Abstract
Objectives
The objective of this study was to evaluate the influence of a long-axial field-of-view (LAFOV) on stage migration using a large single-centre retrospective cohort in lymphoma and non-small cell lung cancer (NSCLC).
Methods
A retrospective study is performed for patients undergoing PET/computed tomography (CT) on either a short-axial field-of-view (SAFOV) or LAFOV PET/CT system for the staging of known or suspected NSCLC or for therapeutic response in lymphoma. The primary endpoint was the Deauville therapy response score for patients with lymphoma for the two systems. Secondary endpoints were the American Joint Committee on Cancer stage for NSCLC, the frequency of cN3 and cM1 findings, the probability for a positive nodal staging (cN1-3) for NSCLC and the diagnostic accuracy for nodal staging in NSCLC.
Results
One thousand two hundred eighteen records were screened and 597 patients were included for analysis (N = 367 for lymphoma and N = 291 for NSCLC). For lymphoma, no significant differences were found in the proportion of patients with complete metabolic response versus non-complete metabolic response Deauville response scores (P = 0.66). For NSCLC no significant differences were observed between the two scanners for the frequency of cN3 and cM1 findings, for positive nodal staging, neither the sensitivity nor the specificity.
Conclusions
In this study use of a LAFOV system was neither associated with upstaging in lymphoma nor NSCLC compared to a digital SAFOV system. Diagnostic accuracy was comparable between the two systems in NSCLC despite shorter acquisition times for LAFOV.
Keywords: digital PET, PET/CT, positron-emission-tomography, total-body, ultra-long FOV PET, whole-body
Introduction
Combined positron emission and computed tomographies (PET/CT) have become the clinical standard of care for chemotherapy response assessment in lymphoma [1], for the staging of non-small cell lung cancer (NSCLC) and the work-up of a suspicious lung nodules [2]. PET/CT technology has undergone rapid development over the last two decades, culminating in the recent introduction of long-axial field-of-view (LAFOV) PET/CT systems with substantial improvements in sensitivity, image quality and noise [3–11]. However, while a number of anecdotal or clinical reports with small cohorts are available evaluating shorter or low-activity protocols LAFOV PET/CT systems [12], few head-to-head comparisons with standard and clinically well-established short-axial field-of-view (SAFOV) systems have been performed which benchmark their clinical performance relative to SAFOV systems [13] and no studies yet demonstrate any improved clinical outcomes when using LAFOV systems. In comparison, a plethora of studies demonstrate that, when using solid-state digital PET/CT systems, improvement in some clinically relevant endpoints can be expected, such as increased detection rate [14–16], detection of smaller structures [17], improved lesion quantification [15], inter-reader agreement and diagnostic certainty [18] and image quality [19] in comparison to previous generation analogue PET/CT systems based on photomultiplier tube technology. Beyond the potential for faster scanning, greater patient throughput or reduction in applied radiopharmaceutical activities [3,4], the clinical impact of this new technology in terms of improved lesion detection or better therapy monitoring has yet to be systematically evaluated.
The replacement of anatomical imaging modalities with PET/CT for the routine staging of NSCLC resulted in a documented stage migration, also known as the ‘Will Rogers phenomenon’ [20]. A similar stage-migration effect has also been recently described to occur as a result of the introduction of low-dose CT screening protocols for patients at high risk of lung cancer in a recent study using trial emulation methodology [21]. Likewise, developments in PET/CT technology and reconstruction software have resulted in well-described effects on Deauville response assessment scores [22–24].
The exquisite sensitivity offered by state-of-the-art LAFOV systems allows, for the first time, previously occult patterns of disease to be detected, such as micro-metastases [25]. Similar improvements in sensitivity and the improved detection of smaller lesions at earlier stages of disease have also been demonstrated when using digital PET/CT systems [14–19]. Currently, there are no published studies which analyse the influence of a LAFOV system on stage migration which this study aims to address.
Ideally, the clinical performance characteristics of any medical device would be tested within the controlled setting of a well-designed and conducted randomised control trial (RCT). However, no large-scale prospective studies assessing the impact of the latest-generation LAFOV-PET/CT systems on the clinical stage are presently available. Instead, real-world evidence is an increasingly recognised evidence source, which might have some additional advantages when compared to a randomised study [26,27]. Retrospective cohort studies are limited by their vulnerability to bias. For example, in retrospective matched-pair cohorts, investigators non-blinded to patient outcome can represent a source of bias in patient selection and few such studies adhere rigorously to pre-determined study protocols or pre-defined statistical power to test hypotheses, limiting their reliability. Therefore, in this study, we assess the influence of LAFOV PET/CT on therapy monitoring for lymphoma and stage migration in NSCLC by means of a retrospective analysis of the largest cohort of patients yet examined using a LAFOV system [21,28].
Materials and methods
Patient cohort and imaging procedures
The study database comprised patients referred for PET/CT at our department for nuclear medicine, between 1 January 2021 and 31 December 2021. This retrospective cohort analysis was approved by the regional ethics review board (KEK 2022-00486). A LAFOV scanner system (Siemens Biograph Vision Quadra, Siemens Healthineers, Erlangen, Germany, aFOV 106 cm) was installed at our centre in October 2020 [29]. Cognisant of previous work demonstrating a learning curve when encountering digital PET/CT systems [18], we exclude patients examined during the first 3 months of operation of the new scanner. Patients referred were examined on either a LAFOV or SAFOV system (Biograph Vision 600, Siemens Healthineers, aFOV 26.3 cm) at random and according to availability with no systematic selection of one scanner over the other. Both scanners are fully digital PET-CT systems, whose performance characteristics are more fully described elsewhere [8,30]. To reduce the risk of claustrophobia or patient choice as a confounder, patients receiving medication for claustrophobia or requiring conscious sedation were excluded from the analysis. Patients were thus assigned to each scanner based on scanner availability and at random.
All 2-[18F]-FDG PET/CT were performed according to extant guidelines [31]. All patients arrived in a fasted state (> 6h), with more than 4 h since their last administration of insulin and with blood glucose < 1.0 mmol/l confirmed by venous sampling prior to administration of the radiopharmaceutical (3.0 MBq/kg weight adjusted activity). Vendor-recommended reconstruction and acquisition parameters were performed as previously published and as per clinical routine [6]. These consisted of an acquisition in continuous bed motion for the SAFOV system (1.1 mm/s, 2 min/bed position equivalent) from skull-base to thighs or a 10 min acquisition in a single bed position [106 cm axial field-of-view (FOV)] for the LAFOV system, with reconstruction parameters as previously described [6]. For an equivalent 106 cm FOV, the total acquisition time for each scanner was therefore 16.06 min for SAFOV and 10 min for LAFOV.
Study design
An institutional database comprising all patients referred to our centre for PET was interrogated for this study. Two investigators (first and second authors) used The National Patient-Centred Clinical Research Network checklist to perform validation of the database and ensure fitness for use of the data. Free text data were checked for completeness and plausibility and numerical data were checked against a pre-defined plausible range. Data outside of these ranges would be considered missing and subject to follow-up by means of scrutiny of their clinical charts; in the end, no patients were found to be lost to follow-up.
All patients referred for PET/CT to our centre for therapy monitoring and follow-up in lymphoma or for the staging or work-up of lung cancer were screened for eligibility. To ensure homogenous and comparable patient cohorts, the following inclusion criteria were applied: referral for PET/CT for patients for therapy response in lymphoma or the work-up or staging of suspected NSCLC. For lymphoma patients, inclusion criteria were patients referred for therapeutic response assessment of a histologically verified lymphoma with 2-[18F]-FDG. Patients undergoing primary staging of lymphoma or work-up of suspected lymphoma (e.g. for identification of metabolically active sites for possible biopsy) or where therapy response assessment was not possible on technical or clinical grounds (Deauville score X) were excluded from the analysis. For NSCLC patients, exclusion criteria were patients presenting for restaging of a known tumour, restaging post-therapy of a known NSCLC or suspected relapse of a previous NSCLC. Patients with SCLC, synchronous, second tumour entities or suspected pulmonary metastasis of a tumour other than NSCLC were also excluded. It is institutional standard to document patients’ general consent for the further use of their health-related data; patients for whom this consent was not documented were excluded.
All patients were documented in the study database which was available to study researchers and with good clinical practice (GCP)-conforming data protection and data traceability using an institutional SharePoint platform. Data records were then scrutinised by the study team. The radiological report was scrutinised for the Deauville staging and cT cN cM stages, which were documented by the reporting board-certified nuclear medicine physician in accordance with the Union for International Cancer Control TNM Classification guidelines (8th Edition). Imaging protocols were as previously described [6].
Endpoints and statistical analysis
The primary endpoint was the Deauville metabolic response score for lymphoma patients undergoing examination for therapy response, which was analysed by means of a proportional odds logistic regression model. Secondary endpoints were: (1) the frequency of obtaining a scan consistent with a complete metabolic response (Deauville scores 1, 2 or 3) in lymphoma patients; (2) The frequency of American Joint Committee on Cancer tumour (cT), nodal (cN) and metastasis (cM) stages for non small cell lung cancer (NSCLC) patients, categorised according to whether they were examined by SAFOV or LAFOV with differences assessed by a chi-squared test; (3) the frequency of a contralateral cN3 stage or cM1 stage for NSCLC; (4) the sensitivity and specificity for nodal staging using endobronchial ultrasound (EBUS) bronchoscopy-guided cytology as the reference standard and with differences assessed by Fisher’s exact test. Binary outcomes were evaluated using logistic regression. P values <0.05 were considered statistically significant. Covariate analysis was performed with applied activity and age as potential confounders.
The null hypothesis was that the distribution of Deauville response scores between the two PET systems does not differ; the alternative hypothesis is that scores differ between the two systems. A power-based approach to the sample size calculation was taken. Assuming four degrees of freedom (Deauville score 1–5 with a pragmatic estimate for the proportion for each score of 0.2) and using a pragmatic estimate of a combined odds ratio (1.8) [32] and at the two-sided 0.05 significance level, a power of 80% is achieved with a target sample size of n = 303 individuals using R Package ‘posamsize’. Owing to the faster speed of scanning with LAFOV, it was expected that more patients would be assigned to the LAFOV than SAFOV, thus a pragmatic estimate of the allocation ratio LAFOV : SAFOV of 2 : 1 was used in the calculation. The expected odds ratio of 1 : 8 corresponds to a small-to-medium effect size (Cohen’s d = 0.32 [33]). All eligible patients were included for analysis to yield maximum statistical power. All statistical analyses were performed in R Foundation for statistical computing (version 4.3.0).
Results
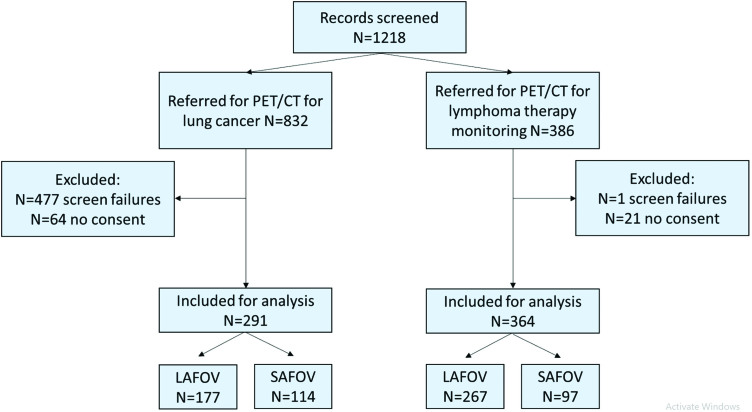
The study flow diagram is shown in Fig. 1. In total 1218 records were screened and 665 patients were included for analysis, with the target sample size (N = 303) being exceeded for the analysis of the primary outcome: N = 364 for lymphoma therapy monitoring (LAFOV N = 267, SAFOV N = 97).
Fig. 1.
Study flow diagram. Screening failure is defined as those patients where one or more exclusion criteria applied, or who did not fulfil the inclusion criteria as described in the materials and methods section.
Primary endpoint
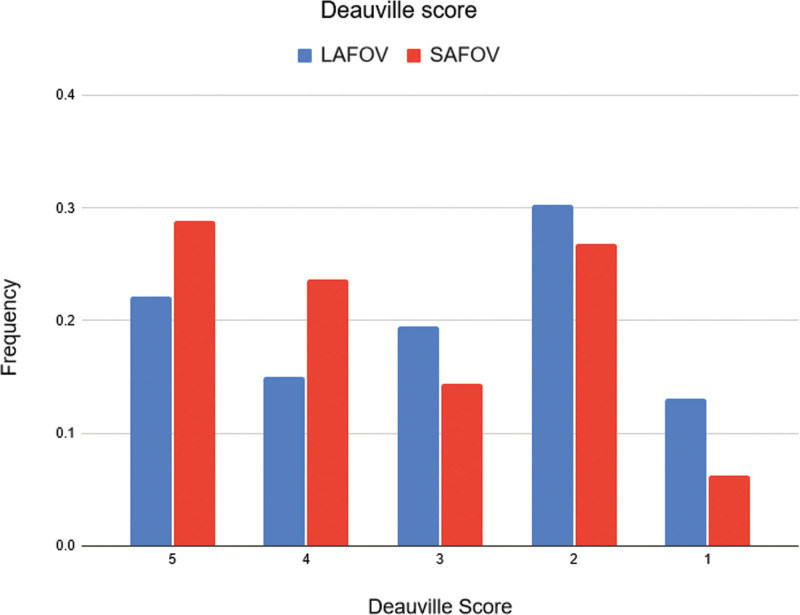
Proportional odds logistic regression for the Deauville response assessment score found no statistically significant difference between the two systems in lymphoma patients [odds ratio (OR) 1.88, 95% confidence interval (CI) 0.01–532.55, P = 0.66]. The frequency of scores is shown in Fig. 2.
Fig. 2.
Frequency of Deauville therapy response scores in lymphoma patients for the LAFOV and SAFOV scanners. LAFOV, long-axial field-of-view; SAFOV, short-axial field-of-view.
Secondary endpoints
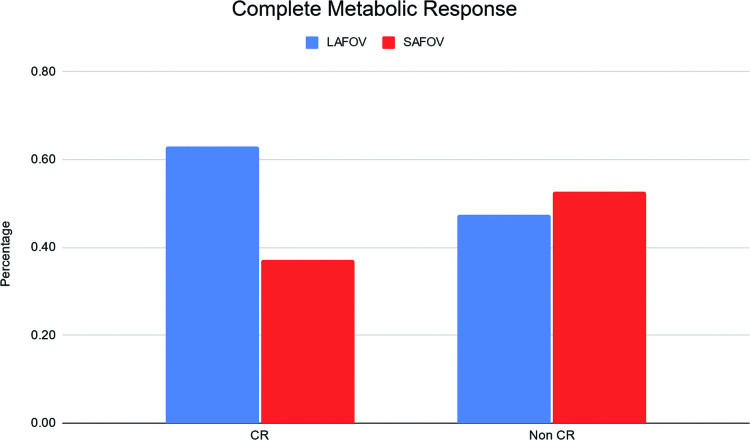
A slightly higher but non-significant proportion of patients presented with complete metabolic response versus non-complete metabolic response on the LAFOV compared to the SAFOV (OR 1.18, 95% CI 0.01–532.55, P = 0.66) is shown in Fig. 3.
Fig. 3.
Frequency of complete metabolic response (Deauville 1 : 3) in lymphoma patients for both scanners.
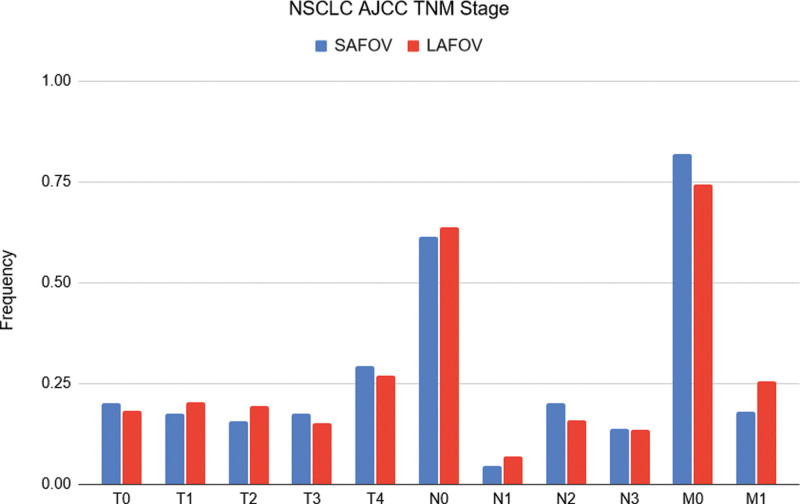
For patients with NSCLC, there were no significant differences between the T, N and M stages between LAFOV and SAFOV systems (for T and N, P > 0.99; for M, P = 0.83). The frequencies of each stage are shown in Fig. 4. A therapeutically relevant cN3 stage was no more likely for patients examined on the LAFOV or SAFOV system (OR = 0.99, 95% CI 0.49–1.98), and a cM1 stage was slightly more likely on the LAFOV system albeit without statistical significance (OR 1.57, 95% CI 0.86–2.83). The OR for a positive nodal staging (i.e. N1-3) was not significantly different between the two systems (OR 1.25, 95% CI 0.8–2.10). EBUS follow-up was available for 47 SAFOV and 63 LAFOV patients. A contingency table is shown in Table 1 which reports the diagnostic accuracy. The sensitivity and specificity for SAFOV were 0.72 (95% CI 0.50–0.88) and 0.88 (95% CI 0.67–0.97) and for LAFOV 0.72 (95% CI 0.50–0.87) and 0.74 (95% CI 0.57–0.86), respectively, with no statistically significant differences observed either for sensitivity (P > 0.99) or for specificity (P = 0.21). Analysis of potential confounders revealed no significant differences in age and applied radiopharmaceutical activity between the two scanners.
Fig. 4.
T, N and M stage in NSCLC patients for LAFOV and SAFOV system. The frequency of N3 findings was the same for both systems and no significant difference in M1 findings was observed. LAFOV, long-axial field-of-view; NSCLC, non-small cell lung cancer; SAFOV, short-axial field-of-view.
Table 1.
Diagnostic accuracy of SAFOV and LAFOV PET/CT (cN) for NSCLC with EBUS cytology as the reference standard
| SAFOV | LAFOV | |
|---|---|---|
| Sensitivity | 0.72 (95% CI 0.50–0.88) | 0.72 (95% CI 0.50–0.87) |
| Specificity | 0.88 (95% CI 0.67–0.97) | 0.74 (95% CI 0.57–0.86) |
| PPV | 0.84 (95% CI 0.59–0.96) | 0.64 (95% CI 0.44–0.81) |
| NPV | 0.78 (95% CI 0.59–0.91) | 0.80 (95% CI 0.63–0.91) |
CT, computed tomography; EBUS, endobronchial ultrasound; LAFOV, long-axial field-of-view; NPV, negative predictive value; NSCLC, non-small cell lung cancer; PPV, positive predictive value; SAFOV, short-axial field-of-view.
Example patients
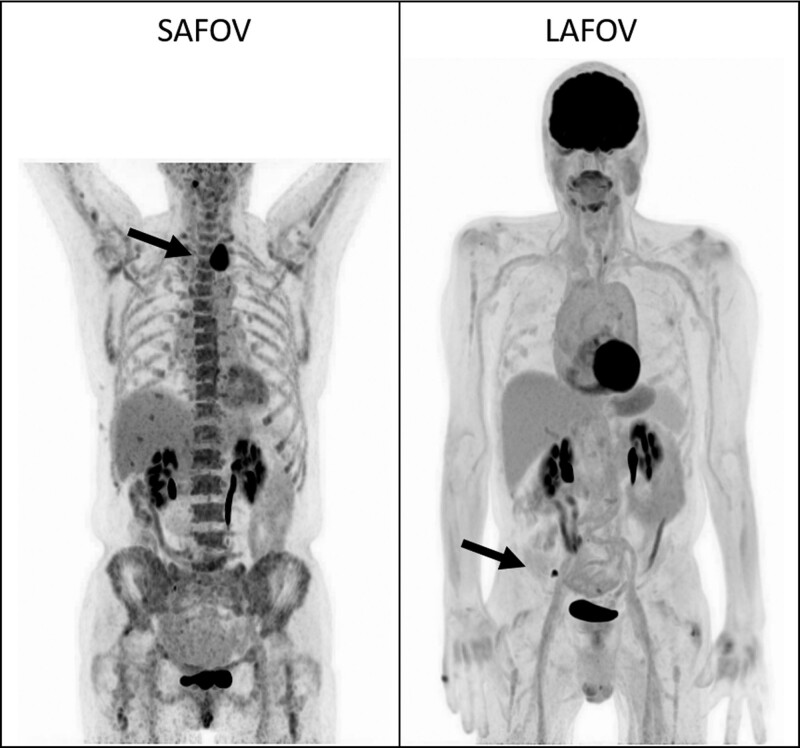
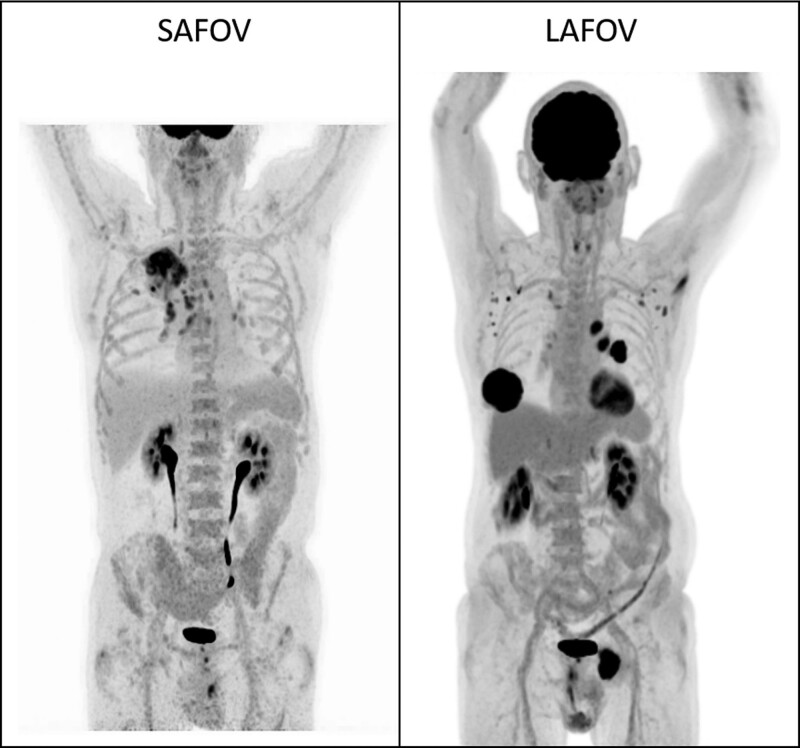
Example images for lymphoma (Fig. 5) and NSCLC (Fig. 6) are presented to demonstrate the image quality and FOV coverage of the two scanners.
Fig. 5.
Example maximum intensity projection (MIP) for random patients undergoing lymphoma assessment on a SAFOV (left) and LAFOV (right) system with hypermetabolic disease in both patients highlighted by the solid arrows. The LAFOV images exhibit improved axial coverage and lower noise compared to the SAFOV (SUV window for both images 0–6). LAFOV, long-axial field-of-view; SAFOV, short-axial field-of-view.
Fig. 6.
Example maximum intensity projection (MIP) images for random patients undergoing staging of NSCLC on a SAFOV (left) and LAFOV (right) system, with hyper-metabolic pulmonary and mediastinal disease readily apparent in both patients. (SUV window for both images 0–6). LAFOV, long-axial field-of-view; SAFOV, short-axial field-of-view.
Discussion
In this study, we compare two cohorts of patients who received either a PET/CT examination on a LAFOV or SAFOV system for assessment of therapy response in lymphoma or for the staging of NSCLC. Appreciable stage-migration effects have previously been described when using PET/CT relative to CT and with modern reconstruction methods in digital PET/CT systems, which can influence both clinical interpretations and have consequences for research studies [1,20,23,24,31]. It is therefore incumbent on operators of state-of-the-art LAFOV systems to benchmark their performance compared to previous-generation SAFOV systems.
Our study compares the Vision Biograph Vision Quadra (LAFOV) with the Biograph Vision 600 (SAFOV) PET/CT system. Other than the axial FOV length (106 vs. 26.3 cm), both systems employ similar detector architecture, image reconstruction and processing software. The longer axial FOV coverage allowed for substantially faster acquisitions when using a LAFOV (10 min per b.p.) compared to the SAFOV (2 min per b.p. in continuous bed motion, 16.06 min total acquisition time).
A number of anecdotal studies and review articles have been published assessing the performance of LAFOV systems, but few if any studies have been performed which demonstrate any improved diagnostic performance or patient outcomes when using these systems. In light of reports demonstrating the detection of micro-metastatic disease in NSCLC using LAFOV PET/CT systems [25], it could be postulated that LAFOV systems might result in stage-migration effects. For example, in NSCLC therapy-defining extra-regional lymph node metastasis or distant metastatic disease might be detected at even earlier stages by this higher sensitivity system. This would result in an upstaging of patients with subsequent migration to higher-stage disease. This hypothesis would be congruent with previous work comparing digital with analogue PET/CT systems: while no studies formally assess the influence of digital PET/CT on stage migration, a number of previous works do demonstrate the detection of smaller lesions and at an earlier stage of disease compared to analogue SAFOV systems [14–19].
However, our data suggest that this does not occur when comparing a latest-generation digital SAFOV system when compared to a LAFOV system. Our interpretation of these findings is that while there is undoubtedly a myriad of benefits for LAFOV PET/CT, including the possibility for ultra-fast [34] or low-activity acquisitions [12], improved quantitative performance [35], improved dynamic range [3,36], improved image quality [6,37] or the ability to perform total-body dynamic scanning [7,38–40], the notion that LAFOV systems furnish increased rates of detection of hyper-metabolic lesions [41], or might increase detection at earlier stages when compared to a state-of-the-art digital PET/CT system cannot be supported by our study. Consequently, statistically significant differences in response score or TNM stage are not seen in a study with pre-defined statistical power to test for these. In keeping with previous findings demonstrating higher image quality and faster overall scan times for LAFOV systems [5,6,12], we demonstrate that LAFOV can deliver faster and comparable results compared to a standard SAFOV system.
One strength of this retrospective study lies in the large sample size with pre-defined power to test for a small to medium effect size. Although randomised control trial (RCT) data are needed to confirm the present findings, it is not necessarily axiomatic that RCT generate better or indeed different data than those obtained from observational studies [42] and RCTs may not always be necessary to make reliable decisions [43]. Indeed, well-conducted observational studies with larger cohorts might be better powered to detect small effect sizes than an underpowered or smaller prospective study. There is therefore increasing recognition of ‘real-world’ data as a rich source of evidence in nuclear medicine [27] and which enjoys increasing recognition by health regulators [26]. Another advantage is the direct comparison between the two systems which differ only according to their axial FOV; the reconstruction algorithms and detection architecture are otherwise equivalent between the LAFOV and SAFOV systems [6]. Follow-up for nodal staging was performed for all NSCLC patients. Although a histological standard of reference for nodal staging was not available in every patient, in those where EBUS was able to confirm or refute the cN staging at PET, no statistically significant differences in sensitivity or specificity could be found between both systems, although a slightly lower specificity was noted for the LAFOV. This might suggest some subtle differences in diagnostic accuracy, for which larger and more dedicated studies would be necessary to assess further.
We note some weaknesses of our study. The data here represent the first calendar year of patients examined with the first installed LAFOV PET/CT system world-wide. Multi-centre data might be collected in the future once a greater number of similar systems are in routine operation. Although observational data cannot entirely replace RCTs, selection bias is considered unlikely with no statistically significant difference in relevant demographics or via patient choice influenced by claustrophobia, and where patients were examined at random according to scanner availability; there is no institutional policy in place which favours assignment to a scanner based on patient characteristics.
Our study was not designed to test for lesion detectability, which may differ as a result of the established higher sensitivity of the LAFOV system [8], and which future studies might address, including in other tumour types. Previous studies demonstrate improved diagnostic confidence and inter-rater reliability when using digital PET/CT systems, which might also be the case in LAFOV systems and which future studies might address [18]. We restrict our analysis to routinely used clinical reconstruction methods, future studies might assess the impact of other reconstruction methods which are known to influence therapy response assessment [1,23,24,31]. Our follow-up period of a minimum of 3 months was insufficient to determine the impact of PET/CT on patient-level outcomes such as overall or progression-free survival. Lymphoma is a highly heterogeneous group of entities with varying 2-[18F]-FDG avidity and a range of therapeutic options from chemotherapy to chimeric antigen receptor therapy. Future prospective studies might interrogate the influence or added benefit of LAFOV PET for particular lymphoma histological types or specific patient groups. Nevertheless, these data represent the as yet largest published cohort of patients as examined on any LAFOV system and are the first data to assess the impact of these systems on clinically relevant outcomes.
Conclusion
We found that therapy assessment in lymphoma and staging of NSCLC were comparable between our LAFOV and SAFOV systems, with no significant stage migration or significantly increased rate of false positive findings for nodal staging in NSCLC. Results were comparable between both scanners with faster examination times on the LAFOV.
Acknowledgements
The cantonal ethics committee approved this study (KEK 2022-00486). All patients provided written informed consent for inclusion in this study. The study was performed in accordance with the Declaration of Helsinki.
Conflicts of interest
H.S. is a full-time employee of Siemens Healthcare AG, Switzerland. A.R. has received research support and speaker honoraria from Siemens. This publication forms part of the doctoral thesis of S.S. For the remaining authors, there are no conflicts of interest
References
- 1.Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging 2017; 44:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003; 348:2500–2507. [DOI] [PubMed] [Google Scholar]
- 3.Badawi RD, Shi H, Hu P, Chen S, Xu T, Price PM, et al. First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med 2019; 60:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. Total-body PET: maximizing sensitivity to create new opportunities for clinical research and patient care. J Nucl Med 2018; 59:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer BA, Berg E, Schmall JP, Omidvari N, Leung EK, Abdelhafez YG, et al. Performance evaluation of the uEXPLORER total-body PET/CT scanner based on NEMA NU 2-2018 with additional tests to characterize pet scanners with a long axial field of view. J Nucl Med 2021; 62:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberts I, Hünermund J-N, Prenosil G, Mingels C, Bohn KP, Viscione M, et al. Clinical performance of long axial field of view PET/CT: a head-to-head intra-individual comparison of the biograph vision quadra with the biograph vision PET/CT. Eur J Nucl Med Mol Imaging 2021; 48:2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sari H, Mingels C, Alberts I, Hu J, Buesser D, Shah V, et al. First results on kinetic modelling and parametric imaging of dynamic (18)F-FDG datasets from a long axial FOV PET scanner in oncological patients. Eur J Nucl Med Mol Imaging 2022; 49:1997–2009. [DOI] [PubMed] [Google Scholar]
- 8.Prenosil GA, Sari H, Furstner M, Afshar-Oromieh A, Shi K, Rominger A, et al. Performance characteristics of the biograph vision quadra PET/CT system with a long axial field of view using the NEMA NU 2-2018 standard. J Nucl Med 2022; 63:476–484. [DOI] [PubMed] [Google Scholar]
- 9.Lan X, Younis MH, Li K, Cai W. First clinical experience of 106 cm, long axial field-of-view (LAFOV) PET/CT: an elegant balance between standard axial (23 cm) and total-body (194 cm) systems. Eur J Nucl Med Mol Imaging 2021; 48:3755–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karp JS, Viswanath V, Geagan MJ, Muehllehner G, Pantel AR, Parma MJ, et al. PennPET explorer: design and preliminary performance of a whole-body imager. J Nucl Med 2020; 61:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moskal P, Rundel O, Alfs D, Bednarski T, Białas P, Czerwiński E, et al. Time resolution of the plastic scintillator strips with matrix photomultiplier readout for J-PET tomograph. Phys Med Biol 2016; 61:2025–2047. [DOI] [PubMed] [Google Scholar]
- 12.Tan H, Sui X, Yin H, Yu H, Gu Y, Chen S, et al. Total-body PET/CT using half-dose FDG and compared with conventional PET/CT using full-dose FDG in lung cancer. Eur J Nucl Med Mol Imaging 2021; 48:1966–1975. [DOI] [PubMed] [Google Scholar]
- 13.Alberts I, Hunermund JN, Prenosil G, Mingels C, Bohn KP, Viscione M, et al. Clinical performance of long axial field of view PET/CT: a head-to-head intra-individual comparison of the biograph vision quadra with the biograph vision PET/CT. Eur J Nucl Med Mol Imaging 2021; 48:2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberts I, Prenosil G, Sachpekidis C, Weitzel T, Shi K, Rominger A, et al. Digital versus analogue PET in [(68)Ga]Ga-PSMA-11 PET/CT for recurrent prostate cancer: a matched-pair comparison. Eur J Nucl Med Mol Imaging 2020; 47:614–623. [DOI] [PubMed] [Google Scholar]
- 15.Fuentes-Ocampo F, Lopez-Mora DA, Flotats A, Paillahueque G, Camacho V, Duch J, et al. Digital vs. analog PET/CT: intra-subject comparison of the SUVmax in target lesions and reference regions. Eur J Nucl Med Mol Imaging 2019; 46:1745–1750. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Mora DA, Flotats A, Fuentes-Ocampo F, Camacho V, Fernandez A, Ruiz A, et al. Comparison of image quality and lesion detection between digital and analog PET/CT. Eur J Nucl Med Mol Imaging 2019; 46:1383–1390. [DOI] [PubMed] [Google Scholar]
- 17.Meyer M, Allenbach G, Nicod Lalonde M, Schaefer N, Prior JO, Gnesin S. Increased (18)F-FDG signal recovery from small physiological structures in digital PET/CT and application to the pituitary gland. Sci Rep 2020; 10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberts I, Hünermund J-N, Sachpekidis C, Mingels C, Fech V, Bohn KP, et al. The influence of digital PET/CT on diagnostic certainty and interrater reliability in [68Ga]Ga-PSMA-11 PET/CT for recurrent prostate cancer. Eur Radiol 2021; 31:8030–8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surti S, Viswanath V, Daube-Witherspoom ME, Conti M, Casey ME, Karp JS. Benefit of improved performance with state-of-the art digital PET/CT for lesion detection in oncology. J Nucl Med 2020; 61:1684–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chee KG, Nguyen DV, Brown M, Gandara DR, Wun T, Lara PN, Jr. Positron emission tomography and improved survival in patients with lung cancer: the Will Rogers phenomenon revisited. Arch Intern Med 2008; 168:1541–1549. [DOI] [PubMed] [Google Scholar]
- 21.Potter AL, Rosenstein AL, Kiang MV, Shah SA, Gaissert HA, Chang DC, et al. Association of computed tomography screening with lung cancer stage shift and survival in the United States: quasi-experimental study. BMJ 2022; 376:e069008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enilorac B, Lasnon C, Nganoa C, Fruchart C, Gac A-C, Damaj G, et al. Does PET reconstruction method affect Deauville score in lymphoma patients? J Nucl Med 2018; 59:1049–1055. [DOI] [PubMed] [Google Scholar]
- 23.Boellaard R, Kobe C, Zijlstra JM, Mikhaeel NG, Johnson PWM, Müller S, et al. Does PET reconstruction method affect Deauville scoring in lymphoma patients? J Nucl Med 2018; 59:1167–1169. [DOI] [PubMed] [Google Scholar]
- 24.Barrington SF, Sulkin T, Forbes A, Johnson PWM. All that glitters is not gold – new reconstruction methods using Deauville criteria for patient reporting. Eur J Nucl Med Mol Imaging 2018; 45:316–317. [DOI] [PubMed] [Google Scholar]
- 25.Fu F, Li X, Wu Y, Xu J, Bai Y, Gao Y, et al. Total-body dynamic PET/CT of micro-metastatic lymph node in a patient with lung cancer. Eur J Nucl Med Mol Imaging 2021; 48:1678–1679. [DOI] [PubMed] [Google Scholar]
- 26.Polak TB, van Rosmalen J, Uyl-de Groot CA. Expanded access as a source of real-world data: an overview of FDA and EMA approvals. Br J Clin Pharmacol 2020; 86:1819–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourla AB, Herrmann K. Real-world data as an evidence source in nuclear medicine. J Nucl Med 2021; 62:156–157. [DOI] [PubMed] [Google Scholar]
- 28.Alberts I, Sari H, Mingels C, Afshar-Oromieh A, Pyka T, Shi K, et al. Long-axial field-of-view PET/CT: perspectives and review of a revolutionary development in nuclear medicine based on clinical experience in over 7000 patients. Cancer Imaging 2023; 23:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascal Gugler. https://www.swissinfo.ch/eng/world-s-fastest-full-body-scanner-turned-on-in-bern/46184876. [Accessed 8 August 2023]
- 30.van Sluis J, de Jong J, Schaar J, Noordzij W, van Snick P, Dierckx R, et al. Performance characteristics of the digital biograph vision PET/CT system. J Nucl Med 2019; 60:1031–1036. [DOI] [PubMed] [Google Scholar]
- 31.Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42:328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum Associates. 1988. [Google Scholar]
- 33.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 2000; 19:3127–3131. [DOI] [PubMed] [Google Scholar]
- 34.Hu P, Zhang Y, Yu H, Chen S, Tan H, Qi C, et al. Total-body (18)F-FDG PET/CT scan in oncology patients: how fast could it be? Eur J Nucl Med Mol Imaging 2021; 48:2384–2394. [DOI] [PubMed] [Google Scholar]
- 35.Leung EK, Berg E, Omidvari N, Spencer BA, Li E, Abdelhafez YG, et al. Quantitative accuracy in total-body imaging using the uEXPLORER PET/CT scanner. Phys Med Biol 2021; 66. doi: 10.1088/1361-6560/ac287c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alberts I, Prenosil G, Mingels C, Bohn KP, Viscione M, Sari H, et al. Feasibility of late acquisition [68Ga]Ga-PSMA-11 PET/CT using a long axial field-of-view PET/CT scanner for the diagnosis of recurrent prostate cancer-first clinical experiences. Eur J Nucl Med Mol Imaging 2021; 48:4456–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Z, Hu D, Ding Y, Dong Y. A comparison of image quality with uMI780 and the first total-body uEXPLORER scanner. J Nucl Med 2019; 60:381. [Google Scholar]
- 38.Viswanath V, Sari H, Pantel AR, Conti M, Daube-Witherspoon ME, Mingels C, et al. Abbreviated scan protocols to capture (18)F-FDG kinetics for long axial FOV PET scanners. Eur J Nucl Med Mol Imaging 2022; 49:3215–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Feng T, Zhao Y, Xu T, Fu F, Huang Z, et al. Whole-body parametric imaging of (18)F-FDG PET using uEXPLORER with reduced scanning time. J Nucl Med 2022; 63:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, Yu H, Shi D, Hu P, Hu Y, Tan H, et al. Short-time total-body dynamic PET imaging performance in quantifying the kinetic metrics of (18)F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging 2022; 49:2493–2503. [DOI] [PubMed] [Google Scholar]
- 41.Abgral R, Bourhis D, Salaun P-Y. Clinical perspectives for the use of total body PET/CT. Eur J Nucl Med Mol Imaging 2021; 48:1712–1718. [DOI] [PubMed] [Google Scholar]
- 42.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000; 342:1878–1886. [DOI] [PubMed] [Google Scholar]
- 43.Yeh RW, Valsdottir LR, Yeh MW, Shen C, Kramer DB, Strom JB, et al. Parachute use to prevent death and major trauma when jumping from aircraft: randomized controlled trial. BMJ 2018; 363:k5094. [DOI] [PMC free article] [PubMed] [Google Scholar]