Abstract
As liposomes are cleared from the circulation to a substantial extent by the phagocytic cells of the mononuclear phagocyte system (MPS), there is a question whether administration of liposome-based therapeutic agents interferes with clearance of infectious organisms by the MPS from blood. In the present study, at first the effect of administration of three types of empty liposomes (devoid of drug), differing in blood residence time, on carbon clearance and bacterial clearance from blood was studied with mice. Classical liposomes (LIP A) and placebo liposomes with lipid composition as in AmBisome (LIP B) or as in Doxil (LIP C) were used. Liposomes were administered intravenously as a single dose. Second, the effect of multiple-dose administration of AmBisome on bacterial blood clearance was studied with rats. AmBisome was administered with two different dosage schedules. The blood clearance capacity of the MPS was monitored at different time points after the last liposome injection. It was shown that the carbon blood clearance capacity of the MPS was impaired only at a high lipid dose of empty classical liposomes. The bacterial blood clearance capacity was never impaired, not even after prolonged treatment with AmBisome administered in a clinically relevant regimen.
The therapeutic value of liposomes as drug carriers, in particular for anticancer, antifungal, and antibacterial agents, has been demonstrated by a substantial number of research groups. Quite recently, various pharmaceutical companies have succeeded in scaling up the production of various liposome-based therapeutic agents, allowing clinical application. Patients treated with liposomal agents are often severely immunocompromised and hence highly susceptible to developing systemic infections. Especially in these immunocompromised patients, the resident phagocytic cells of the mononuclear phagocyte system (MPS) play a major role in the clearance of microorganisms from the blood. Malfunction of the MPS may result in generalization of the infection and hence increased mortality.
Liposomes are also cleared from the circulation by the phagocytic cells of the MPS in liver (Kupffer cells) and spleen (14). The question arises whether intravenous (i.v.) administration of liposomes interferes with the clearance of infectious organisms from the blood. During circulation of liposomes, binding of blood proteins to liposomes (6, 18) might lead to reduced opsonization and clearance of microorganisms, whereas after MPS uptake of liposomes saturation of the phagocytic uptake capacity might be expected (14). Particularly when potentially toxic agents are encapsulated in liposomes, as is the case for the liposomal formulations used in clinical practice, serious damage of the MPS due to release of the entrapped drug within the phagocytic cells should be taken into consideration.
It was previously demonstrated in rats that administration of liposomes that rapidly accumulate in the MPS (classical liposomes) and contain the chemotherapeutic agent doxorubicin (DOX) can result in toxicity towards liver macrophages in terms of impaired phagocytic functions or even depletion of liver macrophages (7). Progress in liposome technology has yielded new types of liposomes that are substantially able to avoid uptake by the MPS and therefore exhibit a relatively long residence time in blood (26). The industrially prepared liposome formulations AmBisome (NeXstar Pharmaceuticals, Inc., San Dimas, Calif.) and Doxil (Sequus Pharmaceuticals, Inc.) indeed show relatively long blood circulation times, although MPS uptake to a certain extent is still observed. As these liposomes carry the potentially toxic agents amphotericin B (AMB) and DOX, respectively, possible implications of i.v. administration of these liposome formulations regarding blood clearance capacity of the MPS need to be investigated thoroughly. It was recently shown by Daemen et al. (9) that DOX encapsulated in long-circulating liposomes was less toxic for the liver macrophage population than was DOX encapsulated in classical liposomes (7). With Doxil, a DOX-containing liposome that shows even more prolonged circulation in blood, MPS toxicity in terms of decreased bacterial blood clearance was not observed, provided that Doxil was administered at clinically relevant intervals (21).
In the present study, the effects of single-dose administration of three types of empty liposomes (devoid of drug), differing in blood residence time, on carbon clearance and bacterial clearance from blood were investigated with mice. The classical parameter for the determination of the phagocytic capacity of the MPS is the clearance of carbon particles from blood. From a clinical point of view, measuring the clearance of bacteria from blood after liposomal administration seems to be much more relevant. As there are no previous reports on the effects of administration of empty liposomes on bacterial blood clearance, we have chosen to determine bacterial blood clearance under experimental conditions similar to those described for carbon clearance (1, 10, 11) to enable a direct comparison in one study.
In a second series of experiments, the effects of AmBisome given in multiple-dose schedules on bacterial clearance from blood were studied with rats, by a method that had been previously described for measuring the toxicity of DOX-containing liposomes (7, 9, 21).
MATERIALS AND METHODS
Animals.
Female BALB/c mice (10 to 13 weeks old, specified pathogen free) were obtained from Iffa Credo (L’Arbresle, France). Female R-strain albino rats (20 to 25 weeks old, specified pathogen free) were obtained from Harlan CPB (Austerlitz, The Netherlands).
Bacteria.
Klebsiella pneumoniae (capsular serotype 2; ATCC 43816) and Staphylococcus aureus (clinical isolate) were used.
Materials.
Egg phosphatidylcholine, phosphatidylserine (PS), hydrogenated soybean phosphatidylcholine (HSPC), distearoylphosphatidylglycerol (DSPG), and polyethylene glycol (PEG) 1900 derivative of distearoylphosphatidylethanolamine (PEG-DSPE) were obtained from Avanti Polar Lipids, Inc. (Alabaster, Ala.). Cholesterol (Chol) was from Sigma (St. Louis, Mo.). Chloroform, methanol, and tert-butanol were from Merck (Darmstadt, Germany). Deferoxamine mesylate (DF) was from Ciba-Geigy (Basel, Switzerland). 67Ga-citrate was from Nordian (Montreal, Canada). Carbon (drawing ink FT) was from Pelikan AG (Hannover, Germany). Lysis buffer (Isolator 10 blood culture system) was from Wampol Laboratories (Cranbury, N.J.). Tryptone soy agar was from Unipath Ltd (Basingstoke, United Kingdom).
Liposomes.
For the clearance studies with mice, three different types of empty liposomes were prepared at our laboratory. Classical liposomes (LIP A), with an average particle size of 300 nm, consisted of egg phosphatidylcholine, PS, and Chol in a molar ratio of 40:10:50; placebo liposomes with lipid composition as in AmBisome (LIP B), with an average particle size of 100 nm, consisted of HSPC, DSPG, and Chol in a molar ratio of 100:40:50; placebo liposomes with lipid composition as in Doxil (LIP C), with an average particle size of 100 nm, consisted of HSPC, PEG-DSPE, and Chol in a molar ratio of 73.5:7:50. Liposomes were prepared in our laboratory as previously described (4, 5, 24). In short, for LIP A and LIP C the lipid mixture in chloroform-methanol was evaporated to dryness in a round-bottom flask, redissolved in tert-butanol, and lyophilized. The lipid film was hydrated with HEPES buffer (10 mM HEPES, 150 mM NaCl, pH 7.4). For LIP B, the lipid mixture in chloroform-methanol was evaporated to dryness and directly hydrated with buffer containing 10 mM sodium succinate and 10% (wt/vol) sucrose (pH 5.5). The liposome suspension was either extruded through polycarbonate filters (LIP A) or sonicated (LIP B and LIP C). Mean particle size was determined by dynamic light scattering (Malvern 4700 system; Malvern, United Kingdom). Phospholipid concentration was determined by a phosphate assay (2).
For the clearance studies with rats, AmBisome was kindly provided by NeXstar Pharmaceuticals, Inc. AmBisome, AMB encapsulated in small unilamellar vesicles (80 nm) consisting of HSPC, DSPG, and Chol in a molar ratio of 100:40:50, was provided as a lyophilized preparation. The powder was reconstituted according to the manufacturer’s instructions to give a liposomal suspension containing 4 g of AMB per liter and 35 g of lipid per liter.
Determination of the time points at which 50% or 10% of injected empty liposome is present in the blood of mice.
The residence time of liposomes in blood was determined with liposomes radiolabeled with a 67Ga-DF complex (67Ga-DF) in the aqueous interior of the liposomes, as described by Woodle (25). As shown by Gabizon et al. (12), this 67Ga-DF complex is appropriate for in vivo tracing of intact liposomes because of advantages of minimal translocation of radioactive label to plasma proteins and the high renal clearance rate when the label is released from the liposomes extracellularly. Radiolabeled liposomes were administered i.v. as a single dose in mice at either 400 μmol of lipid/kg of body weight (LIP A, LIP B, and LIP C) or 80 μmol of lipid/kg (LIP A only). At various time points after administration, 200 μl of blood was collected from the mice in heparinized tubes by retro-orbital bleeding under CO2 anesthesia. Blood samples as well as the injected dosage of liposomes were assayed for 67Ga-DF in a gamma counter (Minaxy 5530; Packard Instruments, Downers Grove, Ill.).
Monitoring of carbon clearance or bacterial clearance from blood in mice treated with empty liposomes.
Liposomes were injected i.v. into mice at a single dose of either 400 μmol of total lipid/kg (LIP A, LIP B, and LIP C) or 80 μmol of total lipid/kg (LIP A only). At different time points after administration of liposomes or buffer (controls), i.e., at 1 min after liposome administration (>90% of liposomes present in blood) and at the times that 50 or 10% of liposomes were present in blood, the blood clearance capacity of the MPS was determined for six mice per time point per treatment. Carbon clearance was monitored by i.v. injection of carbon diluted in phosphate-buffered saline at a dose of 1 mg/mouse. At 1 and 10 min after carbon administration, blood was collected by retro-orbital bleeding under CO2 anesthesia, and blood carbon concentration was determined, after lysis of blood cells with lysis buffer. The percentage of clearance within 9 min was calculated. Bacterial clearance was monitored by i.v. injection of K. pneumoniae at an inoculum of 1.4 × 105 bacteria/kg or S. aureus at an inoculum of 1.4 × 109 bacteria/kg. At 1 and 10 min after bacterial inoculation, blood was collected and from this the number of viable bacteria was determined, after lysis of blood cells. The number of viable bacteria was determined by making plate counts of 10-fold serial dilutions of the blood samples on tryptone soy agar.
Monitoring of bacterial clearance from blood in rats treated with AmBisome.
AmBisome was administered i.v. to rats for either 5 or 10 consecutive doses of 5 mg of AMB/kg (equivalent to 50 μmol of lipid/kg) at 24-h intervals. At two different time points after administration, i.e., 24 and 72 h after the last dose, bacterial blood clearance was determined. Bacterial clearance was monitored for 10 rats per time point per treatment group by i.v. injection of K. pneumoniae at an inoculum of 3.2 × 107 bacteria/kg; at 60 and 120 min after bacterial inoculation, blood was collected and from this the number of viable bacteria was determined.
Statistical analysis.
In the study with empty liposomes, results were expressed as the mean percentages of clearance within 9 min. In the study with AmBisome, results were expressed as the geometric means of numbers of viable bacteria in blood at various time points. In both studies, differences in blood clearance between the various treatment groups were analyzed by the Mann-Whitney test.
RESULTS
Determination of the time points at which 50 or 10% of injected empty liposomes are present in blood of mice.
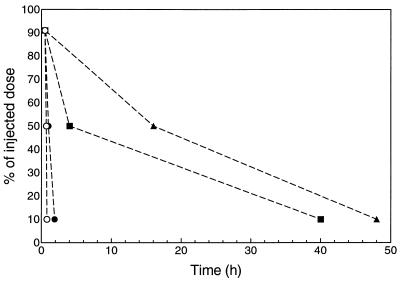
The three types of empty liposomes showed different blood circulation times (Fig. 1). At 1 min after liposome administration, more than 90% of the liposome dose was present in blood for all three liposome types. At the high dose of 400 μmol of lipid/kg, 50% of the liposome dose was present in blood at 50 min for LIP A, at 4 h for LIP B, and at 16 h for LIP C; 10% of the liposome dose was present in blood at 110 min for LIP A, at 40 h for LIP B, and at 48 h for LIP C. At the dose of 80 μmol of lipid/kg, for LIP A 50% of the dose was present in blood at 10 min, and 10% of the dose was present at 30 min.
FIG. 1.
Residence of 67Ga-DF-labeled liposomes in blood after single-dose administration (i.v.) in mice. Only the time points at which >90, 50, or 10% of injected liposomes are present in blood are indicated (n = 3 per time point). ○, LIP A (classical liposomes) at 80 μmol of lipid/kg; •, LIP A at 400 μmol of lipid/kg; ▪, LIP B (placebo liposomes with lipid composition as in AmBisome) at 400 μmol of lipid/kg; ▴, LIP C (placebo liposomes with lipid composition as in Doxil) at 400 μmol of lipid/kg.
Effect of i.v. administration of empty liposomes on carbon clearance from blood in mice.
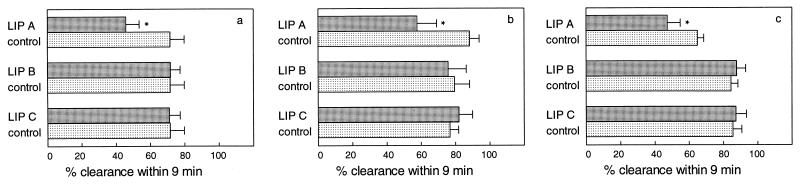
Clearance of carbon from blood in mice after single-dose administration of either LIP A, LIP B, or LIP C at a dose of 400 μmol of lipid/kg is shown in Fig. 2. Due to administration of LIP A (classical liposomes) at the high dose of 400 μmol of lipid/kg, the carbon clearance within 9 min was significantly reduced (P ≤ 0.001) at all time points measured, being 46, 58, and 47% at 1, 50, and 110 min after liposome administration, respectively, whereas in control animals carbon clearance was 72, 88, and 65% at the same time intervals, respectively, after buffer administration. In contrast, when LIP A was administered at the low dose of 80 μmol of lipid/kg the carbon clearance efficiency was unaffected (data not shown). Administration of LIP B (placebo-AmBisome) or LIP C (placebo-Doxil) at the high dose of 400 μmol of lipid/kg did not result in impaired carbon clearance capacity.
FIG. 2.
Clearance of carbon from blood in mice after single-dose administration (i.v.) of empty liposomes at 400 μmol of lipid/kg. LIP A, classical liposomes; LIP B, placebo liposomes with lipid composition as in AmBisome; LIP C, placebo liposomes with lipid composition as in Doxil. Carbon clearance from blood was determined at the time that >90% (a), 50% (b), or 10% (c) of liposomes were present in blood. Results are expressed as the mean percentages (n = 6 per time point per treatment) of clearance ± standard deviations in mice treated with liposomes (░⃞) or buffer ( ). ∗, P ≤ 0.001 versus buffer-treated mice.
Effect of i.v. administration of empty liposomes on bacterial clearance from blood in mice.
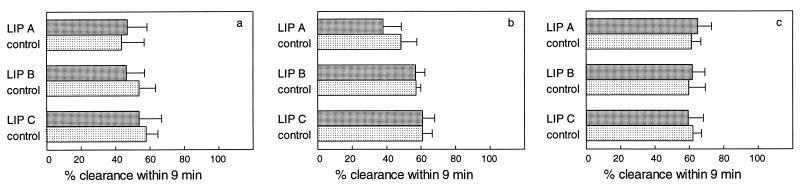
Clearance of K. pneumoniae and S. aureus from blood in mice after single-dose administration of either LIP A, LIP B, or LIP C at a dose of 400 μmol of lipid/kg is shown in Fig. 3 and 4, respectively. In buffer-treated control mice, an average of 56% of K. pneumoniae bacteria was cleared within 9 min. Administration of all three types of liposomes at the high dose of 400 μmol of lipid/kg did not affect the bacterial clearance efficiency at any of the time points.
FIG. 3.
Clearance of K. pneumoniae from blood in mice after single-dose administration (i.v.) of empty liposomes at 400 μmol of lipid/kg. For explanations of symbols and panels, see the legend to Fig. 2.
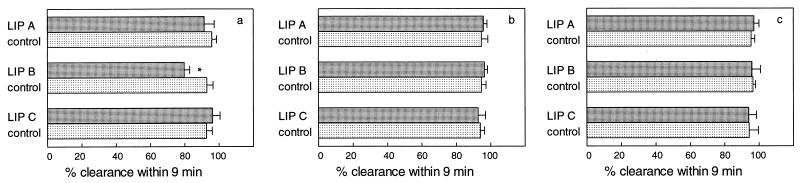
FIG. 4.
Clearance of S. aureus from blood in mice after single-dose administration (i.v.) of empty liposomes at 400 μmol of lipid/kg. For explanations of symbols and panels, see the legend to Fig. 2.
The clearance of S. aureus within 9 min amounted to an average of 95% in buffer-treated controls. Again, the bacterial clearance efficiencies were not different among animals treated with all three types of empty liposomes, with one exception: at 1 min after administration of LIP B (placebo-AmBisome), the clearance capacity was slightly but significantly (P ≤ 0.001) reduced to 80%.
Effect of i.v. administration of AmBisome on bacterial clearance from blood in rats.
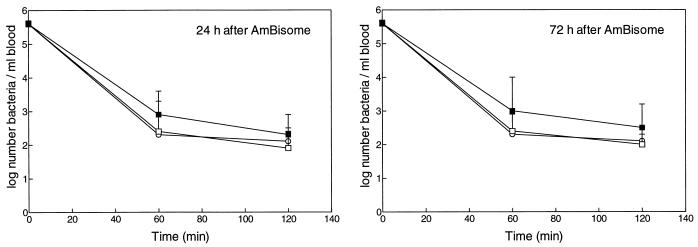
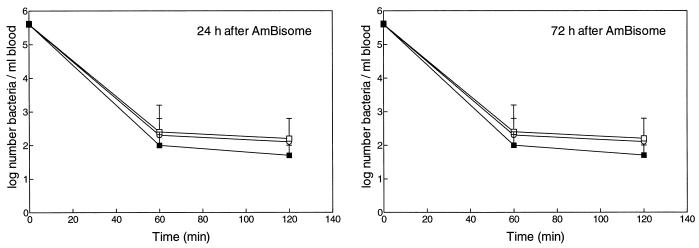
Clearance of K. pneumoniae from blood after daily administration of AmBisome, placebo liposomes, or buffer for either 5 or 10 consecutive days is shown in Fig. 5 and 6, respectively. In buffer-treated control rats, more than 99% of K. pneumoniae bacteria was cleared within 60 min. Administration of AmBisome daily at 5 or 10 doses of 5 mg of AMB/kg each did not affect clearance of K. pneumoniae within 60 or 120 min after inoculation, when determined at 24 or 72 h after the last dosage.
FIG. 5.
Effect of AmBisome (▪) for 5 consecutive days at 5 mg of AMB/kg · day (equivalent to 50 μmol of lipid/kg · day) on clearance of K. pneumoniae from blood in rats. Bacterial blood clearance was determined at 24 as well as 72 h after the last injection of liposomes. Controls were treated with placebo liposomes (□) or buffer (○). Results are expressed as the geometric means of numbers of viable bacteria ± standard deviations for 10 rats per time point per treatment.
FIG. 6.
Effect of AmBisome (▪) for 10 consecutive days at 5 mg of AMB/kg · day (equivalent to 50 μmol of lipid/kg · day) on clearance of K. pneumoniae from blood in rats. For explanations of open symbols, see the legend to Fig. 5.
DISCUSSION
Administration of liposome-based therapeutic agents might affect the blood clearance capacity of the MPS. In the present study, the effects of administration of the liposomal carrier alone (empty liposomes) as well as that of a liposome containing the potentially toxic agent AMB, AmBisome, were investigated. Blood clearance capacity was assessed at various time points after liposome administration, as both during circulation and after uptake by the MPS liposomes might interfere with the clearance of other particles from blood.
As the classical parameter for determination of the phagocytic capacity of the MPS is the clearance of carbon particles (1, 10, 11), this parameter was also used in the present study. In addition, the clearance of bacteria from blood was monitored, as this seems to be a much more relevant parameter from a clinical point of view. Two clinically relevant bacterial pathogens, K. pneumoniae and S. aureus, were chosen. For K. pneumoniae, an inoculum that resulted in a clinically relevant bacterial load in blood (as observed in septicemia) was used. S. aureus is cleared from the blood much more rapidly than K. pneumoniae in this strain of mice. In order to obtain bacterial numbers in blood that could be determined accurately at the indicated time points, a much higher inoculum of S. aureus was necessary.
The first part of the study focused on the effects of three types of empty liposomes, differing in blood residence time. LIP A (classical liposome) is rapidly cleared from the blood by the phagocytic cells of the MPS, due to its strong negative charge (PS), low rigidity of the bilayer, and relatively large particle size. Clearance from blood of LIP B (placebo-AmBisome), which has a small liposome size and increased rigidity of the bilayer, is greatly reduced compared to that of classical liposomes. For LIP C (placebo-Doxil), incorporation of PEG-DSPE results in a hydrophilic PEG coating on the surface of the liposomes, by which binding of blood proteins is substantially reduced. As a result, prolonged blood residence time of liposomes is obtained even at low lipid dosages, small particle size, or low rigidity of the bilayer (26). For LIP A, LIP B, and LIP C, 50% of the injected dose of liposomes was present in blood at 50 min, 4 h, and 16 h after administration in mice, respectively. For that reason, the blood clearance capacity of the MPS was not measured at fixed time points after liposome administration but at the time points at which equal amounts of liposomes, namely, >90, 50, or 10% of the injected dose, were present in blood.
For classical liposomes, carbon clearance was significantly reduced at each of the three different time points after administration of a lipid dose of 400 μmol of lipid/kg. However, bacterial clearance was not reduced. This high lipid dose amply exceeds the lipid doses that are presently used in clinical practice. Therefore, the blood clearance capacity was also monitored at a more clinically relevant dose of 80 μmol of lipid/kg. At this relatively low lipid dose, both carbon clearance and bacterial clearance were not affected. The data obtained clearly demonstrate that the assessment of blood clearance capacity of the MPS is greatly determined by the type of particle that is used.
For carbon as well as for bacteria, the blood clearance was not reduced after administration of empty liposomes representing the size and lipid composition of AmBisome and Doxil, respectively. These findings are not surprising as both types of liposomes are characterized by relatively long circulation half-lives, due to reduced MPS uptake.
In the second part of the study, we examined whether for AMB-containing liposome, AmBisome, similar results were obtained. Until now, the safety of AmBisome has been studied with a focus on infusion-related adverse effects, renal function, and hepatic function (in particular, functioning of hepatocytes) (16, 17, 19). In the present study, the effect of multiple-dose administration of AmBisome on the bacterial blood clearance capacity of the MPS was studied.
AmBisome was evaluated with a clinically relevant dose regimen of 5 mg of AMB/kg · day (15) and was administered daily during a period of 5 or 10 days, as 10 i.v. injections were maximally tolerated by the rats. To measure bacterial clearance capacity, K. pneumoniae was inoculated, as the clearance of this highly encapsulated bacterial strain from blood is rather slow compared to that of other bacterial pathogens. In infections caused by highly encapsulated bacteria, maximal MPS function is very important. Bacterial blood clearance capacity measured over a 120-min period was never impaired, not even after prolonged treatment with AmBisome.
Prolonged treatment schedules, resembling the clinical setting closely, were applied (15). A total of 5 or 10 daily doses of AmBisome in rats is expected to result in high levels of AMB, particularly in the liver and spleen (13, 19, 23). The pattern of tissue distribution of AMB in animals appears quite similar to that determined for humans at autopsy (20). From the animal studies, it is known that the AMB concentrations in liver and spleen remain high for at least 48 h after the last AmBisome administration. It is not known whether these high AMB concentrations represent liposome-associated AMB or AMB released after intracellular degradation of AmBisome. Although intracellular degradation of AmBisome within the phagocytic cells of the MPS is expected to be slow (22), the exact time course of AmBisome uptake, degradation, and AMB release is not known. Therefore, bacterial blood clearance capacity was determined at different time points during an extended period after the last liposome administration.
With respect to the other two industrially produced AMB-lipid formulations, Abelcet (The Liposome Company, Inc.) and Amphocil (Sequus Pharmaceuticals, Inc.), it is evident that these have quite different structural and pharmacokinetic characteristics (15). The relatively large structures of Abelcet as well as the small discoidal particles of Amphocil are rapidly taken up by the MPS. In particular, for these two AMB-lipid formulations potential damage to the MPS should be taken into consideration, and this potential also needs thorough investigation.
Finally, it is important to note that administration of liposomes may have effects on host defense other than interference with phagocytic capacity. It has been described, e.g., for liposomal PS, that it can seriously inhibit the effectiveness of immunomodulating agents acting on macrophages (8). For AmBisome, reduced infusion-related toxicity was associated with reduced cytokine levels in plasma compared to those for conventional AMB (3).
In conclusion, reduction of the blood clearance capacity of the MPS is a major concern, particularly for immunocompromised patients. The data obtained show that the carbon blood clearance capacity of the MPS was impaired only at a high lipid dose of empty classical liposomes. Bacterial blood clearance capacity was never impaired, not even after prolonged treatment with AmBisome administered in a clinically relevant regimen.
REFERENCES
- 1.Allen T M, Murray L, MacKeigan S, Shah M. Chronic liposome administration in mice: effects on reticuloendothelial function and tissue distribution. J Pharmacol Exp Ther. 1984;229:267–275. [PubMed] [Google Scholar]
- 2.Ames B N, Dubin D T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;235:769–775. [PubMed] [Google Scholar]
- 3.Arning M, Kliche K O, Heer-Sonderhoff A H, Wehmeier A. Infusion related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses. 1995;38:459–465. doi: 10.1111/j.1439-0507.1995.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 4.Bakker-Woudenberg I A J M, Lokerse A F, Roerdink F H, Regts J, Michel M F. Free versus liposome-entrapped ampicillin in treatment of infection due to Listeria monocytogenesin normal and athymic (nude) mice. J Infect Dis. 1985;151:917–924. doi: 10.1093/infdis/151.5.917. [DOI] [PubMed] [Google Scholar]
- 5.Bakker-Woudenberg I A J M, ten Kate M T, Stearne-Cullen L E T, Woodle M C. Efficacy of gentamicin or ceftazidime entrapped in liposomes with prolonged blood circulation and enhanced localization in Klebsiella pneumoniae-infected lung tissue. J Infect Dis. 1995;171:938–947. doi: 10.1093/infdis/171.4.938. [DOI] [PubMed] [Google Scholar]
- 6.Chonn A, Semple S C, Cullis P R. Association of blood proteins with large unilamellar liposomes in vivo. J Biol Chem. 1992;267:18759–18765. [PubMed] [Google Scholar]
- 7.Daemen T, Hofstede G, ten Kate M T, Bakker-Woudenberg I A J M, Scherphof G L. Liposomal doxorubicin-induced toxicity: depletion and impairment of phagocyte activity of liver macrophages. Int J Cancer. 1995;61:716–721. doi: 10.1002/ijc.2910610520. [DOI] [PubMed] [Google Scholar]
- 8.Daemen T, Regts J, Scherphof G L. Liposomal phosphatidyl-serine inhibits tumor cytotoxicity of liver macrophages induced by muramyl dipeptide and lipopolysaccharide. Biochim Biophys Acta. 1996;1285:219–228. doi: 10.1016/s0005-2736(96)00164-2. [DOI] [PubMed] [Google Scholar]
- 9.Daemen T, Regts J, Meesters M, ten Kate M T, Bakker-Woudenberg I A J M, Scherphof G L. Toxicity of doxorubicin entrapped within long-circulating liposomes. J Control Release. 1997;44:1–9. [Google Scholar]
- 10.Ellens H, Mayhew E, Rustum Y M. Reversible depression of the reticuloendothelial system by liposomes. Biochim Biophys Acta. 1982;714:479–485. doi: 10.1016/0304-4165(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 11.Fichtner I, Kniest A, Arndt D. Measurement of carbon clearance in mice as toxicity parameter for liposomal preparations. In Vivo. 1992;6:113–118. [PubMed] [Google Scholar]
- 12.Gabizon A, Huberty J, Straubinger R M, Price D C, Papahadjopoulos D. An improved method for in vivo tracing and imaging of liposomes using a gallium 67-deferoxamine complex. J Liposome Res. 1988;1:123–135. [Google Scholar]
- 13.Gondal J A, Swartz R P, Rahman A. Therapeutic evaluation of free and liposome-encapsulated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother. 1989;33:1544–1548. doi: 10.1128/aac.33.9.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregoriadis, G. 1991. Overview of liposomes. J. Antimicrob. Chemother. 28(Suppl. B):39–48. [DOI] [PubMed]
- 15.Janknegt R, van Etten E W M, de Marie S. Lipid formulations of amphotericin B. Curr Opin Infect Dis. 1996;9:403–406. [Google Scholar]
- 16.Meunier, F., H. G. Prentice, and O. Ringdén. 1991. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. J. Antimicrob. Chemother. 28(Suppl. B):73–82. [DOI] [PubMed]
- 17.NeXstar Pharmaceuticals, Inc. AmBisome liposomal amphotericin B. Product monograph. San Dimas, Calif: NeXstar Pharmaceuticals, Inc.; 1994. [Google Scholar]
- 18.Oja C D, Semple S C, Chonn A, Cullis P R. Influence of dose on liposome clearance: critical role of blood proteins. Biochim Biophys Acta. 1996;1281:31–37. doi: 10.1016/0005-2736(96)00003-x. [DOI] [PubMed] [Google Scholar]
- 19.Proffitt, R. T., A. Satorius, S. M. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl. B):49–61. [DOI] [PubMed]
- 20.Ringdén, O., F. Meunier, J. Tollemar, P. Ricci, S. Tura, E. Kuse, M. A. Viviani, N. C. Gorin, J. Klastersky, P. Fenaux, H. G. Prentice, and G. Ksionski. 1991. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J. Antimicrob. Chemother. 28(Suppl. B):73–82. [DOI] [PubMed]
- 21.Storm G, ten Kate M T, Working P K, Bakker-Woudenberg I A J M. Doxorubicin entrapped in sterically stabilized liposomes: effects on bacterial blood clearance capacity of the mononuclear phagocyte system. Clin Cancer Res. 1998;3:111–115. [PubMed] [Google Scholar]
- 22.van Etten E W M, Chander H R, Snijders S V, Bakker-Woudenberg I A J M. Interactions of liposomal amphotericin B with extracellular and intracellular Candida albicans. J Antimicrob Chemother. 1995;36:961–974. doi: 10.1093/jac/36.6.961. [DOI] [PubMed] [Google Scholar]
- 23.van Etten E W M, Otte-Lambillion M, van Vianen W, ten Kate M T, Bakker-Woudenberg I A J M. Biodistribution of liposomal amphotericin B (AmBisome) and amphotericin B-desoxycholate (Fungizone) in uninfected immunocompetent mice and leucopenic mice infected with Candida albicans. J Antimicrob Chemother. 1995;35:509–519. doi: 10.1093/jac/35.4.509. [DOI] [PubMed] [Google Scholar]
- 24.van Etten E W M, van Vianen W, Tijhuis R H G, Storm G, Bakker-Woudenberg I A J M. Sterically stabilized amphotericin B-liposomes: toxicity and biodistribution in mice. J Control Release. 1995;37:123–129. [Google Scholar]
- 25.Woodle M C. 67Gallium-labeled liposomes with prolonged circulation: preparation and potential as nuclear imaging agents. Nucl Med Biol. 1993;20:149–155. doi: 10.1016/0969-8051(93)90107-6. [DOI] [PubMed] [Google Scholar]
- 26.Woodle M C, Lasic D D. Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1113:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]