Abstract
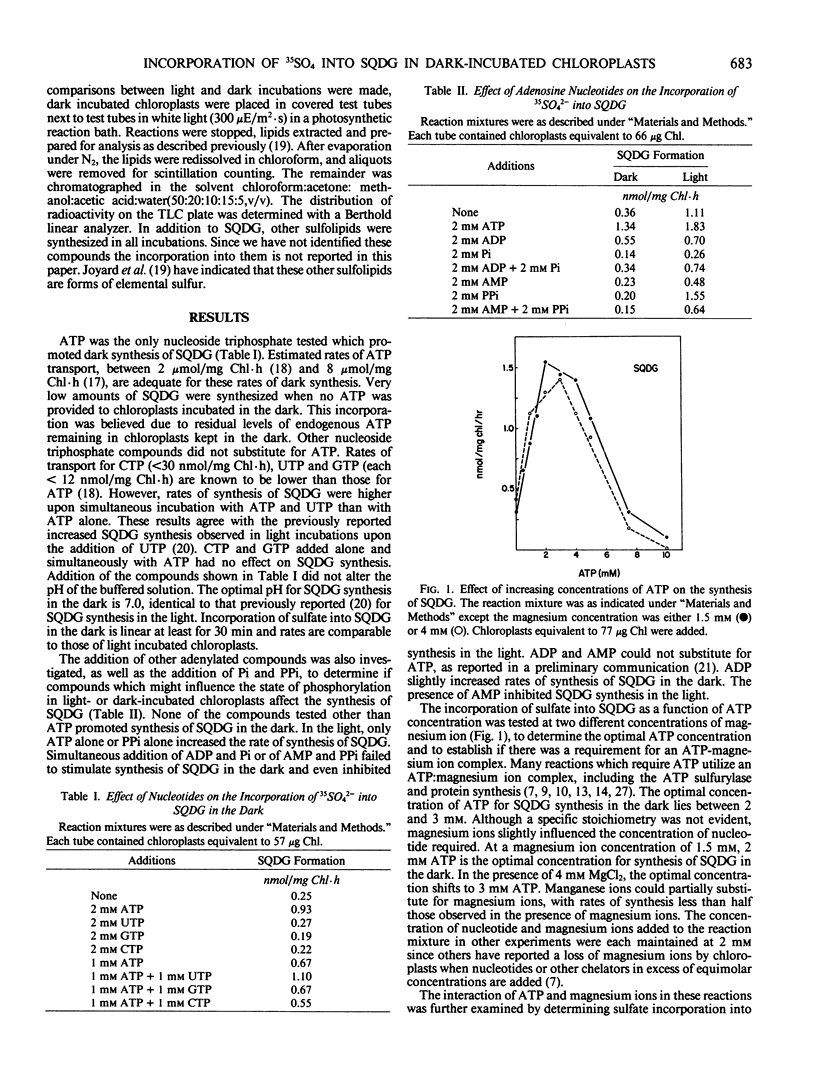
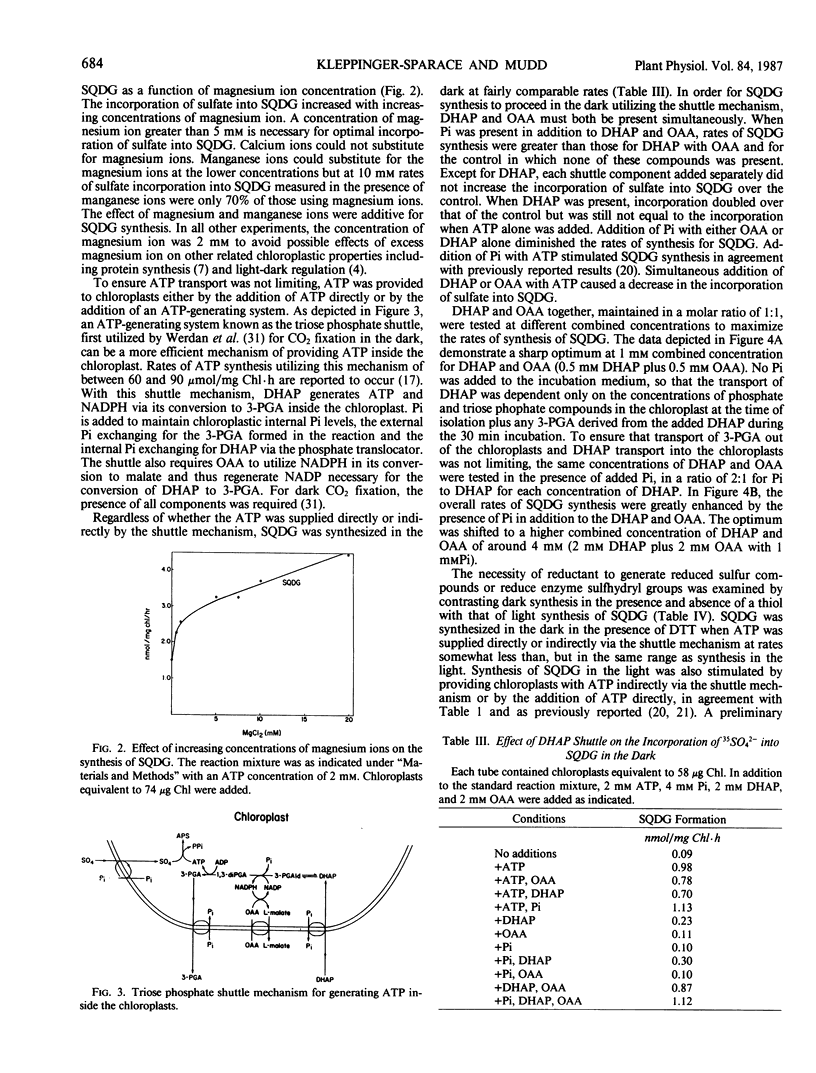
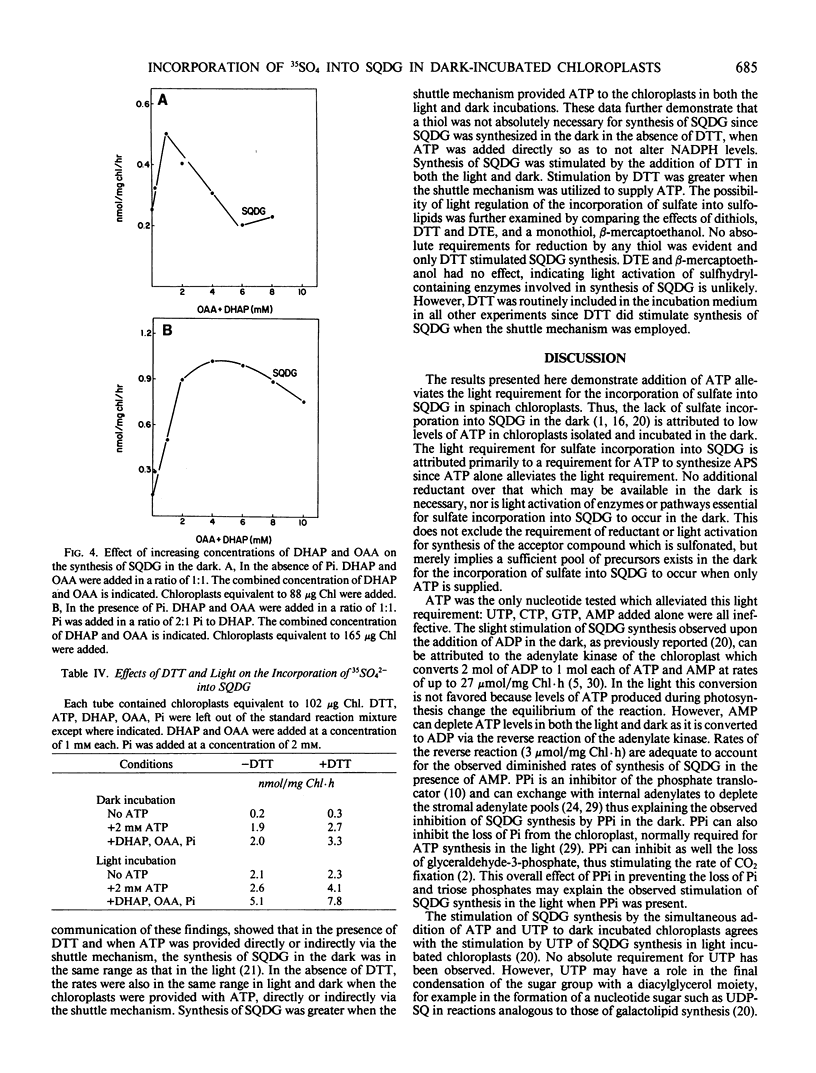
Intact spinach chloroplasts incorporated 35SO42− into sulfoquinovosyldiacylglycerol in the dark at rates equivalent to those previously reported for illuminated chloroplasts provided that either ATP itself or an ATP-generating system was added. No additional reductant was necessary for SQDG synthesis by chloroplasts. The optimal concentration of ATP was between 2 and 3 millimolar. Rates of synthesis up to 2.6 nanomoles per milligram chlorophyll per hour were observed. UTP, GTP, and CTP could not substitute for ATP. Incubation of UTP with ATP (1:1) stimulated synthesis of sulfoquinovosyldiacylglycerol. No additional stimulation of the reaction was observed upon addition of other nucleoside triphosphates with ATP. For the generation of ATP in the chloroplast, addition of dihydroxyacetone phosphate alone did not promote synthesis of sulfoquinovosyldiacylglycerol, but in combination with inorganic phosphate and oxaloacetate, rates of synthesis up to 3.2 nanomoles per milligram chlorophyll per hour were observed. Dark synthesis was optimal in the presence of 2 millimolar dihydroxyacetone phosphate, 2 millimolar oxaloacetate, and 1 millimolar KH2PO4.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM A., BACHHAWATBK Effect of light and streptomycin on the incorporation of sulfate into sulfolipids in Euglena gracilis. Biochim Biophys Acta. 1963 Feb 19;70:104–106. doi: 10.1016/0006-3002(63)90729-7. [DOI] [PubMed] [Google Scholar]
- Bamberger E. S., Ehrlich B. A., Gibbs M. The glyceraldehyde 3-phosphate and glycerate 3-phosphate shuttle and carbon dioxide assimilation in intact spinach chloroplasts. Plant Physiol. 1975 Jun;55(6):1023–1030. doi: 10.1104/pp.55.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver K. A., Hope A. B., Walker D. A. Adenine nucleotide status, phosphoglycerate reduction and photosynthetic phosphorylation in a reconstituted chloroplast system. Biochem J. 1983 Jan 15;210(1):273–276. doi: 10.1042/bj2100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies W. H., Mercer E. I., Goodwin T. W. Some observations on the biosynthesis of the plant sulpholipid by Euglena gracilis. Biochem J. 1966 Feb;98(2):369–373. doi: 10.1042/bj0980369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Fish L. E., Jagendorf A. T. Permeability of chloroplast envelopes to mg: effects on protein synthesis. Plant Physiol. 1984 Apr;74(4):956–961. doi: 10.1104/pp.74.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliege R., Flügge U. I., Werdan K., Heldt H. W. Specific transport of inorganic phosphate, 3-phosphoglycerate and triosephosphates across the inner membrane of the envelope in spinach chloroplasts. Biochim Biophys Acta. 1978 May 10;502(2):232–247. doi: 10.1016/0005-2728(78)90045-2. [DOI] [PubMed] [Google Scholar]
- Gómez-Silva B., Schiff J. A. Synthetic abilities of Euglena chloroplasts in darkness. Biochim Biophys Acta. 1985 Aug 7;808(3):448–454. doi: 10.1016/0005-2728(85)90153-7. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969 Sep;5(1):11–14. doi: 10.1016/0014-5793(69)80280-2. [DOI] [PubMed] [Google Scholar]
- Kleppinger-Sparace K. F., Mudd J. B., Bishop D. G. Biosynthesis of sulfoquinovosyldiacylglycerol in higher plants: the incorporation of 35SO4 by intact chloroplasts. Arch Biochem Biophys. 1985 Aug 1;240(2):859–865. doi: 10.1016/0003-9861(85)90096-7. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Pyrophosphate inhibition of carbon dioxide fixation in isolated pea chloroplasts by uptake in exchange for endogenous adenine nucleotides. Plant Physiol. 1977 Mar;59(3):422–427. doi: 10.1104/pp.59.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic Z. S., Walker D. A. Photosynthesis by isolated pea chloroplasts: some effects of adenylates and inorganic pyrophosphate. Plant Physiol. 1977 Mar;59(3):428–432. doi: 10.1104/pp.59.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Zenker H., Brandt H. P. Netzhautarterienverschluss und Wetter. Klin Monbl Augenheilkd. 1966;148(2):238–244. [PubMed] [Google Scholar]


