Abstract
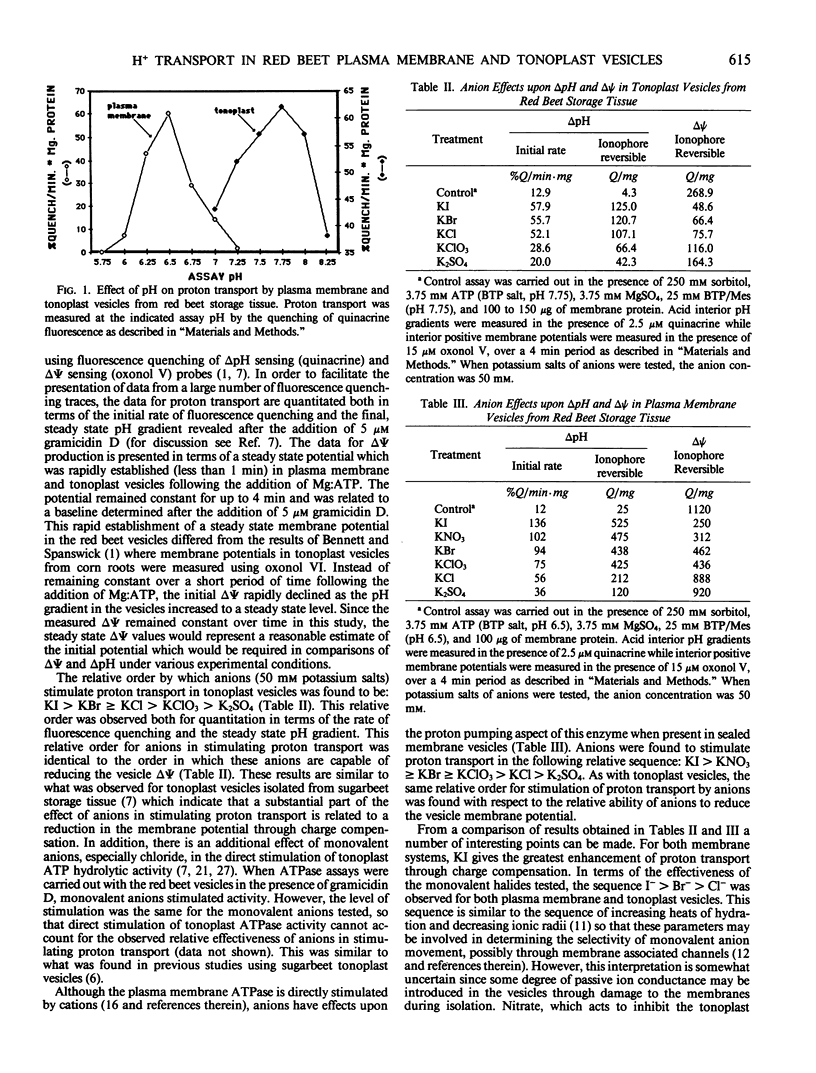
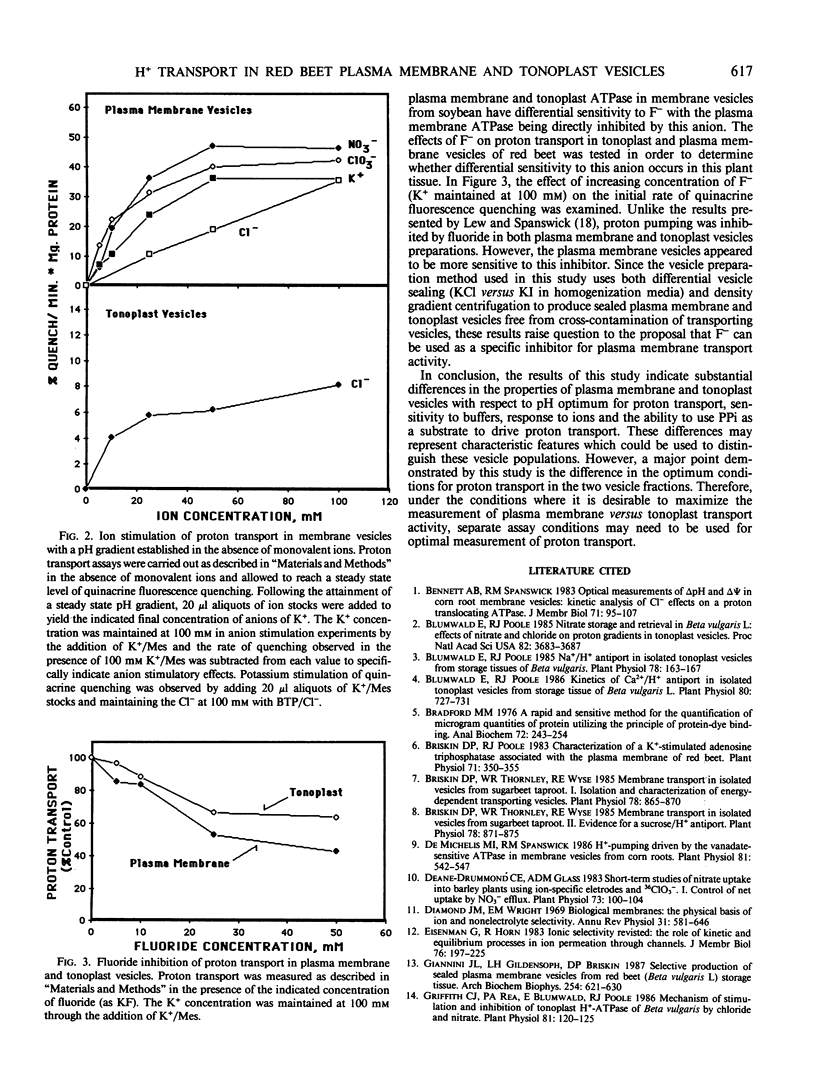
The proton transport properties of plasma membrane and tonoplast vesicles isolated from red beet (Beta vulgaris L.) storage tissue were examined and compared. Membrane vesicles isolated with 250 millimolar KCl in the homogenization media and recovered at low density following sucrose density gradient centrifugation displayed characteristics of proton transport (nitrate inhibition, no inhibition by orthovanadate, pH optimum of 7.75, pyrophosphate-driven proton transport) which were consistent with a tonoplast origin. When the KCl in the homogenization medium was replaced by 250 millimolar KI, sealed membrane vesicles were recovered at higher densities in sucrose gradients and displayed properties (orthovanadate sensitivity, no inhibition by nitrate, pH optimum of 6.5) consistent with a plasma membrane origin. A comparison of anion effects (potassium salts) upon ΔpH and ΔΨ revealed a direct correspondence between the relative ability of anions to stimulate proton transport and reduce ΔΨ. For tonoplast vesicles, the relative order for this effect was KI > KBr ≥ KCl > KClO3 > K2SO4 while for plasma membrane vesicles, a different order KI > KNO3 ≥ KBr ≥ KClO3 > KCl > K2SO4 was observed. Proton transport in plasma membrane and tonoplast vesicles was inhibited by fluoride; however, plasma membrane vesicles appeared to be more sensitive to this anion. In order to correlate anion effects in the two vesicle fractions with anion transport, the kinetics of anion stimulation of steady-state pH gradients established in the absence of monovalent ions was examined. Anions were added as potassium salts and the total potassium concentration (100 millimolar) was maintained through the addition of K+/Mes. For plasma membrane vesicles, chlorate and nitrate displayed saturation kinetics while chloride displayed stimulation of proton transport which followed a linear profile. For tonoplast vesicles, the kinetics of chloride stimulation of proton transport displayed a saturable component. The results of this study indicate differences in proton transport properties of these two vesicle types and provide information on conditions where proton transport in the two fractions can be optimized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985 May;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Nitrate storage and retrieval in Beta vulgaris: Effects of nitrate and chloride on proton gradients in tonoplast vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3683–3687. doi: 10.1073/pnas.82.11.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of a k-stimulated adenosine triphosphatase associated with the plasma membrane of red beet. Plant Physiol. 1983 Feb;71(2):350–355. doi: 10.1104/pp.71.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Thornley W. R., Wyse R. E. Membrane Transport in Isolated Vesicles from Sugarbeet Taproot : II. Evidence for a Sucrose/H-Antiport. Plant Physiol. 1985 Aug;78(4):871–875. doi: 10.1104/pp.78.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Thornley W. R., Wyse R. E. Membrane transport in isolated vesicles from sugarbeet taproot : I. Isolation and characterization of energy-dependent, h-transporting vesicles. Plant Physiol. 1985 Aug;78(4):865–870. doi: 10.1104/pp.78.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michelis M. I., Spanswick R. M. H-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiol. 1986 Jun;81(2):542–547. doi: 10.1104/pp.81.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane-Drummond C. E., Glass A. D. Short Term Studies of Nitrate Uptake into Barley Plants Using Ion-Specific Electrodes and ClO(3): I. Control of Net Uptake by NO(3) Efflux. Plant Physiol. 1983 Sep;73(1):100–104. doi: 10.1104/pp.73.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Briskin D. P. Selective production of sealed plasma membrane vesicles from red beet (Beta vulgaris L.) storage tissue. Arch Biochem Biophys. 1987 May 1;254(2):621–630. doi: 10.1016/0003-9861(87)90145-7. [DOI] [PubMed] [Google Scholar]
- Gunn R. B. Co- and counter-transport mechanisms in cell membranes. Annu Rev Physiol. 1980;42:249–259. doi: 10.1146/annurev.ph.42.030180.001341. [DOI] [PubMed] [Google Scholar]
- Lew R. R., Spanswick R. M. Characterization of Anion Effects on the Nitrate-Sensitive ATP-Dependent Proton Pumping Activity of Soybean (Glycine max L.) Seedling Root Microsomes. Plant Physiol. 1985 Feb;77(2):352–357. doi: 10.1104/pp.77.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Rea P. A., Poole R. J. Identification of 3-O-(4-benzoyl)benzoyladenosine 5'-triphosphate- and N,N'-dicyclohexylcarbodiimide-binding subunits of a higher plant H+-translocating tonoplast ATPase. J Biol Chem. 1985 Oct 5;260(22):12273–12279. [PubMed] [Google Scholar]
- Marrè E., Ballarin-Denti A. The proton pumps of the plasmalemma and the tonoplast of higher plants. J Bioenerg Biomembr. 1985 Feb;17(1):1–21. doi: 10.1007/BF00744985. [DOI] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall S. K., Sze H. Properties of the partially purified tonoplast H+-pumping ATPase from oat roots. J Biol Chem. 1986 Jan 25;261(3):1364–1371. [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C., De Michelis M. I. Electrogenic transport of protons driven by the plasma membrane ATPase in membrane vesicles from radish : biochemical characterization. Plant Physiol. 1985 Jan;77(1):200–205. doi: 10.1104/pp.77.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Chromatographic resolution of h-translocating pyrophosphatase from h-translocating ATPase of higher plant tonoplast. Plant Physiol. 1986 May;81(1):126–129. doi: 10.1104/pp.81.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Calcium transport into the vacuole of oat roots. Characterization of H+/Ca2+ exchange activity. J Biol Chem. 1986 Sep 15;261(26):12172–12178. [PubMed] [Google Scholar]


