Abstract
Acute pancreatitis (AP) is one of the most common acute abdominal conditions, and its incidence has been increasing for years. Approximately 15–20% of patients develop severe AP (SAP), which is complicated by critical inflammatory injury and intestinal dysfunction. AP-associated inflammation can lead to the gut barrier and function damage, causing dysbacteriosis and facilitating intestinal microbiota migration. Pancreatic exocrine deficiency and decreased levels of antimicrobial peptides in AP can also lead to abnormal growth of intestinal bacteria. Meanwhile, intestinal microbiota migration influences the pancreatic microenvironment and affects the severity of AP, which, in turn, exacerbates the systemic inflammatory response. Thus, the interaction between the gut microbiota (GM) and the inflammatory response may be a key pathogenic feature of SAP. Treating either of these factors or breaking their interaction may offer some benefits for SAP treatment. In this review, we discuss the mechanisms of interaction of the GM and inflammation in AP and factors that can deteriorate or even cure both, including some traditional Chinese medicine treatments, to provide new methods for studying AP pathogenesis and developing therapies.
Keywords: acute pancreatitis, cell damage factors, inflammatory responses, intestinal barrier, gut microbiota
Introduction
Multiple organ dysfunction may occur during the early phase of severe acute pancreatitis (SAP), resulting in a high fatality rate. However, over time, the patient enters a second stage, which accompanies the infection and is another cause of the high mortality rate of SAP. 1 Studies have shown that most pancreatic and extra-pancreatic organ infections are caused by the translocation of intestinal bacteria; such infections result in pancreatic necrosis and sepsis, causing late death in patients with SAP. 2 The gut microbiota (GM) is mutualistic with the human body under certain steady states; some gut bacteria can ferment dietary fiber to form short-chain fatty acids (SCFAs), which are then absorbed by the host. 3 The intestinal mucosa can also maintain the stability of the intestinal environment through its barrier function. Once this stability is disrupted by a persistent inflammatory response in SAP, this can lead to intestinal mucosal damage and a change in the status of the intestinal microbiota. 4 Studies have also indicated that various types of intestinal microbiota participate in different pathological conditions, including pancreatic diseases. 5 The role of the intestinal microbiota in the progression of SAP has gradually been clarified in previous studies.
We have searched articles or other types of manuscripts related to the regulatory mechanism of the intestinal microbiota, inflammation, and pathogenesis of SAP or acute pancreatitis (AP) in PubMed and the China National Knowledge Infrastructure, to describe the interactions between the GM and inflammatory responses in AP. We have identified some new methods of AP pathogenesis and the development of therapies. All of our findings are described in the following chapters.
The influence of intestinal flora changes on the occurrence and development of AP
Intestinal flora migration influences the pancreatic microenvironment in AP
Impairment in microcirculation and blood volume reduction during AP can lead to ischemia and reperfusion damage in the intestinal mucosa, causing loss of intestinal barrier integrity and intestinal bacterial translocation and causing local and systemic infections.6 –8 Fewer antimicrobials secreted by the pancreas in AP can also lead to bacterial overgrowth in the small intestine, which further disrupts the balance of the intestinal microbiota. 9 The imbalance of the intestinal microbiota or mucosal damage can increase intestinal permeability, causing the translocation of bacteria from the gut to the blood or nearby tissues, such as the pancreas, increasing the risk of pancreatic infection and aggravating inflammation. 10 A study has found more than one type of bacterial DNA in the peripheral blood of patients with AP, and these DNA molecules are mainly derived from conditional pathogenic bacteria from the gut, such as Escherichia coli, Shigella flexneri, Acinetobacter lwoffii, Bacillus coagulans, and Enterobacter faecalis. 11 Thus, the transfer of bacteria from the gut to the blood may cause infection of necrotic parts of the pancreas.
Recent studies have revealed that nucleotide-binding oligomerization domain 1 (NOD1), an intracellular innate immune receptor, plays a critical role in host defense functions and inflammation. This is because NOD1 can detect small peptide components derived from bacterial wall peptidoglycan and can be excited by intestinal bacteria. 12 On the other hand, NOD1 has been reported to activate innate responses and produce nuclear factor-kappa B (NF-κB) and type 1 interferon-inducing pancreatitis and contribute to the development of pancreatitis.13,14 Thus, NOD1 may be an intermediate regulatory factor of intestinal microbiota interaction with AP.
Previously, the microbial composition of the infected areas of pancreatic necrosis was mainly gram-negative bacteria from the gastrointestinal tract (GIT), such as Enterobacteriaceae. However, recently, Staphylococcus and Enterococcus have become dominant bacteria owing to the widespread use of prophylactic antibiotics. 15 Meanwhile, the prophylactic use of antibiotics does not reduce the risk of infection, and patients with a higher risk of infection in regional pancreatic necrosis are those who have previously received antibiotics.16,17
Intestinal microbiota attenuates the severity of AP
A normal intestinal microbiota constitutes the intestinal mucosal biological barrier that affects intestinal peristalsis, regulates host immunity, and strengthens the epithelial barrier. 18 Studies have shown that intestinal mucosal barrier damage in patients with AP is closely associated with the imbalance of the intestinal microbiota, for example, increased abundances of the intestinal pathogenic bacteria Shigella and Enterococcus and decreased abundances of the beneficial bacteria Lactobacillus and Blautia.19 –21 Deng showed that the bacterial translocation rates of E. coli and Bifidobacterium and the pathological damage score of intestinal tissue were significantly higher in the intestines of SAP rats than in those of the control group, suggesting that the intestinal barrier function of SAP rats was impaired, resulting in an intestinal microbiota disorder. 22 Moreover, the Acute Physiology and Chronic Health Evaluation (APACHE)-II score, the length of the hospital stay, complications such as infections and the incidence of multiple organ dysfunction syndrome were significantly higher in patients with SAP with a GM imbalance than in individuals with intestinal microbiota ratios similar to those of healthy individuals. 9
It has been reported that inter-intestinal probiotics mitigated AP severity by inhibiting the activation of the NOD-like receptor family 3 (NLRP3) inflammasome in the gut,23,24 which might be the mechanism of regulating the intestinal microbiota to reduce the degree of SAP. E. coli has been reported to induce intestinal mucosal barrier damage and aggravate AP through the activation of the toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), and p38 mitogen-activated protein kinase (MAPK) signaling pathways.25,26 Therefore, MAPK inhibitors and TLR4-dependent Phosphoinositide 3-kinase (PI3K), V-akt murine thymoma viral oncogene homolog (AKT), and NF-kB inflammatory signaling pathway inhibitors are important in correcting GM imbalance and mitigating inflammatory responses.27,28 SCFAs, a metabolite of intestinal bacteria, can not only provide growth energy for intestinal mucosal cells but also regulate intestinal pH, maintain the integrity of tight junction proteins between intestinal mucosal epithelial cells, improve intestinal mucosal barrier function, and significantly reduce the severity of SAP.21,24,29 On the other hand, Bacteroides, Escherichia–Shigella and Enterococcus, are the major intestinal microbes in AP, and different levels of AP are associated with different intestinal microbiota disorders. 20 In mild acute pancreatitis (MAP), Finegoldia exhibited the most significant increase, and Brucella was the species of intestinal microbiota that showed the largest decrease. Moderately severe acute pancreatitis (MSAP) patients had the most significant increase in Anaerococcus and the most significant decrease in Eubacterium hallii. The potential biomarkers of MAP are Finegoldia, E. hallii, and Lachnospiraceae. E. hallii and Anaerococcus are potential diagnostic biomarkers for MSAP (Table 1). According to reports, Firmicutes increase while Bacteroidetes decrease in acute patients’ intestines. Enterococcus in Firmicutes can adhere to host cells, invade them, and traverse the epithelial barrier. This can lead to infection and systemic inflammation. Bacteroidetes are capable of producing SCFAs, which have anti-inflammatory effects and help maintain the integrity of the intestinal barrier, thereby protecting it. Certain pathogenic bacteria within Bacteroidetes, such as E. coli and Shigella, can disrupt the intestinal mucosal barrier, resulting in severe colonic inflammation. Therefore, the imbalance between Firmicutes and Bacteroidetes can aggravate the pathogenetic condition of AP. 20 SAP was associated with the most significant increase in the abundance of Enterococcus and the greatest decrease in the abundance of E. hallii. 30 The expression of proinflammatory factors such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α) in the serum of SAP patients was positively correlated with the intestinal aerobic bacteria level but negatively correlated with the level of anaerobic bacteria such as Bifidobacterium. 9 Perhaps modulating the gut flora can reduce the body’s inflammatory response and reduce AP severity.
Table 1.
Effects of alteration of bacteria on AP and GM and their mechanisms.
| Grade of AP | Bacteria | Phylum/family | Change in bacteria | Effect on AP | Effect on GM | Mechanism | References |
|---|---|---|---|---|---|---|---|
| MAP | Faecalibacterium prausnitzii | Firmicutes/Ruminococcaceae | Beneficial and decreased | Aggravation | Negative (migration of intestinal microbiota and overgrowth of intestinal pathogens) | Promote anti-inflammatory properties, such as the production of SCFAs when digesting plant polysaccharides; promotes intestinal epithelial cell proliferation, mucosal repair, and anti-inflammatory responses by inducing IL-10 and regulating T-cell responses in the intestine. | Jandhyala et al., 31 Alkanani et al., 32 Wu et al., 33 Holmes et al., 34 Smith et al., 35 Louis and Flint, 36 Rossi et al., 37 Martín et al. 38 |
| Bacteroides | Bacteroidetes/Bacteroidaceae | Harmful and increased | Aggravation; infectious pancreatic necrosis | Negative | Aggravate inflammation by stimulating the intestinal mucosa to produce cytokines and several toxins; possess high numbers of antibiotic resistance genes and are risk factors for autoimmunity. High-protein and high-fat diets increase the abundance of Bacteroides. Cause a thinning of the mucus layer by transforming lactate into SCFAs, which contribute to a decrease in mucin synthesis and destroy the tight junctions, finally leading to increased gut permeability; alter the intestinal barrier function, which results in bacterial overgrowth and impaired immunity. |
Hamada et al., 5 Wu et al., 33 Sundin et al., 39 de Goffau et al., 40 Smedley et al., 41 Davis-Richardson et al., 42 Mejía-León et al., 43 Brown et al., 44 Tlaskalová-Hogenová et al., 45 Zhang et al. 46 | |
| Bacteroides dorei | Bacteroidetes/Bacteroidaceae | Harmful and increased | Aggravation | Negative | Impair the intestinal epithelial layer, manipulate immune system development, and aggravate inflammation. | Davis-Richardson et al. 42 | |
| Bacteroides vulgatus | Bacteroidetes/Bacteroidaceae | Harmful and increased | Aggravation | Negative | An opportunistic pathogen causing intraabdominal infections and preventing remission. | Davis-Richardson et al., 42 Fujita et al., 47 Rath et al. 48 | |
| Bacteroides fragilis | Bacteroidetes/Bacteroidaceae | Harmful and increased | Aggravation | Negative | Disrupt the tight junctions by proteolytic degradation due to metalloprotease enterotoxins, thus increasing paracellular permeability, with gut inflammation, cell damage, and a loss of microvilli. | Mejía-León et al., 43 Berkes et al. 49 | |
| Streptococcus | Firmicutes/Streptococcaceae | Harmful and increased | Aggravation | Negative | Correlate with malabsorption or decreased levels of pancreatic enzymes associated with pancreatitis. | Hamada et al., 5 Mancabelli et al. 50 | |
| Saccharopolyspora | Actinobacteria/Pseudonocardiaceae | Harmful and increased | Aggravation | Negative | Enhance inflammatory responses by mediating cytokines and chemokines that recruit inflammatory cells and lead to overproduction of interferon-gamma | Riquelme et al., 51 Kim et al. 52 | |
| Proteobacteria | Proteobacteria | Harmful and increased | Aggravation | Negative | LPS-producing bacteria; increase the abundance of certain pathogens and decrease those of probiotics. Induce a tolerogenic immune program by differentially activating select toll-like receptors in monocytic cells and leading to T-cell anergy. |
Zhang et al., 46 Ren et al., 53 Pushalkar et al. 54 | |
| Prevotella | Bacteroidetes/Prevotellaceae | Harmful and increased | Aggravation | Negative | Bacteroides-dominant gut communities and LPS-producing bacteria; carbohydrate-rich diets increase the abundance of Prevotella. Impair the intestinal epithelial barrier function and aggravate inflammation. |
Wu et al., 33 Mejía-León et al., 43 Ren et al. 53 | |
| Clostridium lavalense | Firmicutes/Clostridiaceae | Beneficial and decreased | Aggravation | Negative | A butyrate-producing bacterium, maintaining intestinal epithelial barrier function and anti-inflammation; negatively correlates with serum IL-6 levels. | Hamada et al., 5 Tan et al., 9 Ren et al., 53 Takahashi et al., 55 Hague et al. 56 | |
| Atopobium | Actinobacteria/Coriobacteriaceae | Beneficial and decreased | Aggravation | Negative | Actinobacteria produce many compounds, including antibiotics, anticancer agents, immunosuppressants, anthelmintics, antiviral agents, and extracellular enzymes. | Zhang et al., 46 van Bergeijk et al., 57 Barka et al. 58 | |
| Bifidobacterium adolescentis or B. pseudocatenulatum | Actinobacteria/Bifidobacteriaceae | Beneficial and decreased | Aggravation | Negative | Increase the production of butyrate, which is the main energy source for intestinal epithelial cells; exert anti-inflammatory effects and inhibit bacterial translocation. | de Goffau et al., 40 Duffy 59 | |
| Megamonas | Firmicutes | Beneficial and decreased | Aggravation | Negative | The main metabolite is acetic acid, which promotes intestinal peristalsis and relieves constipation. | Mejía-León et al., 43 Tian et al. 60 | |
| Acidaminococcus intestini | Firmicutes | Harmful and increased | Aggravation | Negative | Positively associated with LPS-stimulated TNF-α production. | Mejía-León et al., 43 Zheng et al. 61 | |
| Finegoldia magna | Firmicutes | Harmful and increased | Aggravation | Negative | An opportunistic pathogen, binding to histones through its surface and the extracellular adhesion protein FAF Finegoldia magna cell wall adhesion protein (FAF) and producing collagenase and gelatinase enzymes to protect bacteria and finally aggravate infection. An anaerobic gram-positive coccus acts as a virulence factor. | Hamada et al., 5 Martín et al., 38 Sundin et al., 39 Murphy et al., 62 Krepel et al. 63 | |
| Blautia | Firmicutes/Lachnospiraceae | Beneficial and decreased | Aggravation | Negative | Reduction in the abundance of Blautia promotes the overgrowth of intestinal bacteria, increases intestinal permeability, and ultimately leads to higher concentrations of endotoxins and the activation of inflammatory cascades. | de Goffau et al., 40 Smedley et al., 41 Davis-Richardson et al. 42 | |
| Lachnospira | Firmicutes/Lachnospiraceae | Beneficial and decreased | Aggravation | Negative | Produce butyrate and influence SCFA levels. Produce reuterin, inhibit pathogens, and suppress inflammation in the gut. | Mejía-León et al. 43 | |
| MSAP | Escherichia–Shigella | Proteobacteria/Enterobacteriaceae | Harmful and increased | Aggravation | Negative | Opportunistic gram-negative bacterial pathogens. Invade the colonic and rectal mucosa, provoking a strong inflammatory response. |
Wu et al., 33 Brown et al. 44 |
| Eubacterium hallii | Firmicutes/Lachnospiraceae | Beneficial and decreased | Aggravation | Negative | A butyrate (SCFA)-producing bacterium. Produces reuterin, inhibits pathogens, and suppresses inflammation in the gut. | Zhang et al., 46 Fujita et al. 47 | |
| MSAP and SAP | Atopobium | Actinobacteria/Coriobacteriaceae | Beneficial and decreased | Aggravation | Negative | Actinobacteria produce many compounds, including antibiotics, anticancer agents, immunosuppressants, anthelmintics, antiviral agents, and extracellular enzymes. | Zhang et al., 46 van Bergeijk et al., 57 Barka et al. 58 |
| Bacteroides | Bacteroidetes/Bacteroidaceae | Harmful and increased | Aggravation | Negative | Aggravate inflammation by stimulating the intestinal mucosa to produce cytokines and several toxins; possess high numbers of antibiotic resistance genes and are risk factors for autoimmunity. High-protein and high-fat diets increase the abundance of Bacteroides. Cause a thinning of the mucus layer by transforming lactate into SCFAs, which contributes to a decrease in the mucin synthesis, destroys the tight junctions, and finally leads to increased gut permeability; alter the intestinal barrier function, which results in bacterial overgrowth and impaired immunity. |
Hamada et al., 5 Wu et al., 33 Sundin et al., 39 de Goffau et al., 40 Smedley et al., 41 Davis-Richardson et al., 42 Mejía-León et al., 43 Brown et al., 44 Tlaskalová-Hogenová et al., 45 Zhang et al. 46 | |
| Firmicutes | Firmicutes | Beneficial and decreased | Aggravation | Negative | Promote anti-inflammatory properties by producing SCFAs when digesting plant polysaccharides; promote intestinal epithelial cell proliferation, mucosal repair, and anti-inflammatory responses by inducing IL-10 and regulating T-cell responses in the intestine. | Jandhyala et al., 31 Alkanani et al., 32 Wu et al., 33 Holmes et al., 34 Smith et al., 35 Louis and Flint, 36 Rossi et al., 37 Martín et al. 38 | |
| Proteobacteria | Proteobacteria | Harmful and increased | Aggravation | Negative | LPS-producing bacteria; Increase the abundance of certain pathogens and decrease that of probiotics. Induce a tolerogenic immune program by differentially activating select toll-like receptors in monocytic cells and leading to T-cell anergy. |
Zhang et al., 46 Ren et al., 53 Pushalkar et al. 54 | |
| Synergistetes | Synergistetes | Harmful and increased | Aggravation | Negative | Related to LPS biosynthesis, considered a putative periodontal pathogen and is associated with several systemic diseases and inflammatory disorders of the gastrointestinal tract. | Pushalkar et al., 54 Hugenholtz et al., 64 Amado et al., 65 Oliveira et al., 66 Deng et al., 67 Kumar 68 | |
| Enterococcus | Firmicutes/Enterococcaceae | Harmful and increased | Aggravation | Negative | Positively correlate with serum IL-6 and endotoxin levels, provoke systemic inflammatory response syndrome and intestinal bacterial translocation. | Tan et al. 9 | |
| Bifidobacterium | Actinobacteria/Bifidobacteriaceae | Beneficial and decreased | Aggravation | Negative | Negatively correlated with serum IL-6 levels; decrease the population of Bacteroides vulgatus in the gut and ameliorate gut inflammation. | Tan et al., 9 Davis-Richardson et al., 42 Shiba et al., 69 Setoyama et al. 70 | |
| Lactobacillus | Firmicutes/Lactobacillaceae | Beneficial and decreased | Aggravation | Negative | Cause dysfunction of immune homeostasis in the gut and periphery and hypofunction of microbial anti-infection, accompanied by downmodulation of inflammation by dendritic cells by inducing polarization of regulatory T cells. | Alkanani et al., 32 Bron et al., 71 Smits et al., 72 Penders et al. 73 | |
| SAP | Staphylococcus | Firmicutes/Staphylococcaceae | Beneficial and decreased | Aggravation | Negative | Promote the growth of anaerobic bacteria, including species of the genera Bifidobacterium, Clostridium, and Bacteroides. | Sahar et al., 15 Alkanani et al. 32 |
| Enterococcus | Firmicutes/Enterococcaceae | Harmful and increased | Aggravation | Negative | Positively correlate with serum IL-6 and endotoxin levels, provoke systemic inflammatory response syndrome and intestinal bacterial translocation. | Tan et al., 9 Marques et al. 74 |
AP, acute pancreatitis; GM, gut microbiota; IL, interleukin; LPS, lipopolysaccharide; MAP, mild acute pancreatitis; MSAP, moderate severe acute pancreatitis; SAP, severe acute pancreatitis; SCFA, short-chain fatty acid; TNF-α, tumor necrosis factor-α.
The influence of AP on intestinal flora changes
Acute pancreatitis-associated gut barrier and functional damage facilitate intestinal flora migration
Intestinal barrier dysfunction was found in both animal models and clinical patients with AP. 75 The mechanism of intestinal microbiota migration in AP is as follows: intestinal barrier damage and a variety of gastrointestinal polypeptide secretions can destroy Cajal mesenchymal cells, 76 decrease gastrointestinal movement, 77 and impair intestinal motility, 78 resulting in the overgrowth of intestinal bacteria in AP. 79 Early fasting in patients with AP can cause intestinal ischemia–reperfusion injury, which can lead to intestinal mucosal microcirculation disorders and abnormal release of inflammatory factors and reactive oxygen species (ROS). These substances can cause the oxidative stress response in the intestinal mucosa, 80 the apoptosis of intestinal epithelial cells, and increased permeability of intestinal capillaries,81,82 ultimately leading to intestinal barrier function disorders and increased intestinal permeability.83,84 The intestinal immune barrier function is compromised in patients with AP, 85 and the level of secretory immunoglobulin A is decreased, 86 which allows bacteria to pass through the intestinal barrier more easily. In addition to these three effects, long-term fasting and the obstruction of the lower bile duct in patients with SAP can result in a significant decrease in bile secretion or ineffective secretion into the intestine. 75 Deoxycholic acid in the bile can selectively inhibit gram-positive bacilli (Bacillus, Clostridium, Lactobacillus, and Streptococcus pneumoniae). The reduction in bile secretion impairs the normal balance of the intestinal microbiota, resulting in the activation of an oxidative stress response and intestinal epithelial cell apoptosis, thus increasing bacterial migration. 87
Effects of secretion of cell damage factors on intestinal flora in AP
Intestinal barrier dysfunction is the most common complication of SAP. Previous clinical studies have shown that elevated serum levels of many inflammatory cytokines in SAP, including TNF-α, 84 IL-1, 88 IL-6, 89 neutrophil elastase (NE), and myeloperoxidase (MPO), 90 are associated with intestinal barrier dysfunction. One of the main cytokines associated with AP is TNF-α, a proinflammatory cytokine, which is found to have elevated levels both locally, in the intestine, and systemically in patients with intestinal barrier dysfunction.91,92 An increase in the TNF-α levels can lead to inflammation in the intestinal mucosa and to intestinal epithelial cell apoptosis,93,94 which can lead to intestinal epithelial mechanical barrier damage and facilitate bacterial displacement. 95 In addition to direct injury, TNF-α can initiate a positive feedback loop that induces the secretion of other cytokines, such as IL-1 and IL-6, to further injure the intestinal mucosa. 96 An increase in the IL-1 levels in AP and the risk associated with IL-1 and the IL-1 receptor (IL-1R) in the pathogenesis of pancreatitis have been reported. 97 IL-1R-deficient mice pretreated with an IL-1R antagonist recombinant human interleukin-1-receptor antagonist (rhIL-1Ra) experience milder pancreatitis after cerulein induction. The activation of IL-1β can also stimulate the local mucosal immune response and cause mucosal injury by stimulating T-cell proliferation and neutrophil entry to the site of injury or infection through the binding of IL-1β and IL-1R.98,99 Serum IL-6 is another reliable indicator of AP severity that can predict both organ failure and SAP. 100 The production of IL-6 can activate several different pathways in the adaptive immune system, thereby exacerbating inflammation and negatively affecting barrier function. 101 Tan et al. 9 also found that serum IL-6 levels in patients with AP were positively correlated with the abundance of Enterobacter and Enterococcus in the intestinal microbiota and negatively correlated with the abundance of XI groups of Bifidobacterium and Clostridium. In pancreatic tissue from a mouse model of SAP, neutrophil extracellular traps (NETs) decorated with MPO and NE were shown to aggravate tissue damage. 102 Many lethal complications of SAP have been shown to be closely related to NETs. According to previous reports, NETs can disrupt the balance of the intestinal microbiota, cause intestinal epithelial cell damage, and even induce apoptosis, leading to gut barrier damage, increased intestinal mucosal permeability, elevated endotoxin secretion, and imbalances in the GM.90,103 –105
Pancreatic exocrine deficiency affects the composition and diversity of GM in AP
Patients with AP exhibit complications such as pancreatic exocrine impairment (PEI) and acinar cell dysfunction, which significantly impact changes in intestinal microbiota composition, 106 and the secretion of many enzymes, such as lactate and bile acids, declines to a certain level. 107 In animal models of PEI, the intestinal microbes E. coli, Lactobacillus, and Bifidobacterium were increased, and the levels of Fusobacterium and Clostridium hiranonis were decreased, inducing a significant difference in the dysbiosis index between affected animals and healthy individuals. 108 Stool samples from PEI patients were analyzed and showed that pancreatic elastase levels significantly correlated with intestinal flora diversity compared with those of normal individuals, and significant differences were found in the abundances of 22 taxa, such as an increase in Pseudomonas spp. and a decrease in Bacillus spp. 106 These results revealed that changes in pancreatic fluid secretion were also significantly correlated with flora diversity.
Antimicrobial peptide changes in AP-affected intestinal flora
Antimicrobial peptides (AMPs) are oligopeptides that are arranged linearly or circularly and are composed of amino acid residues of different lengths (up to 100). AMPs usually form L-amino acids through secondary structures containing alpha helices, beta sheets, or both. 109 These biomolecules exhibit diverse biological activities against gram-positive and gram-negative bacteria, viruses, fungi, protozoa, and even tumors. 110 The Data Repository of AMPs (DRAMP) database includes over 4800 peptides that are antiproteins 111 and contribute to the maintenance of intestinal bacterial homeostasis and intestinal barrier function. 112 AMPs such as the cathelicidin-related AMP (CRAMP) have been reported to be secreted by pancreatic acinar cells, and reduced secretion of pancreatic AMPs can lead to the abnormal growth of intestinal bacteria and the disruption of the intestinal microbiota balance. Moreover, CRAMP deficiency worsens pancreatic inflammation. 113 Decreased expression of ileal terminal AMPs was found in necrotizing pancreatitis. 114 Hypertriglyceridemia (HTG) affects the expression of AMPs, including α-defensin, lysozyme, phospholipase A2, and regenerating islet-derived protein 3α (Reg3A), 115 in Paneth cells, which may exacerbate HTG-related acute necrotizing pancreatitis in intestinal barrier dysfunction. Pancreatic cells secrete a variety of AMPs to regulate the structure of the intestinal microbiota. 116 Lysozyme and α-defensins have activities against gram-negative and gram-positive bacteria, and some experts believe that fecal levels of α-defensins are a surrogate marker for gut microbial homeostasis.117,118 On the other hand, Reg3A, which has powerful bactericidal activity, can antagonize gram-positive bacteria119,120 by limiting the number of mucosal-adherent bacteria to separate the GM from the epithelium and reduce bacterial translocation 121 (Table 2).
Table 2.
AMP changes in AP-affected intestinal microbiota.
| AMPs | Characteristics | Mechanism | Associated with AP | Associated with GM | References |
|---|---|---|---|---|---|
| CRAMP | Reduced | The Orai1 Ca2+ channel, which is needed in pancreatic exocytosis, can suppress the inflammation-associated alteration of intestinal bacteria | Increased mortality in AP | Gastrointestinal inflammation, intestinal bacterial overgrowth or dysbiosis, and systemic infection; impaired immunomodulatory effects | Ahuja et al., 10 Deng et al. 113 |
| RegIIIγ and β-defensins | Reduced | The GM metabolites SCFAs (including butyrate) activate mTOR in IECs and promote IEC RegIIIγ and β-defensins in a GPR43-dependent manner | Serious pancreatic damage and systemic inflammation | Increased intestinal inflammatory responses; decreased SCFA-induced AMP production | Zhao et al. 114 |
| RegIIIγ and β-defensins | Reduced | The GM metabolites SCFAs (including butyrate) activate STAT3 in IECs and promote IEC RegIIIγ and β-defensins in a GPR43-dependent manner | Serious pancreatic damage and systemic inflammation | Inhibition of intestinal immune regulation and intestinal organoid stemness proliferation; decreased AMP secretion | Zhao et al. 114 |
| RegIIIγ and RegIIIβ | Reduced | Microbiota can directly affect AMP production by interfering with TLR-TLR ligand interactions | Serious pancreatic damage and systemic inflammation | Decreased AMP secretion | Zhao et al., 114 Menendez et al., 122 Brandl et al., 123 Vaishnava et al. 124 |
| C-type lectins of the RegIII family | Reduced | Bactericidal activity by binding membrane phospholipids and killing bacteria by forming a hexameric membrane-permeabilizing oligomeric pore | Serious pancreatic damage and systemic inflammation (bactericidal for gram-positive but not for gram-negative bacteria) | Antibacterial effects against enteric pathogens; promoting mutualism with the resident microbiota in orthergasia | Wong 112 , Mukherjee et al., 119 Cash et al. 120 |
| α-Defensin and lysozyme | Reduced | Intestinal microbiota dysbiosis and decreased levels of AMPs in Paneth cells may participate in the pathogenesis of intestinal barrier dysfunction | Serious pancreatic damage and systemic inflammation | Increased intestinal proinflammatory cytokine (TNF-α, IL-1β, and IL-17A) levels in plasma and tissue; weakened resistance against enteric pathogens; dysbiosis of intestinal microbiota structure and aggravated intestinal barrier dysfunction | Huang et al., 115 Clevers and Bevins, 117 Eriguchi et al., 118 Salzman et al., 125 Satoh et al. 126 |
| AMPs secreted by Paneth cells | Reduced | Increase in the concentration of cytosolic Ca2+ accompanies granule secretion responding to bacteria or bacterial products, such as lipopolysaccharide | Serious pancreatic damage and systemic inflammation | Dysbiosis of intestinal microbiota and aggravated intestinal barrier dysfunction | Satoh et al., 126 Ayabe et al. 127 |
AMP, antimicrobial peptide; AP, acute pancreatitis; CRAMP, cathelicidin antimicrobial peptide; GM, gut microbiota; GPR, G protein-coupled receptor; IEC, intestinal epithelial cell; IL, interleukin; MAP, mild acute pancreatitis; MSAP, moderate severe acute pancreatitis; mTOR, mammalian target of rapamycin; Reg, regenerating islet-derived protein; SAP, severe acute pancreatitis; SCFA, short-chain fatty acid; STAT3, signal transducer and activator of transcription 3; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α.
Risk factors that influence the GM and AP
Trillions of microbes live in the gut, and this community plays a vital role in the regulation of both intestinal and pancreatic functions. The underlying common causes of AP, such as biliary obstruction, alcohol misuse, HTG, and a high-fat/sugar diet, may also cause changes in the intestinal flora. 128 These risk factors affect both AP and the intestinal microflora; thus, interactions between the intestinal microflora and the occurrence of AP can be inferred.
Obesity and hyperlipidemia
Obesity typically presents with low-level systemic inflammation, such as increased leukocyte counts and TNF-α, IL-6, and C-reactive protein levels129,130; furthermore, it is characterized by increased secretion of biomarkers by adipocytes and is associated with AP. Moreover, macrophages in adipose tissue have been reported to participate in inflammation in obesity via the secretion of proinflammatory cytokines, such as TNF-α and IL-6, 131 both of which have been proven to affect AP and GM.
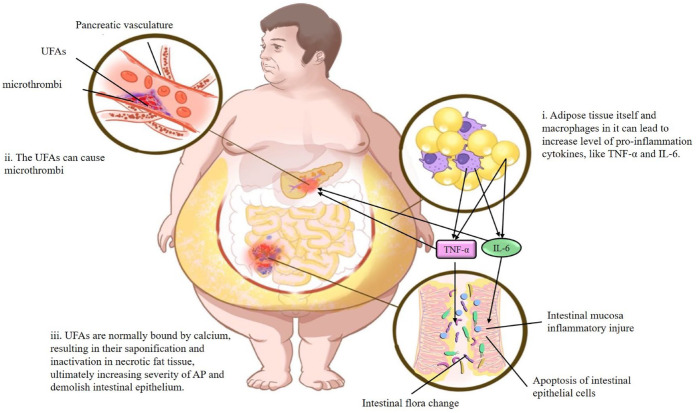
Obesity can also unmask primary HTG due to genetic causes and is a risk factor for secondary HTG, which is associated with pancreatitis.132,133 At present, HTG-induced AP (HTG-AP) has become the second leading cause of AP. 134 Indeed, the proportion of Bacteroides in the intestines of lean mice was found to be higher than that in obese mice after administration of the same diet, while the opposite was true for thick-walled Bacteroides. 135 Rats fed a high-fat diet showed significant increases in serum low-density lipoprotein, total cholesterol, and triacylglycerol, as well as changes in Bifidobacteria, Lactobacilli, Enterococci, Enterobacteria, and Anaphylactic bacteria in the intestinal flora. 136 In an animal model of hyperlipidemic necrotizing pancreatitis, researchers also found intestinal microflora imbalances and decreased AMPs in Paneth cells, further confirming that hyperlipidemia can affect the severity of AP and the intestinal microflora. 115 Unsaturated fatty acids (UFAs) might be an important factor that can affect both AP and GM in obesity and hyperlipidemia, and these factors are mainly transmitted via the lipolysis of circulating triglycerides. 137 The insolubility of UFAs in the aqueous environment of the blood can cause microthrombi formation in the pancreatic vasculature, leading to ischemia and pancreatic infarction. As polar molecules, UFAs usually bind with calcium, resulting in their saponification and inactivation in necrotic fat tissue. 138 Unbound UFAs can increase the serum levels of TNF-α and other inflammatory cytokines, 139 thereby worsening AP and leading to inflammation of the intestinal mucosa and intestinal epithelial cell apoptosis93,94 (Figure 1).
Figure 1.
The negative effect of obesity and hypertriglyceridemia both on AP and GM.
(i) The adipose tissue itself and macrophages in it can lead to increase level of pro-inflammation cytokines, like TNF-α and IL-6. These cytokines cause intestinal mucosa inflammatory injury, apoptosis of intestinal epithelial cells, and intestinal flora alteration. (ii) In the obesity or hyperlipidemia, the UFAs transmitted from lipolysis of circulating triglycerides can cause microthrombi formation in the pancreatic vasculature resulting in ischemia and pancreatic infarction. (iii) UFAs are normally bound by calcium, resulting in their saponification and inactivation in necrotic fat tissue, ultimately increasing severity of AP and demolish intestinal epithelium.
AP, acute pancreatitis; GM, gut microbiota.
In addition to the harmful effects of obesity and hyperlipidemia on the GM, recent studies have shown that the intestinal flora is one of the important environmental factors affecting the occurrence and development of obesity. The intestinal flora can induce adipocytokine gene expression by affecting intestinal epithelial cell fasting, leading to the increased production of triacylglycerols in the body and causing lipid metabolism disorders and the development of obesity. 140 Furthermore, disturbances in the intestinal flora in obese mice may lead to abnormal lipid metabolism, energy metabolism, adipokine synthesis, and cell death, leading to the secretion of a large number of proinflammatory cytokines into the blood and resulting in the exacerbation of pancreatitis. 115 Some studies indicated that intake of probiotic preparations could affect serum cholesterol and high-density lipoprotein levels and indirectly lower blood lipids, suggesting that the establishment of normal intestinal flora can help balance lipid metabolism. 113
Alcohol
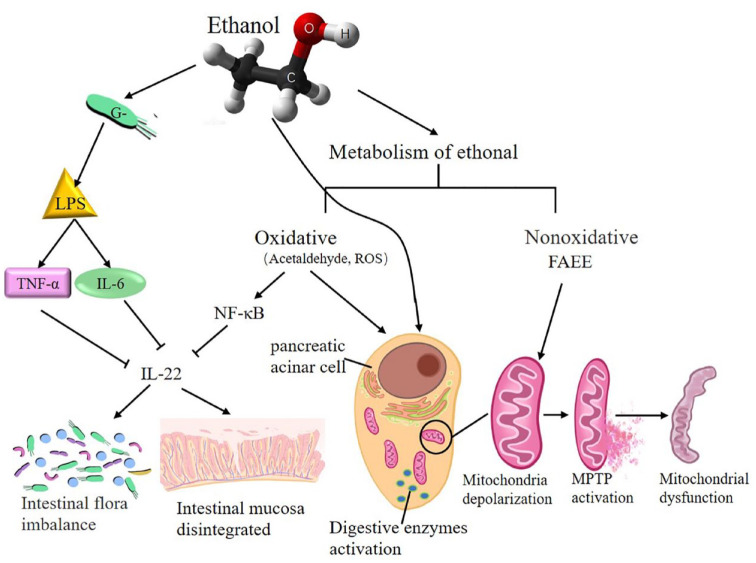
In recent years, due to changes in diet and increases in alcohol consumption, heavy drinking has become a risk factor for AP, and this condition easily progresses to SAP. 141 The toxicity of ethanol is mediated by ethanol itself or its oxidative and nonoxidative metabolism. Oxidative ethanol metabolism potentiates cholecystokinin-induced depolarization by sensitizing pancreatic mitochondria to Ca2+-induced mitochondrial permeability transition pore (MPTP) activation, resulting in mitochondrial dysfunction in pancreatic acini and necrosis in the pancreas. 142 Although the main ethanol metabolism in vivo is oxidation, a smaller part undergoes nonoxidative metabolism.143,144 Incubation of isolated pancreatic acinar cells with fatty acid ethyl esters, one of the nonoxidative ethanol metabolites in vivo, induced mitochondrial depolarization, depletion of cellular adenosine triphosphate, 145 and sustained elevations of intracellular Ca2+ levels ultimately associated with cellular dysfunction and cell death. 146 Both alcohol and its metabolites can activate digestive enzymes early in pancreatic acinar tissue, resulting in pancreatic tissue autodigestive injury, and activate pancreatic stellate cells, leading to fibrosis of the pancreas. 143
On the other hand, alcohol has been shown to have a negative impact on the intestinal flora of healthy people, such as decreasing the biodiversity of the intestinal flora and affecting the overall composition of the microbial community. 145 Disturbances in the intestinal flora may cause disorders of glycolipid energy metabolism and other potential functional pathway changes in the body. 147 Alcohol can lead to changes in the composition of the GIT microbiota and metabolic function, contributing to the well-established association between alcohol-induced oxidative stress and intestinal hyperpermeability to luminal bacterial products.146 –148 Exposure to ethanol can increase the release of enterogenous gram-negative bacteria-derived lipopolysaccharide (LPS), leading to macrophage activation and the secretion of cytokines, including TNF-α, IL-1β, and IL-6. 149 IL-22 is mainly involved in maintaining the integrity of the epithelial barrier and linking intestinal immune activation with epithelial repair and barrier protection.150,151 Under inflammatory conditions, IL-22 can be activated through the IL-23-Janus kinase/signal transducer and activator of transcription signaling pathway, resulting in the production of AMPs. 152 Ethanol metabolism in vivo produces acetaldehyde and ROS, which can activate NF-κB and ultimately stimulate the immune response, 153 decrease intestinal expression of IL-22, and alter gut epithelial integrity, causing an increase in intestinal permeability and bacterial translocation 154 (Figure 2).
Figure 2.
The mechanism and negative effect of ethanol metabolism on AP and GM.
The toxicity of ethanol is mediated by ethanol itself though its oxidative or nonoxidative metabolism. Exposure to ethanol can increase release of enterogenous gram-negative bacteria-derived LPS leading to macrophages activation and cytokines secretion, including TNF-α, IL-6, etc. and inhibit intestinal expression of IL-22, finally damaging intestinal flora balance and intestinal mucosa integrity. Oxidative metabolism of ethanol produces acetaldehyde and ROS which could activate NF-κB, inhibit intestinal expression of IL-22, and also activate trypsin causing pancreatic tissue autodigestive injury. Nonoxidative metabolism of ethanol induced mitochondrial depolarization to Ca2+-induced MPTP activation, resulting in pancreatic mitochondrial dysfunction and trypsin activation.
AP, acute pancreatitis; GM, gut microbiota.
High glucose and insulin resistance
Pancreatic damage, pancreatitis, imbalances in the GM, and blood sugar imbalances may be interrelated. 148 AP exhibits hyperglycemia in the early stage, 155 which can persist as secondary diabetes even after pancreatitis has been resolved. 156 Chronic hyperglycemia may cause oxidative stress, mitochondrial damage, the production of advanced glycation end products (AGEs), and the expression of the receptor for AGEs (RAGE), leading to tissue injury. 157
Researchers have shown that hyperglycemia enhances mitochondrial oxidative stress by increasing ROS production, which is a key step in the pathogenesis of AP, 158 and mediates lipid peroxidation by increasing cytosolic Ca2+.159,160 Furthermore, increased intracellular Ca2+ is also required for premature protease activation, which is an early step in the induction of AP. 158 Elevated glucose levels begin to form covalent conjugates with plasma proteins through a nonenzymatic process called AGE formation. 161 In combination with AGEs, RAGE promotes the development of pancreatitis in part by mediating uninduced nucleosome activation and proinflammatory mediator release via the absence in melanoma 2 (AIM2) inflammasome activation and proinflammatory mediator release in macrophages in an animal model of AP. 162 Under glycoxidative stress, stimulated macrophages can induce oxidative stress and NF-κB activation through activation of the PR21ras and MAPK signaling pathways. 163 Active NF-κB induces the production of TNF-α, which, in turn, leads to enhanced ROS production and more severe damage to tissues. 164 In addition, hyperglycemia was shown to compromise the integrity of the intestinal barrier through glucose transporter 2 (GLUT2)-dependent reprograming of the intestinal epithelial cell transcriptome and disruption of tight and adherence junctions, leading to intestinal flora disorders.165,166 The GM composition of patients and animals with elevated blood glucose was also significantly different from that of normal controls.167,168
Insulin resistance, which is a kind of metabolic dysfunction associated with type 2 diabetes mellitus, is another critical factor that affects both AP and the GM. 169 Observational studies have shown an increased risk of AP among people with diseases linked to insulin resistance.170 –172 Various factors and hormones, such as NF-κB, TNF-α, amylin, leptin, and IL-6, have recently been shown to be increased in patients with insulin resistance, and those factors have been demonstrated to cause AP and intestinal flora disorders.173 –177 In addition, insulin resistance often causes hyperinsulinemia, which can inhibit mucus secretion by promoting fatty acid synthase, to break the integrity of the intestinal barrier, leading to GMs.165,178 Furthermore, insulin resistance has been regarded as a novel risk factor for post-endoscopic retrograde cholangiopancreatography pancreatitis 179 and an independent prognostic factor in patients with AP. 180
Intestinal microbes can also increase insulin resistance by influencing host energy metabolism and the integrity of the intestinal barrier; thus, inflammatory mediators can be transmitted into circulation. 181
Curative substances influence both AP and GM
AP often leads to flora disorder, but some protective cytokines play key roles; for example, IL-22 and IL-23 attenuate intestinal flora disorders.152,182,183 Propolis has recently been reported to reduce the serum levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and increase IL-22 levels, thereby reducing pancreatic neutrophil infiltration and maintaining the intestinal flora in AP rats. 184 Store-operated calcium entry (SOCE) modulators, 185 such as Pyrtriazoles, and the Orai Ca2+ channel inhibitor CM4620, which can reduce endoplasmic reticulum calcium influx, target both parenchymal and immune cells to reduce inflammation in experimental AP. 186 By inhibiting immune cells, SOCE inhibitors can treat imbalances in the GM. Okra pectin could relieve the inflammatory response by inhibiting the expression of proinflammatory mediators, preventing intestinal barrier injury, and regulating the intestinal microbiota by upregulating AMPs and occludin in an AP model. 187 Probiotics have been reported to significantly attenuate pathological injury of the pancreas and reduce the incidence of complications, such as infection, in patients with AP. 188 However, the elevated levels of lactic acid produced by bacterial overgrowth in the small bowel and fermentation of carbohydrates significantly contributed to the high death rate. When considering substituting supplementation for individuals with AP, it is necessary to assess the time, type, appropriate, effective doses of probiotics, and prevent bacterial overgrowth.20,189,190 Some traditional Chinese medicine (TCM) treatments also have effects on both AP and the GM. Saponin A, a monomer of total saikosaponins extracted from Bupleuri Radix, has strong antioxidant properties and can affect the composition of GM by increasing the relative abundance of Lactobacillus and Prevotella species to decrease the development of SAP in rat models. 191 Picroside II is one of the main effective components extracted from Picrorhiza scrophulariiflora Pennell that can improve the intestinal microbiota by inactivating oxidant and inflammatory signals to improve intestinal barrier injury in an SAP rat model. 28 Some studies have reported that berberine can not only repair the gut barrier structure to decrease GM diversity but also reduce blood glucose levels and attenuate insulin resistance; moreover, berberine is regarded as a potential therapeutic agent for AP.192 –194 Meng et al. used acupuncture and moxibustion to stimulate ST36 points to treat SAP based on conventional treatments and found that adjuvant acupuncture treatment could reduce the permeability of intestinal mucosa capillaries, alleviate intestinal dysfunction, and promote recovery in patients 195 (Tables 3 and 4).
Table 3.
Curative substances influencing both AP and GM.
| Substance | Effect on AP | Effect on GM | Mechanism | References |
|---|---|---|---|---|
| IL-22 | Relieves inflammation and tissue injury | Promotes epithelial repair and barrier protection | Activation of inflammation, mediated through the JAK/STAT signaling pathway, results in the production of AMPs, finally repairing barrier damage or controlling pathogenic bacterial expansion | Sonnenberg et al.,150,151 Li et al., 152 Zheng et al., 196 Zindl et al. 197 |
| IL-23 | Relieves inflammation and tissue injury | Promotes epithelial repair and barrier protection | Promotes IL-22 production | Ngo et al., 182 Shih et al. 183 |
| Propolis | Reduces neutrophil infiltration in the pancreas | Reduces intestinal inflammation | Reduces the serum levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and increases that of IL-22 | Al-Hariri et al. 184 |
| SOCE modulators | ||||
| Pyrtriazole | Reduce inflammation in the pancreas | Treat an imbalance of the GM | Reduce calcium influx in the endoplasmic reticulum | Riva et al., 185 Waldron et al. 186 |
| CM4620 | ||||
| Okra pectin | Reduces inflammation in the pancreas | Prevents intestinal barrier inflammatory injury and regulates intestinal microbiota | Relieves inflammatory responses and intestinal barrier injury and regulates intestinal microbiota by inhibiting the expression of proinflammatory mediators or upregulating AMPs and occludin | Xiong et al. 187 |
| Choline | Choline deficiency is related to exocrine pancreatic insufficiency | Choline deficiency is associated with bacterial overgrowth in the small intestine | Choline is a tightly regulated tissue component in the form of phosphatidylcholine and sphingomyelin in all membranes and many secretions | Bernhard 198 |
AMP, antimicrobial peptide; AP, acute pancreatitis; GM, gut microbiota; IL, interleukin; SOCE, store-operated calcium entry; JAK, Janus kinase; STAT, signal transducer and activator of transcription; TNF-α, tumor necrosis factor-α.
Table 4.
Promising prebiotic agent for the treatment of SAP.
| Substance | Effect on AP | Effect on GM | Mechanism | References |
|---|---|---|---|---|
| SCFAs | Anti-inflammatory effects on protecting against severe AP-associated lung injury | Protecting intestinal barrier, decreasing bacterial translocation | SCFAs produced by gut microbiome and has a protective effect against pathogen proliferation, inflammatory response, and intestinal barrier injury | van den Berg et al., 21 Jia et al., 24 Pan et al., 29 Patel et al., 190 Wang et al.,199,200 Zhang et al. 201 |
| Six different strains of probiotic prophylaxis mixture | More multiorgan failure-related deaths | More bowel ischemia | Combination of probiotics had no beneficial effect on the occurrence of infectious complications and been damaged to bowel wall because of inflammatory injury and enteral feeding aggravating intestinal mucosal ischemia | Bongaerts and Severijnen, 189 Besselink et al., 202 Rahman et al., 203 Besselink et al. 204 |
| Glutathione biosynthesis by multispecies probiotics | Reducing pancreatic oxidative stress | Reducing oxidative stress in the ileum | This probiotics mixture increases the biosynthesis of glutathione and reduces oxidative stress both in pancreas and ileum | Lutgendorff et al.,205,206 |
| Probiotics capsules (such as a mixture of Bacillus subtilis and Enterococcus faecium) | Reducing pancreatic injury | Reducing bacterial translocation and increasing food tolerance | Gut microbiome plays important role in the pathogenesis of AP. Probiotics improve intestinal microecology and food tolerance, decrease the inflammation | Hooijmans et al., 188 Zhu et al., 207 Tian et al. 208 |
| Chitosan oligosaccharides (COS) | COS decrease inflammatory infiltration and oxidative stress | Remodeling gut dysbiosis by increasing probiotics Akkermansia and eliminating pathogenic bacteria Escherichia–Shigella and Enterococcus | Lighting oxidative stress, reducing proinflammatory cytokine, and balancing intestinal homeostasis | Mei et al. 209 |
| Bifidobacterium spp. (B. animalis) metabolite lactate | Reducing pancreatic and systemic inflammation | B. animalis metabolite lactate is the energy source for intestinal epithelial cells and inhibits bacterial translocation | B. animalis colonization and B. animalis metabolite lactate administration could relieve macrophage-associated local and systemic inflammation through its metabolite lactate-related TLR4/MyD88- and NLRP3/Caspase1-dependent pathway | Li et al. 210 |
AP, acute pancreatitis; COS, chitosan oligosaccharides; SCFA, short-chain fatty acid; TLR, toll-like receptor.
Future research prospects
SAP is a severe inflammatory disease of the pancreas and results in a high mortality rate when accompanied by multiple organ dysfunction or secondary infection. 211 Studies have shown that most pancreatic and extra-pancreatic organ infections originate in the intestine and induce inflammatory responses, which are major causes of ‘secondary attack’ and increased late death of patients with SAP. 212 Changes in the GM play an important role in intestinal homeostasis and aggravate the inflammatory response under intestinal flora dysfunction in AP.4,5 The migration and proportion of intestinal flora influence the development and severity of AP. However, the molecular mechanism and signaling pathways associated with changes in the intestinal flora in AP are still unclear. 213 The dominant intestinal microbiota species in MAP, MSAP, and SAP were Bacteroides, Escherichia–Shigella, and Enterococcus, respectively. 20 A majority of diseases are accompanied by changes in the microbiota, and whether there is a way to detect GM species could be helpful in predicting or diagnosing SAP. 214
Obesity and hyperlipidemia are regarded as chronic and systemic inflammatory states induced by adipocytes, which secrete a variety of proinflammatory cytokines and act as reservoirs of inflammatory factors. 23 When obesity and hyperlipidemia cause AP and an intestinal microbiota imbalance, 115 the intestinal microbiota also causes disordered lipid metabolism and the development of obesity by mediating adipocytokine gene expression, leading to a vicious cycle. 140 Pancreatic endocrine cells participate in the regulation of blood glucose metabolism. Hyperglycemia exacerbates mitochondrial oxidative stress, increases intracellular Ca2+ levels, and ultimately promotes the progression of AP.158,160 Patients with AP generally have insulin resistance,80,83,86 and gut microbes have been reported to increase insulin resistance. 181 Insulin resistance also causes AP and intestinal microbiota disorders.85,215,216 Glucose and lipids are sources of energy metabolism and are also factors associated with metabolic diseases. The specific GM species in AP combined with the metabolic disorders associated with glucose and lipids need further study. The effect of probiotics on the treatment of AP combined with metabolic disorders associated with glucose and lipids might be worth studying.31,161
There is currently no specific treatment for AP. The intestinal flora attenuates the severity of AP, and personalized probiotic intervention is considered a future trend. 217 The timepoint, dose, and effectiveness of probiotics used for the treatment of AP are worthwhile of further experiments and clinical studies. In addition, the safety issue of probiotic therapy cannot be ignored. 218 TCM, with multiple approaches including decoctions, powders, acupuncture, and moxibustion, has been reported to improve inflammatory or metabolic disorders.219 –222 Whether treatments combining probiotics and TCM could be beneficial for SAP patients or whether the curative factors mentioned above may be used to prevent pancreatitis are unclear, and few studies have focused on this issue.
In conclusion, the interaction between the GM and inflammatory responses provides a new understanding of AP disease progression and treatment. Further studies on the interaction of GM and inflammatory responses in AP are needed.
Acknowledgments
The authors thank Hui-Juan Chen at Chengdu Qiantu Culture Communication Co., Ltd., for preparing the figures included in this paper.
Contributor Information
Linjun Wu, Department of Integrated Traditional Chinese and Western Medicine, West China Hospital, Sichuan University, Chengdu, China; Hospital of Chinese Traditional Medicine of Leshan, Leshan, China.
Jing Hu, Department of Integrated Traditional Chinese and Western Medicine, West China; Hospital, Sichuan University, Chengdu, China; Hospital of Chinese Traditional Medicine of Leshan, Leshan, China.
Xiaolin Yi, Department of Integrated Traditional Chinese and Western Medicine, West China; Hospital, Sichuan University, Chengdu, China; Intensive Care Unit, Suining Municipal Hospital of TCM, Suining, China.
Jianqin Lv, Department of Integrated Traditional Chinese and Western Medicine, West China; Hospital, Sichuan University, Chengdu, China.
Jiaqi Yao, Department of Integrated Traditional Chinese and Western Medicine, West China; Hospital, Sichuan University, Chengdu, China.
Wenfu Tang, Department of Integrated Traditional Chinese and Western Medicine, West China; Hospital, Sichuan University, Chengdu, China.
Shu Zhang, Department of Emergency Medicine, Emergency Medical Laboratory, West China; Hospital, Sichuan University, Guo Xue Road 37, Chengdu 610041, Sichuan, China.
Meihua Wan, Department of Integrated Traditional Chinese and Western Medicine, West China; Hospital, Sichuan University, Guo Xue Road 37, Chengdu 610041, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Linjun Wu: Visualization; Writing – original draft; Writing – review & editing.
Jing Hu: Conceptualization; Project administration; Supervision; Writing – original draft.
Xiaolin Yi: Writing – original draft; Writing – review & editing.
Jianqin Lv: Conceptualization; Project administration; Supervision.
Jiaqi Yao: Project administration; Supervision; Visualization.
Wenfu Tang: Conceptualization; Project administration; Resources; Supervision.
Shu Zhang: Resources; Supervision; Visualization.
Meihua Wan: Conceptualization; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Authors thank the National Natural Science Foundation of China (Nos. 81774160 and 81974552), and the Scientific Research Foundation of the Science and Technology Department of Sichuan Province (Nos. 2020YFS0526 and 2022YFS0417) for providing financial support.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Hu F, Tao X, Zhao L, et al. LncRNA-PVT1 aggravates severe acute pancreatitis by promoting autophagy via the miR-30a-5p/Beclin-1 axis. Am J Transl Res 2020; 12: 5551–5562. [PMC free article] [PubMed] [Google Scholar]
- 2. Hollemans RA, Hallensleben NDL, Mager DJ, et al. Pancreatic exocrine insufficiency following acute pancreatitis: systematic review and study level meta-analysis. Pancreatology 2018; 18: 253–262. [DOI] [PubMed] [Google Scholar]
- 3. Quigley EM. Gut bacteria in health and disease. Gastroenterol Hepatol 2013; 9: 560–569. [PMC free article] [PubMed] [Google Scholar]
- 4. Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr 2015; 174: 151–167. [DOI] [PubMed] [Google Scholar]
- 5. Hamada S, Masamune A, Nabeshima T, et al. Differences in gut microbiota profiles between autoimmune pancreatitis and chronic pancreatitis. Tohoku J Exp Med 2018; 244: 113–117. [DOI] [PubMed] [Google Scholar]
- 6. Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology 2013; 144: 1180–1193. [DOI] [PubMed] [Google Scholar]
- 7. Andersson R, Wang XD. Gut barrier dysfunction in experimental acute pancreatitis. Ann Acad Med Singap 1999; 28: 141–146. [PubMed] [Google Scholar]
- 8. Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet 2008; 371: 143–152. [DOI] [PubMed] [Google Scholar]
- 9. Tan C, Ling Z, Huang Y, et al. Dysbiosis of intestinal Microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas 2015; 44: 868–875. [DOI] [PubMed] [Google Scholar]
- 10. Ahuja M, Schwartz DM, Tandon M, et al. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab 2017; 25: 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Q, Wang C, Tang C, et al. Bacteremia in patients with acute pancreatitis as revealed by 16S ribosomal RNA gene-based techniques. Crit Care Med 2013; 41: 1938–1950. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe T, Sadakane Y, Yagama N, et al. Nucleotide-binding oligomerization domain 1 acts in concert with the cholecystokinin receptor agonist, cerulein, to induce IL-33-dependent chronic pancreatitis. Mucosal Immunol 2016; 9: 1234–1249. [DOI] [PubMed] [Google Scholar]
- 13. Tsuji Y, Watanabe T, Kudo M, et al. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity 2012; 37: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol 2017; 10: 283–298. [DOI] [PubMed] [Google Scholar]
- 15. Sahar N, Kozarek RA, Kanji ZS, et al. The microbiology of infected pancreatic necrosis in the era of minimally invasive therapy. Eur J Clin Microbiol Infect Dis 2018; 37: 1353–1359. [DOI] [PubMed] [Google Scholar]
- 16. Mourad MM, Evans R, Kalidindi V, et al. Prophylactic antibiotics in acute pancreatitis: endless debate. Ann R Coll Surg Engl 2017; 99: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reuken PA, Albig H, Rödel J, et al. Fungal infections in patients with infected pancreatic necrosis and pseudocysts: risk factors and outcome. Pancreas 2018; 47: 92–98. [DOI] [PubMed] [Google Scholar]
- 18. Akshintala VS, Talukdar R, Singh VK, et al. The gut microbiome in pancreatic disease. Clin Gastroenterol Hepatol 2019; 17: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu Y, He C, Li X, et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol 2019; 54: 347–358. [DOI] [PubMed] [Google Scholar]
- 20. Yu S, Xiong Y, Xu J, et al. Identification of dysfunctional gut Microbiota through rectal swab in patients with different severity of acute pancreatitis. Dig Dis Sci 2020; 65: 3223–3237. [DOI] [PubMed] [Google Scholar]
- 21. van den Berg FF, van Dalen D, Hyoju SK, et al. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut 2021; 70: 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deng W-S, Zhang J, Ju H, et al. Arpin contributes to bacterial translocation and development of severe acute pancreatitis. World J Gastroenterol 2015; 21: 4293–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, He C, Li N, et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes 2020; 11: 1774–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia L, Chen H, Yang J, et al. Combinatory antibiotic treatment protects against experimental acute pancreatitis by suppressing gut bacterial translocation to pancreas and inhibiting NLRP3 inflammasome pathway. Innate Immun 2020; 26: 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan LL, Niu W, Fang X, et al. Clostridium butyricum strains suppress experimental acute pancreatitis by maintaining intestinal homeostasis. Mol Nutr Food Res 2019; 63: e1801419. [DOI] [PubMed] [Google Scholar]
- 26. Zheng J, Lou L, Fan J, et al. Commensal Escherichia coli aggravates acute necrotizing pancreatitis through targeting of intestinal epithelial cells. Appl Environ Microbiol 2019; 85: e00059-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wan YD, Zhu RX, Bian ZZ, et al. Improvement of gut microbiota by inhibition of P38 mitogen-activated protein kinase (MAPK) signaling pathway in rats with severe acute pancreatitis. Med Sci Monit 2019; 25: 4609–4616. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Piao X, Liu B, Sui X, et al. Picroside II improves severe acute pancreatitis-induced intestinal barrier injury by inactivating oxidative and inflammatory TLR4-dependent PI3K/AKT/NF-κB signaling and improving gut microbiota. Oxid Med Cell Longev 2020; 2020: 3589497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan X, Fang X, Wang F, et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br J Pharmacol 2019; 176: 4446–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wan YD, Zhu RX, Pan XT, et al. Bile acid supplementation improves murine pancreatitis in association with the gut microbiota. Front Physiol 2020; 11: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jandhyala SM, Madhulika A, Deepika G, et al. Altered intestinal microbiota in patients with chronic pancreatitis: implications in diabetes and metabolic abnormalities. Sci Rep 2017; 7: 43640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alkanani AK, Hara N, Gottlieb PA, et al. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes 2015; 64: 3510–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011; 334: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holmes E, Li JV, Marchesi JR, et al. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab 2012; 16: 559–564. [DOI] [PubMed] [Google Scholar]
- 35. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009; 294: 1–8. [DOI] [PubMed] [Google Scholar]
- 37. Rossi O, van Berkel LA, Chain F, et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep 2016; 6: 18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martín R, Miquel S, Chain F, et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol 2015; 15: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sundin J, Rangel I, Repsilber D, et al. Cytokine response after stimulation with key commensal bacteria differ in post-infectious irritable bowel syndrome (PI-IBS) patients compared to healthy controls. PLoS One 2015; 10: e0134836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Goffau MC, Luopajärvi K, Knip M, et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 2013; 62: 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smedley JG, 3rd, Fisher DJ, Sayeed S, et al. The enteric toxins of Clostridium perfringens. Rev Physiol Biochem Pharmacol 2004; 152: 183–204. [DOI] [PubMed] [Google Scholar]
- 42. Davis-Richardson AG, Ardissone AN, Dias R, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol 2014; 5: 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mejía-León ME, Petrosino JF, Ajami NJ, et al. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci Rep 2014; 4: 3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brown CT, Davis-Richardson AG, Giongo A, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One 2011; 6: e25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tlaskalová-Hogenová H, Šteˇpánková R, Kozáková H, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol 2011; 8: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang XM, Zhang ZY, Zhang CH, et al. Intestinal microbial community differs between acute pancreatitis patients and healthy volunteers. Biomed Environ Sci 2018; 31: 81–86. [DOI] [PubMed] [Google Scholar]
- 47. Fujita H, Eishi Y, Ishige I, et al. Quantitative analysis of bacterial DNA from Mycobacteria spp., Bacteroides vulgatus, and Escherichia coli in tissue samples from patients with inflammatory bowel diseases. J Gastroenterol 2002; 37: 509–516. [DOI] [PubMed] [Google Scholar]
- 48. Rath HC, Wilson KH, Sartor RB. Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus or Escherichia coli. Infect Immun 1999; 67: 2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Berkes J, Viswanathan VK, Savkovic SD, et al. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 2003; 52: 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mancabelli L, Milani C, Lugli GA, et al. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol Ecol 2017; 93: fix153. [DOI] [PubMed] [Google Scholar]
- 51. Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 2019; 178: 795–806.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim YI, Park JE, Brand DD, et al. Protein kinase D1 is essential for the proinflammatory response induced by hypersensitivity pneumonitis-causing thermophilic actinomycetes Saccharopolyspora rectivirgula. J Immunol 2010; 184: 3145–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ren Z, Jiang J, Xie H, et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017; 8: 95176–95191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pushalkar S, Hundeyin M, Daley D, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov 2018; 8: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takahashi K, Nishida A, Fujimoto T, et al. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion 2016; 93: 59–65. [DOI] [PubMed] [Google Scholar]
- 56. Hague A, Butt AJ, Paraskeva C. The role of butyrate in human colonic epithelial cells: an energy source or inducer of differentiation and apoptosis? Proc Nutr Soc 1996; 55: 937–943. [DOI] [PubMed] [Google Scholar]
- 57. van Bergeijk DA, Terlouw BR, Medema MH, et al. Ecology and genomics of actinobacteria: new concepts for natural product discovery. Nat Rev Microbiol 2020; 18: 546–558. [DOI] [PubMed] [Google Scholar]
- 58. Barka EA, Vatsa P, Sanchez L. Taxonomy, physiology, and natural products of actinobacteria. Microbiol Mol Biol Rev 2016; 80: 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Duffy LC. Interactions mediating bacterial translocation in the immature intestine. J Nutr 2000; 130: 432s–436s. [DOI] [PubMed] [Google Scholar]
- 60. Tian Y, Zuo L, Guo Q, et al. Potential role of fecal microbiota in patients with constipation. Therap Adv Gastroenterol 2020; 13: 1756284820968423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zheng J, Hoffman KL, Chen JS, et al. Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br J Nutr 2020; 124: 931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murphy EC, Mohanty T, Frick IM. FAF and SufA: proteins of Finegoldia magna that modulate the antibacterial activity of histones. J Innate Immun 2014; 6: 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krepel CJ, Gohr CM, Walker AP, et al. Enzymatically active Peptostreptococcus magnus: association with site of infection. J Clin Microbiol 1992; 30: 2330–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hugenholtz P, Hooper SD, Kyrpides NC. Focus: Synergistetes. Environ Microbiol 2009; 11: 1327–1329. [DOI] [PubMed] [Google Scholar]
- 65. Amado PPP, Kawamoto D, Albuquerque-Souza E, et al. Oral and fecal microbiome in molar-incisor pattern periodontitis. Front Cell Infect Microbiol 2020; 10: 583761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oliveira RR, Fermiano D, Feres M, et al. Levels of candidate periodontal pathogens in subgingival biofilm. J Dent Res 2016; 95: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Deng ZL, Szafrański SP, Jarek M, et al. Dysbiosis in chronic periodontitis: key microbial players and interactions with the human host. Sci Rep 2017; 7: 3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kumar PS. From focal sepsis to periodontal medicine: a century of exploring the role of the oral microbiome in systemic disease. J Physiol 2017; 595: 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shiba T, Aiba Y, Ishikawa H, et al. The suppressive effect of bifidobacteria on Bacteroides vulgatus, a putative pathogenic microbe in inflammatory bowel disease. Microbiol Immunol 2003; 47: 371–378. [DOI] [PubMed] [Google Scholar]
- 70. Setoyama H, Imaoka A, Ishikawa H, et al. Prevention of gut inflammation by Bifidobacterium in dextran sulfate-treated gnotobiotic mice associated with Bacteroides strains isolated from ulcerative colitis patients. Microbes Infect 2003; 5: 115–122. [DOI] [PubMed] [Google Scholar]
- 71. Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 2011; 10: 66–78. [DOI] [PubMed] [Google Scholar]
- 72. Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol 2005; 115: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 73. Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006; 118: 511–521. [DOI] [PubMed] [Google Scholar]
- 74. Marques TM, Wall R, Ross RP, et al. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol 2010; 21: 149–156. [DOI] [PubMed] [Google Scholar]
- 75. Liu J, Huang L, Luo M, et al. Bacterial translocation in acute pancreatitis. Crit Rev Microbiol 2019; 45: 539–547. [DOI] [PubMed] [Google Scholar]
- 76. Zhou H, Gao J, Wu W, et al. Octreotide ameliorates intestinal dysmotility by interstitial cells of Cajal protection in a rat acute necrotizing pancreatitis model. Pancreas 2011; 40: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 77. Zhou H, Gao J, Zou D, et al. Effect of octreotide on enteric motor neurons in experimental acute necrotizing pancreatitis. PLoS One 2012; 7: e52163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leveau P, Wang X, Soltesz V, et al. Alterations in intestinal motility and microflora in experimental acute pancreatitis. Int J Pancreatol 1996; 20: 119–125. [DOI] [PubMed] [Google Scholar]
- 79. Van Felius ID, Akkermans LM, Bosscha K, et al. Interdigestive small bowel motility and duodenal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol Motil 2003; 15: 267–276. [DOI] [PubMed] [Google Scholar]
- 80. Tian R, Tan JT, Wang RL, et al. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci 2013; 17: 349–355. [PubMed] [Google Scholar]
- 81. Andersson R, Wang X, Ihse I. The influence of abdominal sepsis on acute pancreatitis in rats: a study on mortality, permeability, arterial pressure, and intestinal blood flow. Pancreas 1995; 11: 365–373. [DOI] [PubMed] [Google Scholar]
- 82. Wen W, Zheng H, Jiang Y, et al. Effect of intestinal epithelial autophagy on bacterial translocation in severe acute pancreatitis. Clin Res Hepatol Gastroenterol 2017; 41: 703–710. [DOI] [PubMed] [Google Scholar]
- 83. Wang F, Li Q, Wang C, et al. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PLoS One 2012; 7: e42027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med 1999; 27: 749–755. [DOI] [PubMed] [Google Scholar]
- 85. Braganza JM. Mast cell control: likely modus operandi of panhaematin in experimental pancreatitis. Gut 2012; 61: 632. [DOI] [PubMed] [Google Scholar]
- 86. Zhong Y, Cai D, Cai W, et al. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin Nutr 2009; 28: 575–580. [DOI] [PubMed] [Google Scholar]
- 87. Reinehr R, Becker S, Keitel V, et al. Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology 2005; 129: 2009–2031. [DOI] [PubMed] [Google Scholar]
- 88. Manohar M, Verma AK, Venkateshaiah SU, et al. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther 2017; 8: 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aoun E, Chen J, Reighard D, et al. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology 2009; 9: 777–785. [DOI] [PubMed] [Google Scholar]
- 90. Hu J, Kang H, Chen H, et al. Targeting neutrophil extracellular traps in severe acute pancreatitis treatment. Therap Adv Gastroenterol 2020; 13: 1756284820974913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Horiuchi T, Mitoma H, Harashima S, et al. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology 2010; 49: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reimund JM, Wittersheim C, Dumont S, et al. Increased production of tumour necrosis factor-alpha interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut 1996; 39: 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zeissig S, Bürgel N, Günzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007; 56: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Garcia-Carbonell R, Wong J, Kim JY, et al. Elevated A20 promotes TNF-induced and RIPK1-dependent intestinal epithelial cell death. Proc Natl Acad Sci U S A 2018; 115: E9192–e9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Clark EC, Patel SD, Chadwick PR, et al. Glutamine deprivation facilitates tumour necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocyte fuel substrate. Gut 2003; 52: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16: 448–457. [DOI] [PubMed] [Google Scholar]
- 97. Tanaka N, Murata A, Uda KI, et al. Interleukin-1 receptor antagonist modifies the changes in vital organs induced by acute necrotizing pancreatitis in a rat experimental model. Crit Care Med 1995; 23: 901–908. [DOI] [PubMed] [Google Scholar]
- 98. Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev 2015; 265: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sahoo M, Ceballos-Olvera I, del Barrio L, et al. Role of the inflammasome, IL-1β, and IL-18 in bacterial infections. ScientificWorldJournal 2011; 11: 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sathyanarayan G, Garg PK, Prasad H, et al. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis. J Gastroenterol Hepatol 2007; 22: 550–554. [DOI] [PubMed] [Google Scholar]
- 101. Schoultz I, Keita ÅV. Cellular and molecular therapeutic targets in inflammatory bowel disease-focusing on intestinal barrier function. Cells 2019; 8: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Merza M, Hartman H, Rahman M, et al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology 2015; 149: 1920–1931.e8. [DOI] [PubMed] [Google Scholar]
- 103. Gao X, Hao S, Yan H, et al. Neutrophil extracellular traps contribute to the intestine damage in endotoxemic rats. J Surg Res 2015; 195: 211–218. [DOI] [PubMed] [Google Scholar]
- 104. Liang Y, Wang X, He D, et al. Ameliorating gut microenvironment through staphylococcal nuclease-mediated intestinal NETs degradation for prevention of type 1 diabetes in NOD mice. Life Sci 2019; 221: 301–310. [DOI] [PubMed] [Google Scholar]
- 105. Marin-Esteban V, Turbica I, Dufour G, et al. Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells. Infect Immun 2012; 80: 1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Frost F, Kacprowski T, Rühlemann M, et al. Impaired exocrine pancreatic function associates with changes in intestinal microbiota composition and diversity. Gastroenterology 2019; 156: 1010–1015. [DOI] [PubMed] [Google Scholar]
- 107. Paragomi P, Phillips AE, Machicado JD, et al. Post-acute pancreatitis pancreatic exocrine insufficiency: rationale and methodology of a prospective, observational, multicenter cohort study. Pancreas 2021; 50: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Blake AB, Guard BC, Honneffer JB, et al. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS One 2019; 14: e0224454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sirtori LR, Motta Ade S, Brandelli A. Mode of action of antimicrobial peptide P45 on Listeria monocytogenes. J Basic Microbiol 2008; 48: 393–400. [DOI] [PubMed] [Google Scholar]
- 110. Hancock RE. Peptide antibiotics. Lancet 1997; 349: 418–422. [DOI] [PubMed] [Google Scholar]
- 111. Fan L, Sun J, Zhou M, et al. DRAMP: a comprehensive data repository of antimicrobial peptides. Sci Rep 2016; 6: 24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wong W. Shaping the gut microbiome from the pancreas. Sci Signal 2017; 10: eaan3016. [DOI] [PubMed] [Google Scholar]
- 113. Deng Y-Y, Shamoon M, He Y, et al. Cathelicidin-related antimicrobial peptide modulates the severity of acute pancreatitis in mice. Mol Med Rep 2016; 13: 3881–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhao Y, Chen F, Wu W, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol 2018; 11: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Huang C, Chen J, Wang J, et al. Dysbiosis of intestinal microbiota and decreased antimicrobial peptide level in Paneth cells during hypertriglyceridemia-related acute necrotizing pancreatitis in rats. Front Microbiol 2017; 8: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011; 9: 356–368. [DOI] [PubMed] [Google Scholar]
- 117. Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 2013; 75: 289–311. [DOI] [PubMed] [Google Scholar]
- 118. Eriguchi Y, Nakamura K, Hashimoto D, et al. Decreased secretion of Paneth cell α-defensins in graft-versus-host disease. Transpl Infect Dis 2015; 17: 702–706. [DOI] [PubMed] [Google Scholar]
- 119. Mukherjee S, Zheng H, Derebe MG, et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014; 505: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cash HL, Whitham CV, Behrendt CL, et al. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006; 313: 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Vaishnava S, Yamamoto M, Severson KM, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011; 334: 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Menendez A, Willing BP, Montero M, et al. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell α-defensins. J Innate Immun 2013; 5: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Brandl K, Plitas G, Schnabl B, et al. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med 2007; 204: 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Vaishnava S, Behrendt CL, Ismail AS, et al. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA 2008; 105: 20858–20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010; 11: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Satoh Y, Habara Y, Ono K, et al. Carbamylcholine- and catecholamine-induced intracellular calcium dynamics of epithelial cells in mouse ileal crypts. Gastroenterology 1995; 108: 1345–1356. [DOI] [PubMed] [Google Scholar]
- 127. Ayabe T, Satchell DP, Wilson CL, et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 2000; 1: 113–118. [DOI] [PubMed] [Google Scholar]
- 128. Adolph TE, Mayr L, Grabherr F, et al. Pancreas-microbiota cross talk in health and disease. Annu Rev Nutr 2019; 39: 249–266. [DOI] [PubMed] [Google Scholar]
- 129. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548–2556. [DOI] [PubMed] [Google Scholar]
- 130. Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep 2005; 5: 70–75. [DOI] [PubMed] [Google Scholar]
- 131. Bastard J-P, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 2006; 17: 4–12. [PubMed] [Google Scholar]
- 132. Lloret Linares C, Pelletier AL, Czernichow S, et al. Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas 2008; 37: 13–12. [DOI] [PubMed] [Google Scholar]
- 133. Deng LH, Xue P, Xia Q, et al. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol 2008; 14: 4558–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Guo YY, Li HX, Zhang Y, et al. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discov Med 2019; 27: 101–109. [PubMed] [Google Scholar]
- 135. Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Our Nat 2006; 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 136. Santos-Marcos JA, Perez-Jimenez F, Camargo A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J. Nutr. Biochem 2019; 70: 1–27. [DOI] [PubMed] [Google Scholar]
- 137. Mössner J, Bödeker H, Kimura W, et al. Isolated rat pancreatic acini as a model to study the potential role of lipase in the pathogenesis of acinar cell destruction. Int. J. Pancreatol 1992; 12: 285–296. [DOI] [PubMed] [Google Scholar]
- 138. Dettelbach MA, Deftos LJ, Stewart AF. Intraperitoneal free fatty acids induce severe hypocalcemia in rats: a model for the hypocalcemia of pancreatitis. J Bone Miner Res 1990; 5: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 139. Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med 2011; 3: 107ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U.S.A 2004; 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yuan S, Giovannucci EL, Larsson SC. Gallstone disease, diabetes, calcium, triglycerides, smoking and alcohol consumption and pancreatitis risk: Mendelian randomization study. NPJ Genomic Med 2021; 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shalbueva N, Mareninova OA, Gerloff A, et al. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology 2013; 144: 437–446.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Apte MV, Pirola RC, Wilson JS. Mechanisms of alcoholic pancreatitis. J. Gastroenterol. Hepatol 2010; 25: 1816–1826. [DOI] [PubMed] [Google Scholar]
- 144. Heier C, Xie H, Zimmermann R. Nonoxidative ethanol metabolism in humans-from biomarkers to bioactive lipids. IUBMB Life 2016; 68: 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rendon JL, Li X, Akhtar S, et al. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock 2013; 39: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Engen PA, Green SJ, Voigt RM, et al. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res 2015; 37: 223–236. [PMC free article] [PubMed] [Google Scholar]
- 147. Sublette MG, Cross TL, Korcarz CE, et al. Effects of smoking and smoking cessation on the intestinal microbiota. J. Clin. Med 2020; 9: 2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kim DB, Paik CN, Lee JM, et al. Association between increased breath hydrogen methane concentration and prevalence of glucose intolerance in acute pancreatitis. J. Breath Res 2020; 14: 026006. [DOI] [PubMed] [Google Scholar]
- 149. Ansari RA, Husain K, Rizvi SA, et al. Role of transcription factors in steatohepatitis and hypertension after ethanol: the epicenter of metabolism. Biomolecules 2016; 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol 2011; 12: 383–390. [DOI] [PubMed] [Google Scholar]
- 151. Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv. Immunol 2010; 107: 1–29. [DOI] [PubMed] [Google Scholar]
- 152. Li LJ, Gong C, Zhao M-H, et al. Role of interleukin-22 in inflammatory bowel disease. World J Gastroenterol 2014; 20: 18177–18188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. González-Reimers E, Santolaria-Fernández F, Martín-González MC, et al. Alcoholism: a systemic proinflammatory condition. World J. Gastroenterol 2014; 20: 14660–14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hendrikx T, Duan Y, Wang Y, et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 2019; 68: 1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Czakó L, Hegyi P, Rakonczay Z, Jr, et al. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology 2009; 9: 351–359. [DOI] [PubMed] [Google Scholar]
- 156. Das SL, Singh PP, Phillips AR, et al. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut 2014; 63: 818–831. [DOI] [PubMed] [Google Scholar]
- 157. Khan MS, Ikram M, Park TJ, et al. Pathology, risk factors, and oxidative damage related to type 2 diabetes-mediated Alzheimer's disease and the rescuing effects of the potent antioxidant anthocyanin. Oxid. Med. Cell Longev 2021; 2021: 4051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Halangk W, Lerch MM. Early events in acute pancreatitis. Clin. Lab. Med 2005; 25: 1–15. [DOI] [PubMed] [Google Scholar]
- 159. Kamboj SS, Sandhir R. Protective effect of N-acetylcysteine supplementation on mitochondrial oxidative stress and mitochondrial enzymes in cerebral cortex of streptozotocin-treated diabetic rats. Mitochondrion 2011; 11: 214–222. [DOI] [PubMed] [Google Scholar]
- 160. Yu T, Jhun BS, Yoon Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid. Redox Signal 2011; 14: 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ciciliot S, Albiero M, Campanaro S, et al. Interplay between gut microbiota and p66Shc affects obesity-associated insulin resistance. FASEB J 2018; 32: 4004–4015. [DOI] [PubMed] [Google Scholar]
- 162. Kang R, Chen R, Xie M, et al. The receptor for advanced glycation end products activates the AIM2 inflammasome in acute pancreatitis. J. Immunol 2016; 196: 4331–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem 1994; 269: 9889–9897. [PubMed] [Google Scholar]
- 164. Halliwell B. The wanderings of a free radical. Free Radic. Biol. Med 2009; 46: 531–542. [DOI] [PubMed] [Google Scholar]
- 165. Zhang C, Wu W, Xin X, et al. Extract of ice plant (Mesembryanthemum crystallinum) ameliorates hyperglycemia and modulates the gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. Food Funct 2019; 10: 3252–3261. [DOI] [PubMed] [Google Scholar]
- 166. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012; 490: 55–60. [DOI] [PubMed] [Google Scholar]
- 167. Tilg H, Zmora N, Adolph TE, et al. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol 2020; 20: 40–54. [DOI] [PubMed] [Google Scholar]
- 168. Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018; 359: 1376–1383. [DOI] [PubMed] [Google Scholar]
- 169. Arora A, Behl T, Sehgal A, et al. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci 2021; 273: 119311. [DOI] [PubMed] [Google Scholar]
- 170. Noel RA, Braun DK, Patterson RE, et al. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 2009; 32: 834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lai SW, Muo CH, Liao KF, et al. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol 2011; 106: 1697–1704. [DOI] [PubMed] [Google Scholar]
- 172. Girman CJ, Kou TD, Cai B, et al. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab 2010; 12: 766–771. [DOI] [PubMed] [Google Scholar]
- 173. Rakonczay Z, Jr, Hegyi P, Takács T, et al. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut 2008; 57: 259–267. [DOI] [PubMed] [Google Scholar]
- 174. Li H, Zhang Z, Feng D, et al. PGRN exerts inflammatory effects via SIRT1-NF-κB in adipose insulin resistance. J Mol Endocrinol 2020; 64: 181–193. [DOI] [PubMed] [Google Scholar]
- 175. Phillips AR, Abu-Zidan FM, Bonham MJ, et al. Amylin and severe acute pancreatitis. Pancreas 2000; 20: 105–106. [DOI] [PubMed] [Google Scholar]
- 176. Tukiainen E, Kylanpaa ML, Ebeling P, et al. Leptin and adiponectin levels in acute pancreatitis. Pancreas 2006; 32: 211–214. [DOI] [PubMed] [Google Scholar]
- 177. Gillies N, Pendharkar SA, Asrani VM, et al. Interleukin-6 is associated with chronic hyperglycemia and insulin resistance in patients after acute pancreatitis. Pancreatology 2016; 16: 748–755. [DOI] [PubMed] [Google Scholar]
- 178. Wei X, Yang Z, Rey FE, et al. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe 2012; 11: 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Koksal AR, Boga S, Alkim H, et al. Insulin resistance as a novel risk factor for post-ERCP pancreatitis: a pilot study. Dig Dis Sci 2016; 61: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 180. Cho SK, Huh JH, Yoo JS, et al. HOMA-estimated insulin resistance as an independent prognostic factor in patients with acute pancreatitis. Sci Rep 2019; 9: 14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Upadhyaya S, Banerjee G. Type 2 diabetes and gut microbiome: at the intersection of known and unknown. Gut Microbes 2015; 6: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ngo VL, Abo H, Maxim E, et al. A cytokine network involving IL-36γ, IL-23, and IL-22 promotes antimicrobial defense and recovery from intestinal barrier damage. Proc Natl Acad Sci U S A 2018; 115: E5076–e5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Shih VF, Cox J, Kljavin NM, et al. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22-mediated containment of commensal microbiota. Proc Natl Acad Sci U S A 2014; 111: 13942–13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Al-Hariri MT, Eldin TG, Hashim T, et al. Propolis modulates inflammatory mediators and improves histopathology in male rats with L-arginine-induced acute pancreatitis. Sultan Qaboos Univ Med J 2019; 19: e103–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Riva B, Griglio A, Serafini M, et al. Pyrtriazoles, a novel class of store-operated calcium entry modulators: discovery, biological profiling, and in vivo proof-of-concept efficacy in acute pancreatitis. J Med Chem 2018; 61: 9756–9783. [DOI] [PubMed] [Google Scholar]
- 186. Waldron RT, Chen Y, Pham H, et al. The Orai Ca2+ channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J Physiol 2019; 597: 3085–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Xiong B, Zhang W, Wu Z, et al. Okra pectin relieves inflammatory response and protects damaged intestinal barrier in caerulein-induced acute pancreatic model. J Sci Food Agric 2021; 101: 863–870. [DOI] [PubMed] [Google Scholar]
- 188. Hooijmans CR, de Vries RB, Rovers MM, et al. The effects of probiotic supplementation on experimental acute pancreatitis: a systematic review and meta-analysis. PLoS One 2012; 7: e48811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bongaerts GPA, Severijnen RSVM. A reassessment of the Propatria study and its implications for probiotic therapy. Nat Biotechnol 2016; 34: 55–63. [DOI] [PubMed] [Google Scholar]
- 190. Patel BK, Patel KH, Bhatia M, et al. Gut microbiome in acute pancreatitis: a review based on current literature. World J Gastroenterol 2021; 27: 5019–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Li J, Han J, Lv J, et al. Saikosaponin A-Induced gut Microbiota changes attenuate severe acute pancreatitis through the activation of Keap1/Nrf2-ARE antioxidant signaling. Oxid Med Cell Longev 2020; 2020: 9217219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zhang X, Zhao Y, Zhang M, et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One 2012; 7: e42529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Fan CF, Guo YS, Wang JH, et al. [Effect of berberine on insulin resistance in diabetic rats]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2020; 36: 561–564. [DOI] [PubMed] [Google Scholar]
- 194. Tarasiuk A, Pawlik L, Fichna J. [Berberine as a potential therapeutic agent in the treatment of acute pancreatitis]. Postepy Biochem 2019; 65: 224–230. [DOI] [PubMed] [Google Scholar]
- 195. Meng J-B, Jiao Y-N, Xu XJ, et al. Electro-acupuncture attenuates inflammatory responses and intraabdominal pressure in septic patients: a randomized controlled trial. Medicine 2018; 97: e0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zheng Y, Valdez PA, Danilenko DM, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med 2008; 14: 282–289. [DOI] [PubMed] [Google Scholar]
- 197. Zindl CL, Lai JF, Lee YK, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A 2013; 110: 12768–12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Bernhard W. Choline in cystic fibrosis: relations to pancreas insufficiency, enterohepatic cycle, PEMT and intestinal microbiota. Eur J Nutr 2021; 60: 1737–1759. [DOI] [PubMed] [Google Scholar]
- 199. Wang Z, Liu J, Li F, et al. The gut-lung axis in severe acute Pancreatitis-associated lung injury: the protection by the gut microbiota through short-chain fatty acids. Pharmacol Res 2022; 182: 106321. [DOI] [PubMed] [Google Scholar]
- 200. Wang C, Xiao Y, Yu L, et al. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J Adv Res 2022; 36: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zhang T, Gao G, Sakandar HA, et al. Gut dysbiosis in pancreatic diseases: A causative factor and a novel therapeutic target. Front Nutr 2022; 9: 814269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Besselink MG, van Santvoort HC, Renooij W, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg 2009; 250: 712–719. [DOI] [PubMed] [Google Scholar]
- 203. Rahman SH, Ammori BJ, Holmfield J, et al. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg 2003; 7: 26–36. [DOI] [PubMed] [Google Scholar]
- 204. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371: 651–659. [DOI] [PubMed] [Google Scholar]
- 205. Lutgendorff F, Trulsson LM, van Minnen LP, et al. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2008; 295: G1111–G1121. [DOI] [PubMed] [Google Scholar]
- 206. Lutgendorff F, Nijmeijer RM, Sandström PA, et al. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLoS One 2009; 4: e4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhu Y, Mei Q, Fu Y, et al. Alteration of gut microbiota in acute pancreatitis and associated therapeutic strategies. Biomed Pharmacother 2021; 141: 111850. [DOI] [PubMed] [Google Scholar]
- 208. Tian X, Pi YP, Liu XL, et al. Supplemented use of pre-, pro-, and synbiotics in severe acute pancreatitis: an updated systematic review and meta-analysis of 13 randomized controlled trials. Front Pharmacol 2018; 9: 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mei QX, Hu JH, Huang ZH, et al. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta Pharmacol Sin 2021; 42: 942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Li H, Xie J, Guo X, et al. Bifidobacterium spp. and their metabolite lactate protect against acute pancreatitis via inhibition of pancreatic and systemic inflammatory responses. Gut Microbes 2022; 14: 2127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Banks PA. Acute pancreatitis: landmark studies, management decisions, and the future. Pancreas 2016; 45: 633–640. [DOI] [PubMed] [Google Scholar]
- 212. Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas 2003; 26: 122–129. [DOI] [PubMed] [Google Scholar]
- 213. Kotzampassi K, Giamarellos-Bourboulis EJ. Probiotics for infectious diseases: more drugs, less dietary supplementation. Int. J. Antimicrob. Agents 2012; 40: 288–296. [DOI] [PubMed] [Google Scholar]
- 214. Surana NK, Kasper DL. Moving beyond microbiome-wide associations to causal microbe identification. Our Nat 2017; 552: 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Sun JK, Mu XW, Li WQ, et al. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol 2013; 19: 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Fishman JE, Levy G, Alli V, et al. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock 2014; 42: 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Zmora N, Zilberman-Schapira G, Suez J, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018; 174: 1388–1405.e21. [DOI] [PubMed] [Google Scholar]
- 218. Cohen PA. Probiotic safety-no guarantees. JAMA Intern Med 2018; 178: 1577–1578. [DOI] [PubMed] [Google Scholar]
- 219. Sun Z, Li J, Dai Y, et al. Indigo naturalis alleviates dextran sulfate sodium-induced colitis in rats via altering gut Microbiota. Front Microbiol 2020; 11: 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Hu AL, Song S, Li Y, et al. Mercury sulfide-containing Hua-Feng-Dan and 70W (Rannasangpei) protect against LPS plus MPTP-induced neurotoxicity and disturbance of gut microbiota in mice. J Ethnopharmacol 2020; 254: 112674. [DOI] [PubMed] [Google Scholar]
- 221. Hong Y, Li B, Zheng N, et al. Integrated metagenomic and metabolomic analyses of the effect of Astragalus polysaccharides on alleviating high-fat diet-induced metabolic disorders. Front Pharmacol 2020; 11: 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Si YC, Miao WN, He JY, et al. Regulating gut flora dysbiosis in obese mice by electroacupuncture. Am J Chin Med 2018; 46: 1481–1497. [DOI] [PubMed] [Google Scholar]