Abstract
A rapid and simple purification method was used to separate and purify nitrate reductases (NR) from Williams soybean leaves. Blue Sepharose columns were sequentially eluted with 50 millimolar NADPH and 50 millimolar NADH, thus separating NAD(P)H:NR from NADH:NRs. Subsequent purification of the collected peaks on a fast protein liquid chromatography-Mono Q column enabled separation of two NADH:NRs. Sodium dodecyl sulfate polyacrylamide gel electrophoresis revealed that the subunit relative molecular mass for all three NR forms (constitutive NAD(P)H:NR [pH 6.5], EC 1.6.6.2; constitutive NADH:NR [pH 6.5], EC not assigned; and inducible NADH:NR [pH 7.5], EC 1.6.6.1) was approximately 107 to 109 kilodaltons. All three NRs showed similar spectra with absorption maxima at 413 and 273 nanometers in the oxidized state, and with the characteristics of a cytochrome b type heme upon reduction with NADH (absorption maxima at 556, 527, and 424 nanometers). The technique developed provides an improved separation of the three NR forms from soybean leaves. The similarity of the NRs with regard to their cytochrome b556 type heme content and in relative molecular mass indicated that other differences must exist to account for the different kinetic and physical properties previously reported.
Full text
PDF
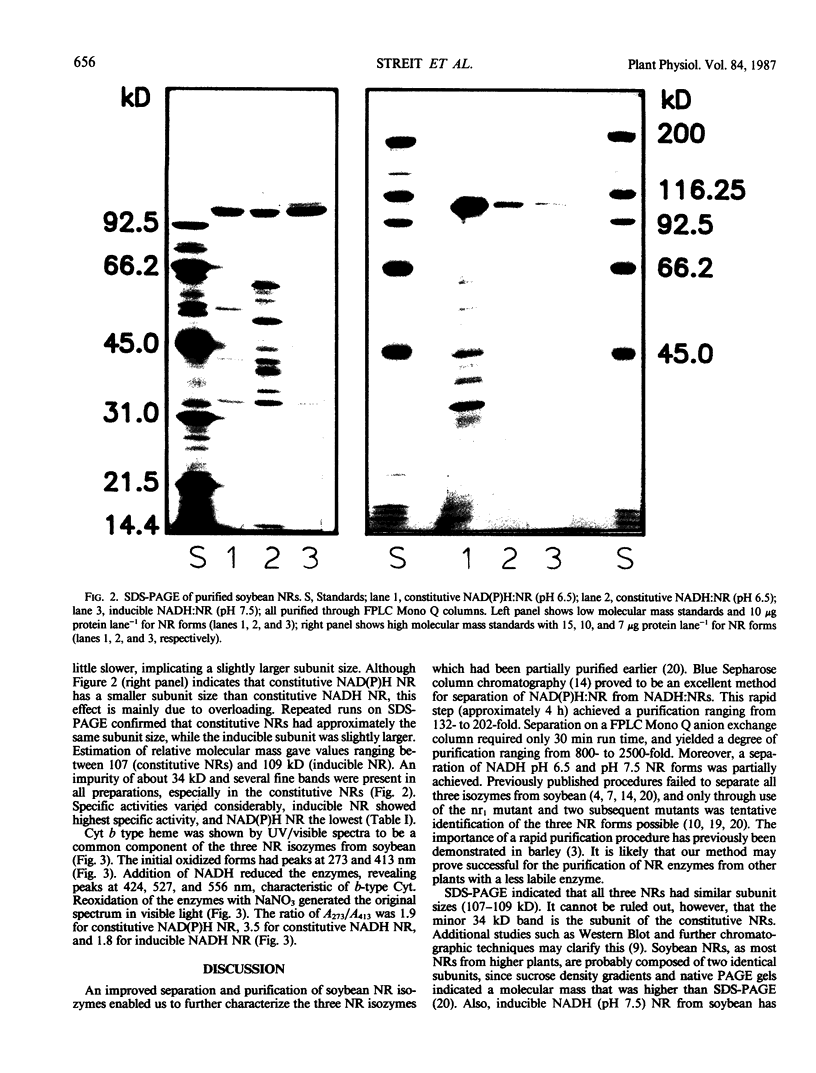

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- Carroll B. J., Gresshoff P. M. Isolation and Initial Characterization of Constitutive Nitrate Reductase-Deficient Mutants NR328 and NR345 of Soybean (Glycine max). Plant Physiol. 1986 Jun;81(2):572–576. doi: 10.1104/pp.81.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., Campbell W., Tolbert N. E. NADPH- and NADH-nitrate reductases from soybean leaves. Arch Biochem Biophys. 1976 Jun;174(2):431–439. doi: 10.1016/0003-9861(76)90371-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nelson R. S., Streit L., Harper J. E. Nitrate Reductases from Wild-Type and nr(1)-Mutant Soybean (Glycine max [L.] Merr.) Leaves : II. Partial Activity, Inhibitor, and Complementation Analyses. Plant Physiol. 1986 Jan;80(1):72–76. doi: 10.1104/pp.80.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuel-Iranzo B., Campbell W. H. Development of NAD(P)H: and NADH:Nitrate Reductase Activities in Soybean Cotyledons. Plant Physiol. 1980 Apr;65(4):595–599. doi: 10.1104/pp.65.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Campbell W. H. Quaternary structure and composition of squash NADH:nitrate reductase. J Biol Chem. 1985 Mar 25;260(6):3380–3385. [PubMed] [Google Scholar]
- Robin P., Streit L., Campbell W. H., Harper J. E. Immunochemical Characterization of Nitrate Reductase Forms from Wild-Type (cv Williams) and nr(1) Mutant Soybean. Plant Physiol. 1985 Jan;77(1):232–236. doi: 10.1104/pp.77.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarrelli J., Campbell W. H. Immunological approach to structural comparisons of assimilatory nitrate reductases. Plant Physiol. 1981 Dec;68(6):1226–1230. doi: 10.1104/pp.68.6.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Barber M. J., Howard W. D., Johnson J. L., Rajagopalan K. V. Electron paramagnetic resonance studies on the molybdenum center of assimilatory NADH:nitrate reductase from Chlorella vulgaris. J Biol Chem. 1984 Jan 25;259(2):849–853. [PubMed] [Google Scholar]
- Streit L., Harper J. E. Biochemical Characterization of Soybean Mutants Lacking Constitutive NADH:Nitrate Reductase. Plant Physiol. 1986 Jun;81(2):593–596. doi: 10.1104/pp.81.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit L., Nelson R. S., Harper J. E. Nitrate Reductases from Wild-Type and nr(1)-Mutant Soybean (Glycine max [L.] Merr.) Leaves : I. Purification, Kinetics, and Physical Properties. Plant Physiol. 1985 May;78(1):80–84. doi: 10.1104/pp.78.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]