0). Frequencies, percentages, means, and standard deviations were calculated for descriptive analysis. Categorical variables were tested using the chi-squared test or Fisher's exact test where appropriate. Paired t-tests were used for pre-and post-intervention comparisons of each group, and two-sample t-tests were used to compare differences between groups. For non-normally distributed data, median (interquartile range) was used for descriptive analysis, Mann-Whitney U tests for between-group comparisons, and Wilcoxon signed rank tests for within-group comparisons. P < 0.05 was considered statistically significant.
The interview data were audio-recorded and then transcribed verbatim. The interviews were analyzed using the conventional content analysis method. Two researchers separately coded an initial sample of transcripts, and discrepant coding was discussed until inter-rater reliability was achieved (Cohen's kappa > 0.8). The quotes used in this paper were translated into English by the first author and later discussed and compared with the original wording by the research group.
Results
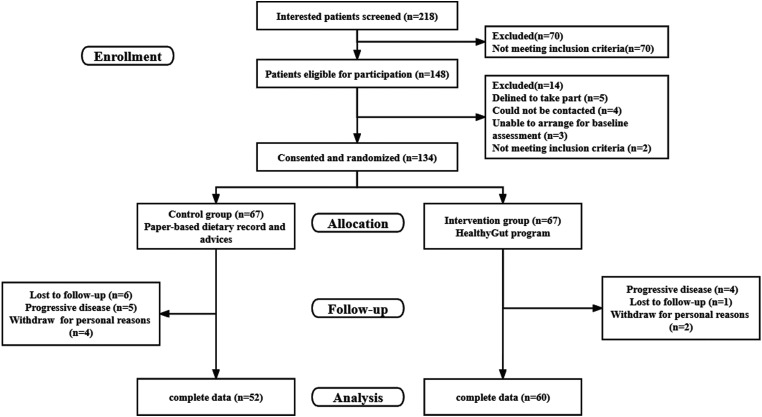
From March 2021 to September 2022, a total of 218 patients with UC were evaluated for inclusion, of whom 70 (32.1%) were excluded. Of 148 enrolled participants, 134 consented and were randomly assigned to one of the two groups using random tables, with 67 participants in each group. A flowchart of the patient recruitment is presented in Figure 2.
Figure 2.
The flow chart of participants’ recruitment.
The baseline characteristics of participants in each group are summarized in Table 1. Most participants were in remission (n = 96, 85.7%), and the average age was 37.90 years. A total of 95 individuals had received dietary guidance from healthcare providers in the last year, but only 14.3% (n = 16) had kept a paper or electronic food diary. No significant differences existed between the two groups regarding the baseline demographic and clinical characteristics.
Table 1.
The baseline characteristics of participants.
| Variables | Total (n = 112) | Intervention group (n = 60) | Control group (n = 52) | p-value |
|---|---|---|---|---|
| Gerder, n(%) | ||||
| Male | 59(52.7) | 36(60.0) | 23(44.2) | 0.096 |
| Female | 53(47.3) | 24(40.0) | 29(55.8) | |
| Age, years, mean (SD) | 37.90(11.35) | 37.45(12.32) | 38.42(10.20) | 0.297 |
| Marital status, n (%) | ||||
| married | 91(81.3) | 46(76.7) | 45(86.5) | 0.182 |
| Single or divorced | 21(18.7) | 14(23.3) | 7(13.5) | |
| Educational level, n (%) | ||||
| Elementary | 3(2.7) | 2(3.3) | 1(1.9) | 0.330 |
| Middle or high school | 27(24.1) | 12(20.0) | 15(28.9) | |
| Bachelor | 73(65.2) | 43(71.7) | 30(57.7) | |
| Master | 9(8.0) | 3(5.0) | 6(11.5) | |
| Living place, n (%) | ||||
| Urban | 92(82.1) | 50(83.4) | 42(80.8) | 0.348 |
| Suburban | 13(11.6) | 5(8.3) | 8(15.4) | |
| Rural | 7(6.3) | 5(8.3) | 2(3.8) | |
| Clinical disease activity | ||||
| Remission | 96(85.7) | 52(86.7) | 44(84.6) | 0.757 |
| Active disease | 16(14.3) | 8(13.3) | 8(15.4) | |
| BMI, years, mean (SD) | 21.75(3.02) | 21.78(3.00) | 21.71(3.08) | 0.492 |
| Disease duration, years, mean (SD) | 7.41(5.68) | 7.43(6.26) | 7.38(4.99) | 0.073 |
| medication currently used | ||||
| None | 11(9.8) | 6(10.0) | 5(9.6) | 0.946 |
| 5-aminosaliculic acid | 57(50.9) | 30(50.0) | 27(51.9) | 0.839 |
| steroids | 26(23.2) | 14(23.3) | 12(23.1) | 0.974 |
| immunomodulators Biologics | 20(17.9) | 10(16.7) | 10(19.2) | 0.724 |
| Have received dietary guidance from HCP in the last 12 months, n (%) | 95(84.8) | 50(83.3) | 45(86.5) | 0.637 |
| Dietary advice given by, n (%) | ||||
| Gastroenterologist | 83(87.4) | 42(84.0) | 41(91.1) | 0.298 |
| Dietician | 18(18.9) | 8(16.0) | 10(22.2) | 0.440 |
| Nurse | 32(33.7) | 18(36.0) | 14(31.1) | 0.615 |
| General practitioner | 7(7.4) | 4(8.0) | 3(6.7) | 0.804 |
| Have kept a paper or electronic food diary, n (%) | 16(14.3) | 9(15.0) | 7(13.5) | 0.751 |
| Self-rated confidence in using technology in general (Score 1-10), mean (SD) | 7.98(0.85) | 8.02(0.83) | 7.94(0.87) | 0.646 |
Compliance was reasonable among the intervention group members, with 55 (82.1%, 55/67) providing complete dietary records for at least three consecutive days, per month for three consecutive months and a further five participants provided four days of dietary intake. A total of 37 participants (61.7%) completed compliance logs to record their food intake without a reminder, while 18.3%, 5%, and 15% completed dietary records after one, two, and three reminders, respectively. The mean number of days logged for food was higher in the intervention group, with an average of 15.1 days (SD 5.9 days), whereas the control group had a lower mean of 7.2 days (SD 2.8 days). No related or unexpected adverse events were reported.
At the end of the study, seven participants (10.4%) assigned to the intervention group dropped out for reasons such as progressive disease (n = 4), loss to follow-up (n = 1), and withdrawal for personal reasons (n = 2). Participants who remained until the end of the study did not display any significant differences in any of their demographic and clinical variables compared to participants who dropped out.
This study was designed to be completed within 12 weeks. More than four-fifths of participants (n = 50) in the intervention group used all three modules, and eight (13.3%) used at least two modules. Most participants in the intervention group (n = 41, 68.3%) used the applet to upload photographs and texted food information, and 31.7% of participants reported weighed dietary records at each meal. The mean number of photographs submitted per day for participants was 3.29 (SD 1.50). The results demonstrated good concordance between the estimations of dietary intake by the two analysts, with a mean bias of 15.70 grams (0,30). The average time spent by analysts to complete the analyses and advice was 29.39 min (SD 13.00) per day per participant.
The usability of HealthyGut was rated high, with a mean System Usability Scale score of 79.63 (SD 7.39). Table 2 displays that participants’ experiences with HealthyGut were mostly positive. Finally, three topics and nine related themes are summarized in Table 2.
Table 2.
Qualitative feedback on intervention acceptability
| Topic | Themes | Representative participant quotes |
|---|---|---|
| User experience | Accessibility of dietary recording and tracking | “I kept a paper food diary for a few days, but then I gave it up because it is too complicated and a waste of time…It (the HealthyGut) was easy to use, and since I carry my phone with me wherever I go.” (ID 7) |
| “I like to post on my Wechat moments, so I can take photos and record what I eat (in the HealthyGut).”(ID 11) | ||
| Real-time feedback and mentoring | “The nurse had taught me to keep a dietary diary on a public platform in Wechat, but I do not know how it works, and no one gave me feedback…Now that I know, you’re gonna tell me how to eat healthier.”(ID 13) “…Just like you have someone telling you how to eat…”(ID 6) |
|
| Easy to understand and follow | “I cannot finish all the tasks (of dietary management). For example, I cannot intake enough calories. You know, it is hard for me to calculate those so accurately. In other word, there is probably a number, it is impossible to finish it exactly. After all, we are not the nutritionist… Just tell me how to eat better, right? I think it's pretty good right now.”(ID 4) “Their (doctors’) dietary advices are very generic, such as liquid diet, low residue diet…It (the feedback from HealthyGut) is more specific, so I’ll try to stick to it… “ (ID 16) “We are not specialized in scientific research, and I don’t think it's appropriate for patients to read these things (papers), which always confuse us… You guys are good. Just tell me how to eat healthier.” (ID 14) |
|
| Individualized and targeted dietary advice | “First, he (doctor) gave me a rough list of suggestions, dividing food into different categories, such as staple food, fruit, meat, whatever, and then he has a suggestion in each of them… This is for reference only; everybody is different… Now I know whether this is sufficient for me… ” (ID 2) “It is actually very difficult to meet everyone needs. For example, people coming from Jiangsu, Guangdong, Shanghai and Nanjing have different eating habits, different families have different living habits, so it is impossible to unify this… I can adjust it myself according to this report, and I think it’s fine.” (ID 1) |
|
| Perceived benefits | Improved awareness and skills in dietary management | “It helps me a lot. Now I look at what I haven’t eaten today and replenish it in the evening to keep a balanced diet.” (ID 8) “The doctor told me not to eat spicy, allergic food and seafood… Now at least I know how about my dinner.” (ID 15) “I would show my doctor what I eat at home, like a food diary, because they cannot monitor me.” (ID 5) |
| Reduction in unnecessary dietary restriction | “I didn’t drink milk, because I thought I am intolerant of lactose… And then when you told me it was changing, I tried to drink a little…And I think I can try… ” (ID 4) “They are just telling me not to eat this, or eat that… But I think it’s all ready to eat.” (ID 16) “It is very important to keep a balanced diet, but not special diet. I will try to eat more as long as it does not violate the principles…yes, I know, I can’t drink.” (ID 10) |
|
| Promoted self-efficacy in dietary management | “I’m feeling, I’m still feeling pretty confident (in dietary management), because, you know, I’m just trying to eat as much as I can eat… Nutrition should be balanced.” (ID 13) “It (the HealthyGut) is just like a nutritionist so that I feel more confident (in dietary management).” (ID 3) |
|
| Suggestions/ Improvement | Recommendation of healthy recipes | “I desire to learn about how to cook healthy recipes at home, like what to eat in the morning, in the afternoon and in the evening. In remission, I want to know what kind of diet should there be and I can follow the recipe.” (ID 5) “I’ll always try to think positively and adjust my mindset as much as I can. But my family is always exhausted because we need two recipes…I mean, it is not a normal pattern… all because of my disease.” (ID 10) |
| Peer support | “(laughs)Yes, I love it, I love chatting with other patients, especial those like sharing delicious food and happy life. I like talking to these people, and it’s good for me.” (ID 17) “I like to share delicious food on my WeChat moments, and the people who liked me most were my fellow patients. Newly diagnosed patients can get positive feedback from happy life of other patients…You know, they always want to see more of this.” (ID 11) |
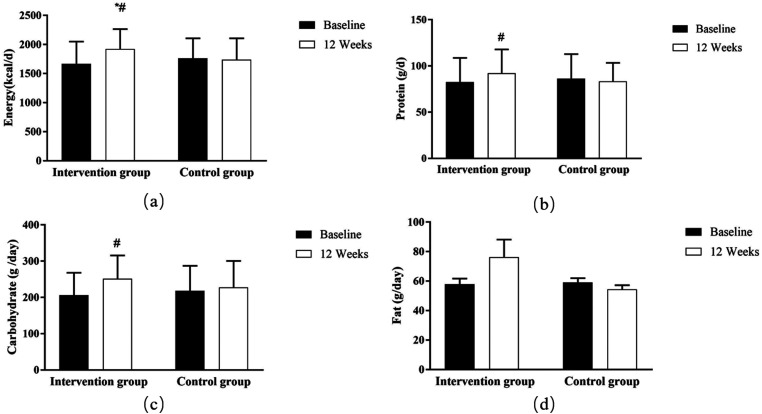
The pilot RCT could not detect clinically significant differences in the treatment efficacy. Data from dietary records revealed a significantly higher daily intake of energy (Z = −4.965, p = 0.001), carbohydrates (Z = −4.145, p = 0.001), and proteins (Z = −2.959, p = 0.003) in the intervention group than in the control group at week 12. To compare the difference between the two groups, intervention responders increased energy intakes (Z = −3.089, p = 0.002) relative to the control group at week 12 (Figure 3).
Figure 3.
Changes in intakes of energy and macronutrients throughout the study. * p < 0.05, within group comparison;# p < 0.05, between groups comparison.
Discussion
To the best of our knowledge, this is the first pilot feasibility study of a mobile-based dietary intervention in a Chinese population with UC. This pilot study has successfully demonstrated the high feasibility and acceptance of HealthyGut, suggesting its potential to positively influence healthy eating habits among patients with UC.
Our recruitment rates were comparable to or slightly higher than those observed in other studies targeting patients with chronic diseases,32,33 thus confirming the feasibility of our approach. The satisfactory recruitment and retention rates achieved in this study may be attributed to the effective research methods and data collection strategies employed. Notably, a significant portion of participant interactions following initial recruitment were conducted through the utilization of HealthyGut. These methods offer added advantages for younger participants, who may favor the convenience of engaging in research from their own environments, at their own convenience, and without the requirement of travel. Furthermore, the participants in our study possess a high level of education and exhibit greater acceptance towards eHealth. An additional aspect arising from the findings pertains to the geographical setting of the study visits, specifically whether visits conducted in a clinical setting would be more suitable and well-received by participants in comparison to those conducted in a non-clinical setting. This finding is consistent with the conclusions drawn by Lee and colleagues. 34
Continuous dietary self-monitoring and modification are both challenging tasks and important factors in the long-term treatment prognosis for patients with UC. Reduction of barriers to adherence is critical for chronic patients to maintain long-term adherence to dietary management.35–37 Compliance among applet users was reasonable, with 82.1% achieving basic goals of dietary record. Compared with those in the control group, participants in the intervention group completed a greater number of dietary records. Possible reasons for this are twofold.
On the one hand, potential benefit of utilizing text messages is their capacity to serve as useful reminders. Previous researches have demonstrated the positive impact of text message reminders on enhancing adherence to electronic health programs. 38 Furthermore, the manner and frequency of reminders have been found to influence response rates. For instance, a study involving individuals diagnosed with colorectal cancer indicated that the use of interrogative grammatical structures resulted in a higher level of engagement compared to declarative forms. 39 Consequently, future research should explore diverse reminder formats and frequencies tailored to patients at varying stages of healthy behavior to optimize participant retention and engagement.
On the other hand, our study employed a supplementary photo documentation approach, rather than solely relying on diet history questionnaires or weighed dietary records as in previous studies. 40 The food intake data were reviewed by experienced dietitians to ensure the accuracy and precision of energy and nutrient estimates and reduce the total time required to complete the food intake record of patients, which may result in a higher retention rate and documented dietary adherence of the participants. However, the review process of these images poses a significant burden on dietitians, thereby limiting the generalizability of HealthyGut to larger samples over an extended period. Consequently, future studies should contemplate the inclusion of standardized portion sizes as a frequently utilized option for users, aiming to enhance the frequency of self-recording.
Furthermore, the analysis of both quantitative and qualitative data indicated that the participants exhibited a considerable degree of acceptability towards the HealthyGut tool. A majority of the participants expressed their belief that HealthyGut has the potential to be a valuable tool for managing their diet. They also expressed their willingness to utilize HealthyGut again in the future and recommended it to other patients. These findings align with previous research, which has demonstrated that applet-based dietary recording offers the advantages of real-time dietary tracking and personalized feedback when compared to traditional paper diet diaries. These features are likely to be crucial factors contributing to the participants’ adoption of eHealth practices and their positive perception of HealthyGut's acceptability. Real-time dietary recording has the potential to mitigate recall bias among participants, while simultaneously enhancing the user experience through the provision of reliable, evidence-based nutritional information. This finding aligns with the Technology Acceptance Model, 41 which posits that the acceptability of novel applications is primarily influenced by users’ perceptions of ease of use and usefulness. An additional factor that warrants consideration is the adherence to a user-centered design methodology throughout the development process of HealthyGut, ensuring that the technology effectively facilitates tasks, possesses user-friendly operability, and provides value to its users. Prior investigations have also revealed that engaging in co-creation with the targeted end-users can significantly enhance the adoption of such a novel smartphone application.42,43
Besides, our study demonstrated that HealthyGut holds promise in facilitating the adoption of healthier behaviors. Several participants reported that the utilization of HealthyGut resulted in enhanced awareness and proficiency in dietary management, a decrease in unnecessary dietary restrictions, and increased confidence in effectively managing their diet. These outcomes are crucial for the sustenance of healthy behaviors.
Additionally, this study yielded a plethora of valuable information that can be utilized to enhance future iterations. Firstly, it was observed that users often exhibit a preoccupation with identifying suitable foods or recipes. While dietitians currently provide healthy recipes on a monthly basis, it is hoped that a greater variety of recipes specifically tailored for patients with UC will be made available. Newly diagnosed patients, particularly, express a desire to acquire knowledge about dietary restrictions and recommendations through recipe resources. Additionally, the potential of peer support in facilitating self-management of chronic diseases is noteworthy. In accordance with the recommendations put forth by participants, the incorporation of more practical modules for information exchange will enable users to establish connections with their family, friends, or local community, thereby mitigating feelings of isolation and loneliness.
Despite the short follow-up period, preliminary comparative data demonstrated some improvement in the dietary composition of patients with UC after using HealthyGut. Specifically, there was an increase in energy intake in the intervention group compared to the control group at week 12. The examination of initial dietary records indicated inadequate energy and macronutrient consumption among the participants included in the study. Patients with UC are recognized as being highly susceptible to malnutrition and sarcopenia, primarily due to excessive avoidance of food and restrictive dietary habits resulting from misinformation and apprehension of adverse bowel symptoms. 44 In comparison to interventions solely focused on dietary education, the HealthyGut program has the potential to enhance participants’ awareness of their dietary habits, encourage thoughtful consideration of their food choices, and potentially facilitate improved decision-making regarding food. These findings align with previous research studies that have reported similar outcomes.40,45,46
Limitations
This study has several limitations. First, the conclusions based on pilot trials were prone to significant bias. The convenience sample of the Chinesepopulation with UC from four clinics, together with the fact that participation in this trial was voluntary, may have resulted in selection bias. Another possible limitation is that dietary intake was self-reported, which could lead to reporting bias. Finally, no data were collected regarding participants’ previous dietary intake. However, it is worth noting that no significant differences were found in baseline nutrition knowledge between the two groups.
Conclusions
In summary, the HealthyGut, a newly developed dietary management applet consisting of four primary functional modules (user management, diet recording, nutritional feedback, and dietary advice), has been successfully developed and validated. This preliminary investigation has demonstrated that HealthyGut is a viable and well-received dietary management tool, with the potential to enhance healthy eating habits among Chinese patients with UC. A larger randomized controlled trial study on the long-term effects of the HealthyGut on eating behavior and health outcomes of patients with UC will be designed in the future to provide better and timely dietary decision support.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076231205741 for Mobile-based program improves healthy eating of ulcerative colitis patients: A pilot study by Wenjing Tu, Shuxia Yan, Tingting Yin, Sumin Zhang, Wenjing Xu, Ping Zhang and Guihua Xu in DIGITAL HEALTH
Supplemental material, sj-docx-2-dhj-10.1177_20552076231205741 for Mobile-based program improves healthy eating of ulcerative colitis patients: A pilot study by Wenjing Tu, Shuxia Yan, Tingting Yin, Sumin Zhang, Wenjing Xu, Ping Zhang and Guihua Xu in DIGITAL HEALTH
Supplemental material, sj-docx-3-dhj-10.1177_20552076231205741 for Mobile-based program improves healthy eating of ulcerative colitis patients: A pilot study by Wenjing Tu, Shuxia Yan, Tingting Yin, Sumin Zhang, Wenjing Xu, Ping Zhang and Guihua Xu in DIGITAL HEALTH
Acknowledgements
The authors would like to appreciate the efforts of physicians, nurses and staff at the participating hospitals who facilitated the study and all the study participants.
Footnotes
Contributorship: Wenjing Tu conceived the study, obtained funding and obtained ethical approval. Wenjing Tu,Tingting Yin, Sumin Zhang, Wenjing Xu and Ping Zhang were involved in patient recruitment. Wenjing Tu and Shuxia Yan analysed data and wrote the first draft of the manuscript. Guihua Xu revised critically the article. All authors reviewed and approved the final version of the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the Ethics Committee of the Affiliated Hospital (Project approval number: KY2021081). The trial was registered in the Chinese trial register (trial-ID: ChiCTR2100051539).
Funding: This research was supported by National Natural Science Foundation Youth Program of China, Ministry of Education Humanities and Social Sciences Youth Project of China, Nanjing University of Chinese medicine supporting National Natural Science Foundation Youth Program of China (grant number 72204124, 19YJCZH147, XPT72204124).
Guarantor: Wenjing Tu and Guihua Xu.
ORCID iD: Wenjing Tu https://orcid.org/0000-0002-6854-0869
Supplemental material: Supplemental material for this article is available online.
References
- 1.Okobi OE, Udoete IO, Fasehun OO, et al. A review of four practice guidelines of inflammatory bowel disease. Cureus J Med Science 2021; 13: e16859. DOI: 10.7759/cureus.16859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J, Cheon JH. Incidence and prevalence of inflammatory bowel disease across Asia. Yonsei Med J 2021; 62: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin A, Micic D. Nutrition considerations in inflammatory bowel disease. Nutr Clin Pract 2021; 36: 298–311. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa H, Nakamura S, Miyazaki T, et al. Inflammatory bowel disease and sarcopenia: its mechanism and clinical importance. J. Clin Med 2021; 10: 4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff SC, Escher J, Hébuterne X, et al. ESPEN Practical guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr 2020; 39: 632–653. [DOI] [PubMed] [Google Scholar]
- 6.Levine A, Rhodes JM, Lindsay JO, et al. Dietary guidance from the international organization for the study of inflammatory bowel diseases. Clin Gastroenterol H 2020; 18: 1381–1392. [DOI] [PubMed] [Google Scholar]
- 7.Sood A, Ahuja V, Kedia S, et al. Diet and inflammatory bowel disease: the Asian working group guidelines. Indian J Gastroenter 2019; 38: 220–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campmans-Kuijpers MJE, Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the Groningen anti-inflammatory diet (GrAID). Nutrients 2021; 13: 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rondanelli M, Lamburghini S, Faliva MA, et al. A food pyramid, based on a review of the emerging literature, for subjects with inflammatory bowel disease. Endocrinología, Diabetes y Nutrición 2021; 68: 17–46. [DOI] [PubMed] [Google Scholar]
- 10.Wark G, Samocha-Bonet D, Ghaly S, et al. The role of diet in the pathogenesis and management of inflammatory bowel disease: a review. Nutrients 2021; 13: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasson AN, Ingram RJM, Zhang Z, et al. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol Hepatol 2021; 6: 754–769. [DOI] [PubMed] [Google Scholar]
- 12.Shafiee NH, Manaf ZA, Mokhtar NM, et al. Anti-inflammatory diet and inflammatory bowel disease: what clinicians and patients should know? Intest Res 2021; 19: 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu E, Oleynick C, Raman M, et al. Optimizing inpatient nutrition care of adult patients with inflammatory bowel disease in the 21st century. Nutrients 2021; 13: 1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crooks B, McLaughlin J, Matsuoka K, et al. The dietary practices and beliefs of people living with inactive ulcerative colitis. Eur J Gastroen Hepat 2021; 33: 372–379. [DOI] [PubMed] [Google Scholar]
- 15.Guida L, Di Giorgio FM, Busacca A, et al. Perception of the role of food and dietary modifications in patients with inflammatory bowel disease: impact on lifestyle. Nutrients 2021; 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamp KJ, Pennings B, Javelli D, et al. Dietary patterns, beliefs and behaviours among individuals with inflammatory bowel disease: a cross-sectional study. J Hum Nutr Diet 2021; 34: 257–264. Journal Article; Research Support, Non-U.S. Gov't; Research Support, N.I.H., Extramural. DOI: 10.1111/jhn.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters V, Tigchelaar-Feenstra EF, Imhann F, et al. Habitual dietary intake of IBD patients differs from population controls: a case–control study. Eur J Nutr 2021; 60: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shafiee NH, Manaf ZA, Mokhtar NM, et al. An assessment of dietary intake, food avoidance and food beliefs in patients with ulcerative colitis of different disease status. Intest Res 2020; 18: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garnweidner-Holme L, Henriksen L, Torheim LE, et al. Effect of the pregnant+ smartphone app on the dietary behavior of women with gestational diabetes Mellitus: secondary analysis of a randomized controlled trial. Jmir Mhealth Uhealth 2020; 8: e18614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn SL, Kaciroti N, Eisenberg D, et al. Introducing dietary self-monitoring to undergraduate women via a calorie counting app has No effect on mental health or health behaviors: results from a randomized controlled trial. J Acad Nutr Diet 2021; 121: 2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putz P, Kogler B, Bersenkowitsch I. Reliability and validity of assessing energy and nutrient intake with the Vienna food record: a cross-over randomised study. Nutr J 2019; 18. DOI: 10.1186/s12937-019-0431-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontana JM, Pan Z, Sazonov ES, et al. Reproducibility of dietary intake measurement from diet diaries, photographic food records, and a novel sensor method. Front Nutr 2020; 7. DOI: 10.3389/fnut.2020.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elamin S, Cohen J. Telenutrition for inflammatory bowel disease: a tipping point for dietary wellness. Crohn's Colitis 360 2021; 3(2): 1–3. DOI: 10.1093/crocol/otab017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JM, Franco-Arellano B, Froome H, et al. The content, quality, and behavior change techniques in nutrition-themed Mobile apps for children in Canada: app review and evaluation study. Jmir Mhealth Uhealth 2022; 10: e31537. Journal Article; Research Support, Non-U.S. Gov't; Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinon P, Saliasi I, Bourgeois D, et al. Nutrition-Related Mobile apps in the French app stores: assessment of functionality and quality. Jmir Mhealth Uhealth 2022; 10: e35879. Journal Article; Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bucher Della Torre S, Carrard I, Farina E, et al. Development and evaluation of e-CA, an electronic Mobile-based food record. Nutrients 2017; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montag C, Becker B, Gan C. The multipurpose application WeChat: a review on recent research. Front Psychol 2018: 9. DOI: 10.3389/fpsyg.2018.02247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinese society of parenteral and enteral nutrition, inflammatory bowel disease committee of chinese medical education association. Consensus on nutritional diagnosis and treatment of inflammatory bowel disease in China. Chinese J Dig Dis Imaging 2021; 11: 8–15. [Google Scholar]
- 29.Yang Y. China Food Composition Table. 6nd ed. Peking: Peking University Medical Press, 2019. [Google Scholar]
- 30.Brooke J. SUS – a quick and dirty usability scale. United Kingdom: Usability Evaluation in Industry, 1996. [Google Scholar]
- 31.Hyzy M, Bond R, Mulvenna M, et al. System usability scale benchmarking for digital health apps: meta-analysis. Jmir Mhealth Uhealth 2022; 10: e37290. Journal Article; Meta-Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gan T, Cheng H, Tse MMY. Feasibility, acceptability, and effects of behavior change interventions for improving multiple dietary behaviors among cancer survivors: a systematic review. Support Care Cancer 2022; 30: 2877–2889. [DOI] [PubMed] [Google Scholar]
- 33.Salas-Groves E, Galyean S, Alcorn M, et al. Behavior change effectiveness using nutrition apps in people with chronic diseases: scoping review. Jmir Mhealth Uhealth 2023; 11: e41235. Journal Article; Review; Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SS, McGrattan A, Soh YC, et al. Feasibility and acceptability of a dietary intervention to reduce salt intake and increase high-nitrate vegetable consumption in Malaysian middle-aged and older adults with elevated blood pressure: findings from the DePEC-nutrition trial. Nutrients 2022; 14: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim JS, Heo JE, Kim HC. Factors associated with dietary adherence to the guidelines for prevention and treatment of hypertension among Korean adults with and without hypertension. Clin Hypertens 2020; 26: 5. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shim JS, Oh K, Jung SJ, et al. Self-Reported diet management and adherence to dietary guidelines in Korean adults with hypertension. Korean Circ J 2020; 50: 432–440. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh H, Cinnirella M, Bradley C. Support systems for and barriers to diabetes management in South Asians and Whites in the UK: qualitative study of patients’ perspectives. Bmj Open 2012; 2. Journal Article. DOI: e001459. 10.1136/bmjopen-2012-001459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulhanek A, Lukavska K, Gabrhelik R, et al. Comparing reminders sent via SMS text messaging and email for improving adherence to an electronic health program: randomized controlled trial. Jmir Mhealth Uhealth 2022; 10: e31040. Journal Article; Randomized Controlled Trial; Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagoel L, Stein N, Rennert G, et al. Better ask than tell: responses to mHealth interrogative reminders and associations with colorectal cancer screening subsequent uptake in a prospective cohort intervention. Jmir Mhealth Uhealth 2019; 7: e9351. Journal Article; Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamers CR, van Erp LW, Slotegraaf AI, et al. Web-based dietary assessment and advice helps inflammatory bowel disease patients to improve their diet quality. Brit J Nutr 2023; 129(2): 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckingham SA, Walker T, Morrissey K. The feasibility and acceptability of digital technology for health and wellbeing in social housing residents in Cornwall: a qualitative scoping study. Digit Health 2022; 8: 2012837451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turesson C, Liedberg G, Bjork M. Development of a digital support application with evidence-based content for sustainable return to work for persons with chronic pain and their employers: user-centered Agile design approach. JMIR Hum Factors 2022; 9: e33571. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fareed N, Swoboda C, Singh P, et al. Developing and testing an integrated patient mHealth and provider dashboard application system for type 2 diabetes management among medicaid-enrolled pregnant individuals based on a user-centered approach: mixed-methods study. Digit Health 2023; 9: 579787893. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day AS, Yao CK, Costello SP, et al. Food avoidance, restrictive eating behaviour and association with quality of life in adults with inflammatory bowel disease: a systematic scoping review. Appetite 2021; 167: 105650. [DOI] [PubMed] [Google Scholar]
- 45.Recio-Rodriguez J, Agudo Conde C, Calvo-Aponte M, et al. The effectiveness of a smartphone application on modifying the intakes of macro and micronutrients in primary care: a randomized controlled trial. The EVIDENT II study. Nutrients 2018; 10: 1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rollo ME, Haslam RL, Collins CE. Impact on dietary intake of two levels of technology-assisted personalized nutrition: a randomized trial. Nutrients 2020; 12: 3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076231205741 for Mobile-based program improves healthy eating of ulcerative colitis patients: A pilot study by Wenjing Tu, Shuxia Yan, Tingting Yin, Sumin Zhang, Wenjing Xu, Ping Zhang and Guihua Xu in DIGITAL HEALTH
Supplemental material, sj-docx-2-dhj-10.1177_20552076231205741 for Mobile-based program improves healthy eating of ulcerative colitis patients: A pilot study by Wenjing Tu, Shuxia Yan, Tingting Yin, Sumin Zhang, Wenjing Xu, Ping Zhang and Guihua Xu in DIGITAL HEALTH
Supplemental material, sj-docx-3-dhj-10.1177_20552076231205741 for Mobile-based program improves healthy eating of ulcerative colitis patients: A pilot study by Wenjing Tu, Shuxia Yan, Tingting Yin, Sumin Zhang, Wenjing Xu, Ping Zhang and Guihua Xu in DIGITAL HEALTH