Obesity is a chronic disease, characterized by both abnormal and/or excess body fat accumulation, that is multifactorial in origin and influenced by various genetic, behavioral, and environmental factors1 This state of hyperlipidosis adversely affects someone’s health, increasing their risk for a range of comorbid conditions and premature mortality, and reducing their overall quality of life.2 Life-altering and oftentimes life-threatening comorbid conditions that have been empirically linked to obesity include type 2 diabetes mellitus (T2DM),3–5 cardiovascular disease,5–8 sleep apnea,9,10 chronic kidney disease,11,12 and at least 13 distinct forms of cancer that, among others, include breast, colorectal, hepatocellular, ovarian, and pancreatic malignancies and multiple myeloma.13,14 More recently, obesity has been empirically documented to be an independent risk factor for adverse health outcomes, including death, in persons with coronavirus disease 2019 (COVID-19).15–18 For all these reasons, obesity is now considered a leading cause of chronic disease, disability, morbidity, and both direct and indirect health care costs worldwide.
Tragically, prevalence rates for obesity are increasing globally and in all age groups, including children and adolescents.19–22 This said, how quickly these rates have been increasing over the past decade has varied geographically. Consequently, geographical origins and ethnicity are considered important factors in the pathophysiology of obesity and its associated diseases, and interventions targeting obesity and its comorbidities must take such links into consideration to optimize their effectiveness.23
Much of the diminished general health and life quality that individuals living with obesity experience stems from this extensive array of comorbid health conditions that influence virtually every organ system and both physical and psychological health. Besides T2DM, cardiovascular disease, sleep apnea, renal disease and cancer, such conditions include metabolic syndrome,24,25 liver disease,26–28 gallbladder disease,29,30 pancreatitis,29,30 venous thromboemboli,31 urinary stress incontinence,32,33 idiopathic intracranial hypertension,34,35 osteoarthritis,36 and psychiatric disorders like depression and anxiety.37–41 It is crucial that such conditions are recognized for several reasons that include (a) their potential for severe and even life-threatening consequences, and (b) how many of these conditions, including diabetes and cardiovascular disease, have been documented to improve or even abate altogether following successful metabolic and bariatric surgery (MBS) or bariatric endoscopy. In contrast, certain other conditions, like the risk of certain cancers, may or may not decline after MBS.
Diagnosing, managing, and monitoring comorbid conditions are among numerous valid arguments for health care practitioners to adopt a multidisciplinary team approach to managing patients with obesity. Another is that the management of obesity has changed dramatically in recent decades with the emergence of a broad array of procedural (eg, surgical and endoscopic) therapies that have proven more effective than conservative therapy alone—with respect to achieving and maintaining weight loss, reducing comorbidities, and improving patients’ overall quality of life.42–45 Yet another rationale for multidisciplinary management is that the presence of obesity-associated physical and psychiatric conditions, their severity, and how well they are being controlled can all influence decisions both about whether surgical therapy is indicated and safe for a given patient, and which operative procedures to consider.
It was with this in mind that a multidisciplinary board of advisors—including members of both the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) and the World Gastroenterology Organisation (WGO)—was created in the latter part of 2020 for the primary purpose of constructing and ultimately publishing consensus guidelines for the management of obesity and its associated comorbid conditions. Drafting these guidelines relied on (a) a thorough literature review conducted by a multidisciplinary team—consisting of bariatric surgeons and endoscopists, internists specializing in either endocrinology or hepatology, nutritionists/dieticians, and psychology/behavioral health care professionals—all members having extensive experience in obesity management; (b) a 3-stage, online consensus (Delphi) survey to identify areas of consensus and nonconsensus in obesity management among 94 international experts spanning all the fields of expertise listed above and 6 continents; and (c) the drafting of guidelines, by the same multidisciplinary team. A full copy of the guidelines and all Delphi survey results have been published on both the IFSO (https://www.ifso.com) and WGO (https://worldgastroenterology.org) websites. A paper summarizing the Delphi survey’s design and results has also been published elsewhere.23 This paper summarizes the main points of the consensus guidelines.
INITIAL ASSESSMENT OF PATIENTS WITH OBESITY
For obesity management to be successful, a multidisciplinary approach to both its assessment and treatment is required2,46–48; and such a multidisciplinary approach should begin with a comprehensive evaluation of each patient’s physical health and fitness, psychological health, nutritional health, dietary practices, and personal beliefs, goals, and expectations. Such is true whether patients are being considered for conservative therapy (eg, diet, exercise, counseling, medication) alone or combined with either an endoscopic or surgical bariatric procedure. Through these evaluations, patients typically learn about and are determined to be either eligible or ineligible for bariatric surgery by designated medical, psychology/behavioral health, and nutrition specialists. Since patients are expected to schedule and attend appointments at which they will be interviewed and examined and may undergo procedures to determine if they are healthy enough to withstand bariatric surgery,49 this evaluation period also may help to predict their likely compliance and success in their obesity management program.
A trained psychotherapist, preferably with considerable expertise managing patients with obesity, should play a major role in this initial assessment. Such a psychological evaluation has several purposes. Among them is identifying dysfunctional eating behaviors—like binge-eating disorder, emotional eating, and food addiction—that could undermine the effectiveness of any therapeutic approach.50 Though the concept of “food addiction” remains unproven and controversial,51 since obesity manifests many of the same symptoms, it also is important to assess for behavioral factors that might place patients at elevated risk of developing problems associated with alcohol and/or other substances and/or behavioral abuse over the course of treatment, especially if a more invasive and permanently life-altering approach like MBS is being considered.52
Patients with a severe psychiatric disorder, like schizophrenia or bipolar disorder, must have it identified. However, the presence of such a condition, in itself, is not an absolute contraindication to MBS. Rather, it is the severity of psychiatric symptoms and how well they are being controlled that predict bariatric surgery outcomes, in terms of both weight loss and mental health consequences.53 In other words, even patients with a major psychiatric diagnosis like schizophrenia can be considered for MBS, if their psychiatric symptoms are well controlled.
Early psychological evaluations also need to assess each individual’s perceptions of their obesity and how stigmatized they feel because of it. This is because weight bias, obesity stigma, and discrimination all are experienced by a sizeable percentage of persons with obesity,54,55 even within general health care settings.56,57 Even health care providers who provide obesity management often hold biased beliefs and attitudes about obesity and people with obesity.58 To combat this, every member of an obesity management team must treat obesity as the chronic disease it is now recognized to be, both to counter patient perceptions that it is merely the result of weak willpower and to reinforce to patients the importance of regular, lifelong follow-up and adherence to treatment. Such health care providers must be especially vigilant regarding their own potential weight bias and recognize that patients who perceive such bias might become averse to adhering to ongoing follow-up and the overall treatment plan. It is also important for health care professionals performing initial psychological assessments to help patients establish realistic goals for weight loss and other outcomes—like diabetes control—early on, lest failure to achieve unrealistic levels of weight loss leads to later discouragement and either reduced patient compliance with, or dropout from, the treatment plan.
Obesity management also requires a detailed nutritional assessment and prolonged nutritional follow-up, even if surgery is elected as the cornerstone of therapy. As with psychological assessments, there are several reasons for this. First, as adjunctive therapy, dietary measures enhance surgical outcomes. Second, potentially life-threatening nutritional deficiencies may occur in patients who elect either for or against MBS.59–62 Several recent clinical practice and best practices guidelines have been published that encompass nutrition care in patients who either intend to undergo or already have undergone MBS, including recommendations for a preoperative medical workup and having a registered dietitian perform a nutritional assessment and provide education and ongoing monitoring.49,59,63–66 It also is well established that the care of any patient undergoing MBS must begin preoperatively and that this must include preoperative screening for micronutrient deficiencies if excellent patient outcomes are to be achieved.59,63,64,66 Obesity management should, therefore, begin with a thorough assessment of every patient’s nutritional status and dietary practices and any nutritional deficits that are identified must be corrected before MBS.
Exercise is another essential component of therapy, even if MBS is undertaken, as it induces health benefits like weight loss, reduced blood pressure, improved physical function, enhanced lipid profile, lower fasting glucose levels, improved mental health, and better overall quality of life.67–69 Studies also have revealed a 16% to 30% reduction in all-cause mortality risk in moderately active individuals, versus those who are sedentary, irrespective of a patient’s body mass index (BMI) and waist circumference. Consequently, like their psychological and nutritional status, patients’ current level of physical fitness, exercise interests, and capacity for different exercise regimes must be assessed early on.
As a general principle and, again, irrespective of whether surgery is selected or rejected, all aspects of nonsurgical management must be tailored to each individual patient, as no one diet, behavior, exercise program, or medication will be accepted by or effective in all patients, and none has been documented as first-line or superior to all others. Long-term and preferably lifelong monitoring of all nonoperative components of obesity management also is required to continuously assess the effects of treatment, identify treatment nonresponse and/or intolerance, and detect any adverse effects that might have arisen from the treatments chosen.
Associated diseases—including T2DM, obstructive sleep apnea, hypertension, and dyslipidemia—also must be identified, be evaluated for severity, and have appropriate treatment initiated preoperatively. Since obesity is a common risk factor for 13 different types of cancer, the importance of cancer screening should be reinforced, in accordance with national guidelines.46,47,49,70 In patients considering MBS, a preoperative upper gastrointestinal (UGI) endoscopic evaluation also is recommended if either a history or symptoms suggestive of gastroesophageal reflux disease (GERD) or other UGI pathology is reported, or if patients are on chronic antiacid therapy.71 In present times, a patient’s COVID-19 status also is considered crucial,23 given the findings of several studies that have identified obesity as a significant, independent determinant of COVID-19 severity.72–76 Two special patient populations that warrant further discussion are seniors and adolescents, as elaborated in the next section.
SENIORS AND ADOLESCENTS
Several observational studies have demonstrated that the overall risk of bariatric surgery in seniors is low, in terms of mortality and other severe outcomes.77,78 However, the literature is contradictory regarding whether that risk is increased relative to that observed in younger adults. For example, in one meta-analysis of nine studies encompassing 4391 individuals who underwent Roux-en-Y gastric bypass (RYGB) (366 and 4025 >60-y-old and ≤60-y-old, respectively), significant rate elevations were detected among seniors for both morbidity (odds ratio=1.88; 95% CI: 1.07, 3.30; P=0.03) and mortality (odds ratio=4.38; 1.25, 15.31; P=0.02).79 In contrast, another meta-analysis uncovered comparable complication rates in patients older than 60 versus 60 or younger, independent of the type of procedure performed.80 Certain specific complications may be more common among seniors, including some nutritional deficiencies,81 rendering close, long-term follow-up a necessity. And though data are scarce comparing the different bariatric procedures, in terms of both efficacy and safety, numerous studies have identified laparoscopic RYGB as a viable option in elderly patients.79,82–85 Interestingly, though total weight loss may be less in older versus younger patients, the reverse appears to be true for metabolic response and comorbidity amelioration rates.86
According to statistics published by the World Health Organization (WHO), >340 million individuals 19 years old or under are currently affected by either overweight or obesity, including 39 million children under the age of 5.20 As in adults, obesity in childhood is empirically linked to several adverse physical and mental health outcomes, including T2DM, steatohepatitis, sleep apnea, cardiovascular disease, and polycystic ovary syndrome,87–89 as well as to negative societal outcomes, like poor self-esteem, reduced academic performance, depression, and decreased quality of life.88,89 In addition, most adolescents with obesity continue to live with obesity as adults,90 with severe obesity in youths a particular concern. Risks of severe obesity during adolescence include several-year reductions in both life expectancy and quality years of life.91 With respect to treatment, short-term studies have shown that the results of MBS in adolescents are like those achieved in adults, in terms of efficacy, major complications, readmission rates, and mortality.23 Durable weight loss and improvements in both obesity-related comorbidities and quality of life are often achieved. Laparoscopic sleeve gastrectomy is the procedure most commonly performed in adolescents, followed by RYGB, while biliopancreatic diversion (duodenal switch) and one-anastomosis gastric bypass are generally not recommended in this age group.92 Unfortunately, despite a sizeable body of published empirical evidence confirming MBS as the most effective therapy for severe obesity in adolescents, the number of MBS procedures performed in adolescents is lagging behind the rapidly increasing prevalence of severe obesity worldwide in this age group.92–94 Likely, insufficient physician and public knowledge and the dearth of published long-term results on MBS in adolescents remain barriers preventing the referral of these youths for MBS.95
ENDOSCOPIC METABOLIC AND BARIATRIC THERAPY (EMBT)
One alternative to bariatric surgery that may be considered in select patients is EMBT, which includes a range of procedural therapies that rely on one of 3 predominant mechanisms of action. These mechanisms are restriction (reducing gastric capacity), biliopancreatic diversion (sectionally separating duodenal and upper jejunal mucosa and preventing the exposure of food to digestive juices), and the percutaneous aspiration of already-ingested gastric contents.96,97 Forms of EMBT also can be categorized as either gastric or small intestinal.96,97 Currently, only EBMTs that restrict gastric capacity—like various models of intragastric balloon (IGB) and endoscopic sleeve gastroplasty (ESG)—are being used in routine clinical practice. The current indication spectrum for EBMTs is a BMI ranging from 30 kg/m2 to just under 40 kg/m2; or a BMI >27 kg/m2 in patients with 1 or more concomitant, obesity-associated comorbidities.
In general, EMBTs are considered as safe, if not safer than MBS, though long-term data remain scarce. Advantages that EMBTs have over MBS are that most can be both repeated and reversed easily. Many EMBTs are, by their very nature (eg, IGBs), transient. Reported weight loss with EMBT generally ranges from 10% to roughly 20% of total body weight. As such, they generally are recommended for use only in patients with less severe (class I or II) obesity or as bridge therapy in patients with more severe obesity awaiting MBS.23 More recently, the FDA has approved ESG for patients with a BMI from 30 to 50 kg/m2. Long-term data up to 5 years reveal weight loss averaging 15% of total body weight.98 Further details regarding currently practiced EMBT procedures are depicted and summarized in Table 1.
TABLE 1.
Specific EMBT Procedures
| Primary EMBTs | Illustrations | Description | Efficacy | SAE rate | FDA/CE mark status |
|---|---|---|---|---|---|
| Gastric volume restriction | |||||
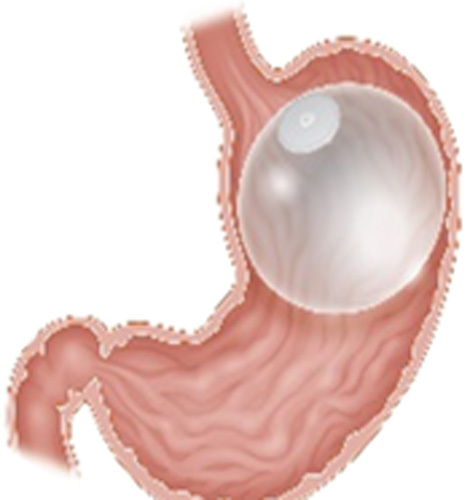
| Orbera Gastric Balloon (Apollo Endosurgery, Austin, TX) |
|
Single fluid-filled balloon Endoscopic placement and removal at 6-12 mo Filled with 400-700 mL of saline |
11.3% TWL at 1 y | 1.6% Migration, perforation, death |
FDA approved in 2015 CE mark BMI 30-40 kg/m2 Age 22 or older |
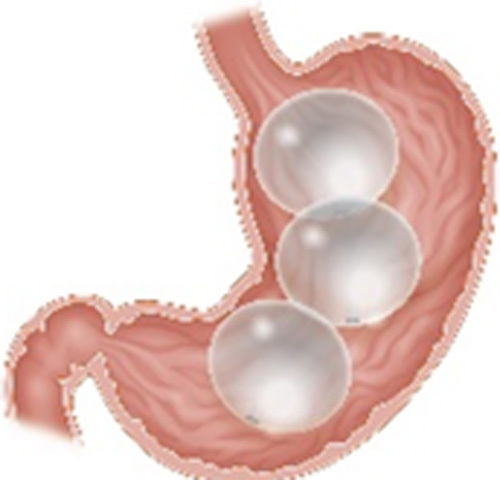
| Obalon Balloon System (ReShape Lifesciences, San Clemente, CA) |
|
Gas-filled balloon Swallowable placement and endoscopic removal at 6 mo Three balloons administered over a 9- to 12-wk period Each balloon filled with 250 mL of a nitrogen mix gas |
10% TWL at 6 o | 0.15% Severe pain, perforation |
FDA approved in 2016 CE mark BMI 30-40 kg/m2 Age 22 or older |
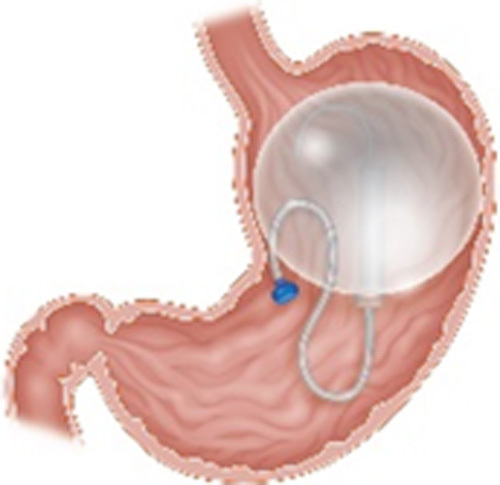
| Spatz3 Adjustable Balloon System (Spatz Medical, Great Neck, NY) |
|
Single fluid-filled balloon with a connecting tube for volume adjustment Endoscopic placement and removal at 8-12 mo Filled with 400-550 mL of saline with methylene blue Volume may be adjusted down to 300 mL or up to 800 mL |
15.0% TWL at 8 mo | 4% Persistent accommodative GI symptoms |
FDA approved in 2021 CE mark BMI 30-40 kg/m2 Age 22 or older |
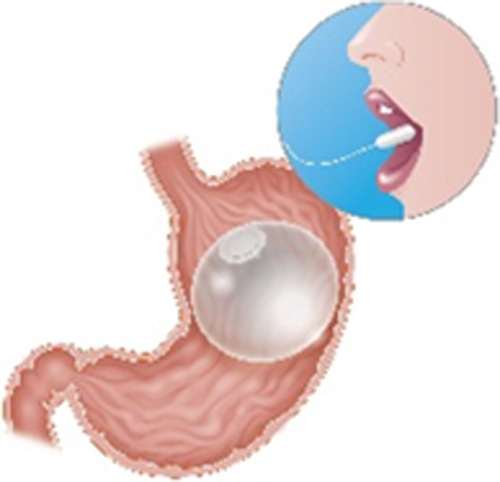
| Elipse Balloon (Allurion Technologies, Wellesley, MA) |
|
Single fluid-filled balloon Swallowable with fluoroscopic guidance for placement and self-emptying mechanism at 4 mo for removal Filled with 550 mL of saline |
Data pending pivotal trial |
NA | Under FDA review CE mark Pivotal trial underway |
| Primary Obesity Surgery Endoluminal (POSE) (USGI Medical, San Clemente, CA) |
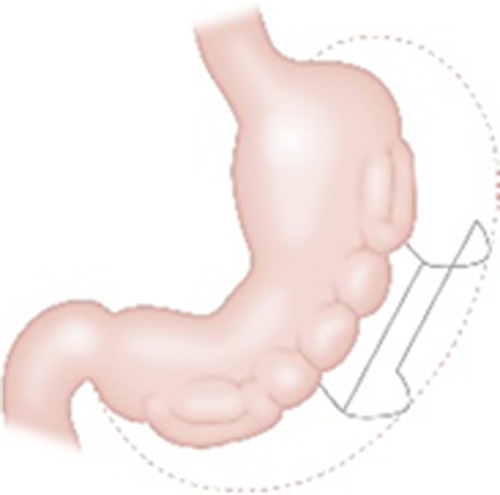
|
One of the applications of the Incisionless Operating Platform (IOP) Endoscopic plications of the fundus (traditional) or gastric body (Distal POSE/POSE 2.0) |
13.2% at 12-15 mo (traditional) 15%-17.5% TWL at 6-9 mo (Distal POSE/POSE 2.0) | 3.2% Chest pain, low-grade fever, extragastric bleeding, and hepatic abscess |
Cleared in 2006 for tissue apposition CE mark In the US clinical trial Pending FDA approval |
| Endoscopic Sutured/Sleeve Gastroplasty (ESG) (Apollo Endosurgery, Austin, TX) |
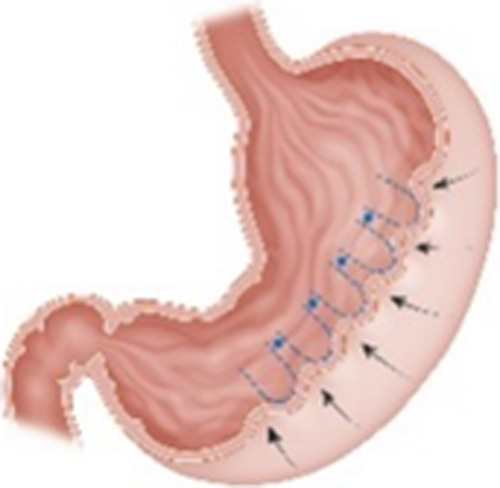
|
One of the applications of the Overstitch Endoscopic Suturing System Endoscopic suturing along the greater curvature of the stomach to create a sleeve-like structure |
16.5% TWL at 1 y9 | 2.2% Severe pain, nausea, GI bleeding, leak, fluid collection |
Cleared in 2008 for tissue apposition CE mark FDA approved in 2022 (BMI 30-50) |
| Delayed gastric emptying | |||||
| Transpyloric Shuttle (BAROnova Inc., Goleta, CA) |
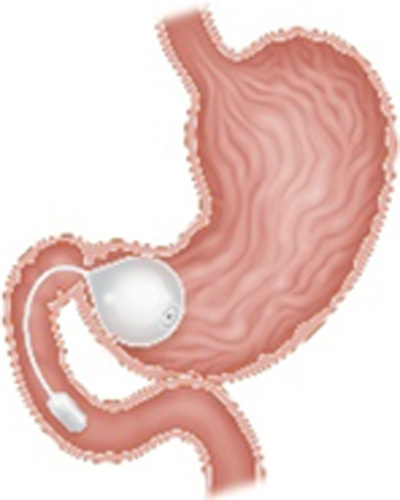
|
A spherical bulb tethered to a smaller cylindrical bulb Endoscopic placement and removal at 12 mo Located across the pylorus creating intermittent obstruction |
9.5% TWL at 1 y | 2.8% Device impaction, esophageal rupture, pneumothorax, pain, ulcer, vomiting |
FDA approved in 2019 BMI 30-40 kg/m2 |
| Gastric aspiration | |||||
| Aspiration Therapy (Aspire Bariatrics, King of Prussia, PA) |
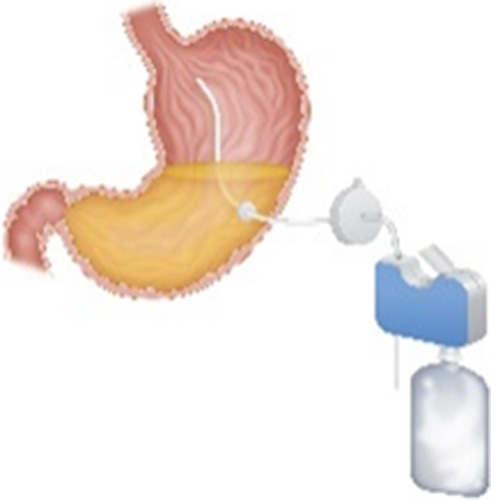
|
A 26-Fr gastrostomy tube with 15 cm internal fenestrated drainage catheter Endoscopic placement and removal Patients aspirate 25% to 30% of ingested calories 30 min after meals |
17.8% TWL at 1 y | 4.1% Buried bumper, peritonitis, severe pain, ulcer, product malfunction |
FDA approved in 2016, withdrawn 2021 CE mark BMI 35-55 kg/m2 Age 22 or older |
| Small bowel bypass | |||||
| Duodenal-Jejunal Bypass Liner (GI Dynamics, Boston, MA) |
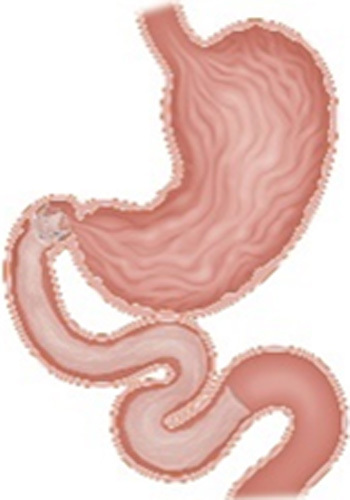
|
A 60-cm fluoropolymer liner anchored at the duodenal bulb and ending at the jejunum Endoscopic placement and removal at 12 mo |
Data pending pivotal trial |
NA | Not currently FDA approved CE mark under review In the US clinical trial |
| Duodenal Mucosal Resurfacing (Fractyl, Lexington, MA) |
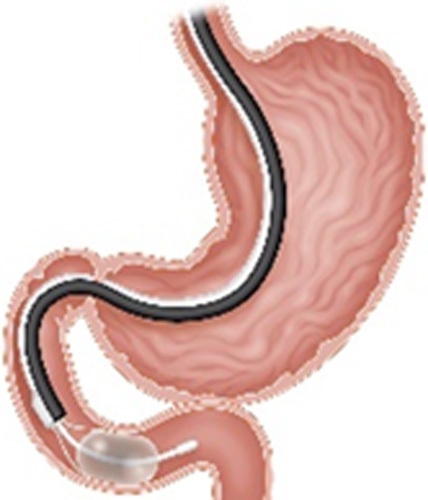
|
Endoscopic thermal ablation of the duodenal mucosa using a balloon filled with heated water | Data pending pivotal trial |
NA | Not currently FDA approved CE mark In the US clinical trial |
BMI indicates body mass index; CE, Conformitè Europëenne; EMBT, endoscopic metabolic and bariatric therapy; FDA, US Food and Drug Administration; GI, gastrointestinal; NA, not available; SAE, serious adverse event; TWL, total weight loss.
Adapted from Jirapinyo and Thompson.100 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Of the various EMBTs available, by far the most supportive evidence has been published for IGBs, with both randomized clinical trials and meta-analyses demonstrating statistically significant weight loss and relatively low rates of serious adverse events.101–106 The most commonly reported side effect and rationale for treatment discontinuation is nausea, with fluid-filled balloons tending to be slightly less well tolerated in this respect.107 In contrast, in one meta-analysis comparing fluid-filled and gas-filled IGBs, fluid-filled balloons were linked to statistically greater and more consistent weight loss than gas-filled balloons.99 Several IGBs have already been approved by the US Food and Drug Administration (FDA) and received a CE (Conformitè Europëenne) mark.
ESG—which involves endoscopic placement of full-thickness running sutures along the greater curvature of the stomach—is another approach employed to reduce stomach volume endoscopically. One system—called the OverStitch Endoscopic Suturing System (Apollo Endosurgery)—has achieved both FDA and CE mark approval. In several meta-analyses comparing ESG against the more-invasive laparoscopic sleeve gastrectomy, findings generally indicate less weight loss but a tendency (albeit, not statistically significant) towards fewer adverse events with the former.99,108–110 This said, meta-analysis authors have consistently recommended restricting the use of ESG to patients with mild to moderate (class I or II) obesity.98,99,108–110
Far less supportive evidence exists for gastric delay and gastric aspiration procedures and their use remains limited, though specific approaches to both procedures have received FDA approval (Table 1). To date, neither FDA nor CE mark approval has been afforded to any small bowel bypass procedure.
MBS
Despite the emergence of EMBT, over the past few decades, a growing body of evidence has established MBS as the most effective treatment for obesity, with respect to reducing weight, improving numerous comorbid conditions that have been empirically linked to BMI, enhancing overall patient quality of life, and decreasing patient mortality.111 Among the various surgical approaches that are currently in use, sleeve gastrectomy (SG) and RYGB are currently the most commonly performed worldwide, in that order, though newer procedures, like one-anastomosis gastric bypass,112 show promise. Which procedure is employed should largely be decided on a patient-by-patient basis, that decision influenced by various patient characteristics—for example, the evidence favours utilizing RYGB over SG in patients with GERD113–116—as well as by the operating surgeon’s level of experience with each surgical approach. Regardless of which operation is chosen, patients must be thoroughly assessed by a multidisciplinary team preoperatively to determine their suitability for surgery and identify any issues that may require addressing.
As stated previously, preoperative patient preparation for MBS involves ensuring that each patient has realistic goals and expectations pertaining to the benefits and potential problems that might arise from surgery and that all psychosocial and behavioral barriers to adherence are addressed. Patients also must be alerted to any nutritional deficiencies and have such deficiencies corrected preoperatively. Cessation of tobacco, alcohol, and drugs is mandatory and should be maintained lifelong.23 Patients also should be assessed for and instructed in an exercise program that they can realistically resume postoperatively. In addition, during a life-threatening pandemic like COVID-19, suitable precautions must be taken to protect patients with obesity awaiting and undergoing MBS, because they are particularly vulnerable to severe COVID symptoms and mortality.72–76
OUTCOMES AND FOLLOW-UP AFTER MBS
For MBS to be successful in enhancing patient health appreciably and long-term, both patients and their health care providers need to make a lifelong commitment to ongoing treatment and monitoring. This includes patients being monitored closely throughout the perioperative period for perioperative complications; then followed, essentially for the remainder of their life, preferably by the multidisciplinary obesity management team thus far involved in their assessment and management. This is because MBS alters so many facets of their life and physiology, potentially impacting them physically, psychologically, and socially. Some of these changes (eg, weight loss, enhanced diabetes control) are desirable; while others (eg, food intolerances, gastrointestinal discomfort, loose skin) are not. After MBS, for example, patients have an increased risk of developing such conditions as gallstones,117–120 gout,121–124 and nephrolithiasis.125–128 Nutritional deficits also may develop, some of them potentially catastrophic, including but not limited to central and peripheral nervous system disorders,129,130 severe protein malnutrition,62,131 osteoporosis and osteomalacia secondary to both rapid weight loss and vitamin D deficiency,132–135 iron-deficiency anemia,136,137 and immunocompromise.138 Such deficiencies have been documented to occur in as many 87% and 70% of patients undergoing RYGB and SG, respectively.139,140 Consequently, besides monitoring, postoperative follow-up needs to include ensuring that patients adhere to nutritional guidelines and to taking vitamin and mineral supplements, as prescribed. Lifelong abstinence from tobacco, alcohol, and all recreational drugs also must be emphasized.
Ongoing changes may need to be made to patients’ medications and other treatments, as well, for a variety of reasons that include (a) either improvement or complete resolution of certain obesity-associated comorbidities—like reduced or eliminated insulin requirements for T2DM, and changes in night-time continuous positive airway pressure settings for obstructive sleep apnea; and (b) anatomical changes induced by both MBS and EMBT that can appreciably alter the absorption of certain pharmaceuticals. Consequently, before MBS, medications that might be impacted by surgery need to be identified by the obesity management team. Then, after MBS and before patients’ discharge from the hospital, clear instructions on required postoperative medication changes and monitoring must be communicated both to patients themselves and to their primary physicians. Moreover, even if comorbid conditions appear to resolve postoperatively, patients must continue to be monitored for them lifelong, since disease recurrence may occur, sometimes independent of the patient’s weight loss trajectory.
Also as stated above, UGI endoscopic evaluation is recommended in patients with a history of reflux disease and in those undergoing gastric bypass surgery, both preoperatively and every 5 years postoperatively. Since obesity is a risk factor for 13 different types of cancer, MBS patients also must continue to be screened for cancer postoperatively, in accordance with national guidelines. Nutritional intake, activity levels, adherence with multivitamin and mineral supplements, current weight, and both comorbidity assessments and blood tests should be done annually by the obesity management team.
Once a patient has undergone MBS, the center where the surgery was conducted needs to, thus, relay a comprehensive postoperative health management plan to primary care providers,23 which must include which procedures, blood tests, and long-term vitamin supplements are required, any medication changes and/or monitoring that may be necessary, and when patients should be referred back to the MBS center. Reasons for referral back to the MBS center or to a local specialist include persistent gastrointestinal symptoms, nutritional issues, pregnancy, a need for psychological support, appreciable weight regain, and other medical issues requiring bariatric care.
With respect to weight regain, it is crucial that patients and their primary health care providers understand that some degree of weight regain is typical,141 especially after 2 years postoperatively, and that even appreciable weight regain must never be considered treatment “failure,” as such a perception can exert detrimental effects on patients’ self-perception, motivation to continue treatment, compliance with further monitoring and treatment and, ultimately, their health outcomes.142,143 Instead, just like patients who experience disease recurrence after cancer therapy, patients presenting with significant weight regain after MBS require an extensive evaluation, including anatomical studies (eg, UGI endoscopy, UGI barium studies) and being assessed by the multidisciplinary team.144,145
Finally, weight regain is not the only clinical outcome that can warrant investigation after MBS. For example, patients presenting with GERD symptoms, with or without weight regain after MBS, also require an objective assessment to identify or rule out GERD, including pH studies with or without manometry.146
CONCLUSIONS
Obesity has been called the world’s most extensive pandemic, and its prevalence, distribution, and costs continue to rise. To stem this rising tide of obesity and its numerous complications and costs, health care providers, insurers, and public officials must now work together, systematically, to increase public awareness about both the adverse health risks associated with obesity and the potential amelioration of such risks achieved when nonoperative and operative therapy are combined. They also must work to eliminate the stigma associated with obesity, since such stigmatization can prevent individuals from seeking appropriate treatment and from adhering to such treatment once sought. This requires that everyone recognizes and treats obesity as the chronic disease it is now known to be, using a multidisciplinary team approach like that used for other chronic diseases, like diabetes, heart disease, and cancer. It is only through such concerted efforts that the steadily worsening obesity pandemic can be reversed.
Footnotes
The authors declare that they have nothing to disclose.
These guidelines are being co-published by Springer Nature (Obesity Surgery, doi 10.1007/s11695-023-06757-2) and Wolters Kluwer (Journal of Clinical Gastroenterology, doi 10.1097/MCG.0000000000001916).
REFERENCES
- 1. Bray GA, Frühbeck G, Ryan DH, et al. Management of obesity. Lancet. 2016;387:1947–1956. [DOI] [PubMed] [Google Scholar]
- 2. Rueda-Clausen CF, Poddar M, Lear A, et al. Canadian Adult Obesity Clinical Practice Guidelines: assessment of people living with Obesity 2020;192:E875–E891. [Google Scholar]
- 3. Abdullah A, Peeters A, de Courten M, et al. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89:309–319. [DOI] [PubMed] [Google Scholar]
- 4. Maggio CA, Pi-Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin North Am. 2003;32:805–822; viii. [DOI] [PubMed] [Google Scholar]
- 5. Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126:1477–1500. [DOI] [PubMed] [Google Scholar]
- 6. van Dis I, Kromhout D, Geleijnse JM, et al. Body mass index and waist circumference predict both 10-year nonfatal and fatal cardiovascular disease risk: study conducted in 20,000 Dutch men and women aged 20-65 years. Eur J Cardiovasc Prev Rehabil. 2009;16:729–734. [DOI] [PubMed] [Google Scholar]
- 7. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–1770. [DOI] [PubMed] [Google Scholar]
- 8. Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meurling IJ, Shea DO, Garvey JF. Obesity and sleep: a growing concern. Curr Opin Pulm Med. 2019;25:602–608. [DOI] [PubMed] [Google Scholar]
- 10. Crummy F, Piper AJ, Naughton MT. Obesity and the lung: 2. Obesity and sleep-disordered breathing. Thorax. 2008;63:738–746. [DOI] [PubMed] [Google Scholar]
- 11. Lakkis JI, Weir MR. Obesity and kidney diseASE. Prog Cardiovasc Dis. 2018;61:157–167. [DOI] [PubMed] [Google Scholar]
- 12. Silva Junior GB, Bentes AC, Daher EF, et al. Obesity and kidney disease. J Bras Nefrol. 2017;39:65–69. [DOI] [PubMed] [Google Scholar]
- 13. Avgerinos KI, Spyrou N, Mantzoros CS, et al. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. [DOI] [PubMed] [Google Scholar]
- 14. Colditz GA, Peterson LL. Obesity and cancer: evidence, impact, and future directions. Clin Chem. 2018;64:154–162. [DOI] [PubMed] [Google Scholar]
- 15. Ho JSY, Fernando DI, Chan MY, et al. Obesity in COVID-19: a systematic review and meta-analysis. Ann Acad Med Singap. 2020;49:996–1008. [PubMed] [Google Scholar]
- 16. Poly TN, Islam MM, Yang HC, et al. Obesity and mortality among patients diagnosed with COVID-19: a systematic review and meta-analysis. Front Med (Lausanne). 2021;8:620044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sales-Peres SHC, de Azevedo-Silva LJ, Bonato RCS, et al. Coronavirus (SARS-CoV-2) and the risk of obesity for critically illness and ICU admitted: meta-analysis of the epidemiological evidence. Obes Res Clin Pract. 2020;14:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Y, Lu Y, Huang YM, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NCD-Risk-Factor-Collaboration . Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. NCD-Risk-Factor-Collaboration . Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NCD-Risk-Factor-Collaboration . Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. Lancet. 2020;396:1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kow L, O’Kane M, White KP, et al. Methodology and results of a joint IFSO-WGO Delphi Survey of 94 intercontinental, interdisciplinary experts in obesity management 2023. [publication pending]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. [DOI] [PubMed] [Google Scholar]
- 25. Vega GL. Obesity and the metabolic syndrome. Minerva Endocrinol. 2004;29:47–54. [PubMed] [Google Scholar]
- 26. Scheen AJ, Luyckx FH. Obesity and liver disease. Best Pract Res Clin Endocrinol Metab. 2002;16:703–716. [DOI] [PubMed] [Google Scholar]
- 27. Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28(suppl 1):68–76. [DOI] [PubMed] [Google Scholar]
- 28. Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48:97–113. [DOI] [PubMed] [Google Scholar]
- 29. Bonfrate L, Wang DQ, Garruti G, et al. Obesity and the risk and prognosis of gallstone disease and pancreatitis. Best Pract Res Clin Gastroenterol. 2014;28:623–635. [DOI] [PubMed] [Google Scholar]
- 30. Cruz-Monserrate Z, Conwell DL, Krishna SG. The impact of obesity on gallstone disease, acute pancreatitis, and pancreatic cancer. Gastroenterol Clin North Am. 2016;45:625–637. [DOI] [PubMed] [Google Scholar]
- 31. Nimeri AA, Gamaleldin MM, McKenna KL, et al. Reduction of venous thromboembolism in surgical patients using a mandatory risk-scoring system: 5-year follow-up of an American College of Surgeons National Surgical Quality Improvement Program. Clin Appl Thromb Hemost. 2017;23:392–396. [DOI] [PubMed] [Google Scholar]
- 32. Rodrigues AFS, Korkes F, Bezerra DSD, et al. Impact of bariatric surgery in patients with stress urinary incontinence. Einstein (Sao Paulo). 2021;19:eAO5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheridan W, Da Silva AS, Leca BM, et al. Weight loss with bariatric surgery or behaviour modification and the impact on female obesity-related urine incontinence: a comprehensive systematic review and meta-analysis. Clinical Obes. 2021;11:e12450. [DOI] [PubMed] [Google Scholar]
- 34. Kalyvas A, Neromyliotis E, Koutsarnakis C, et al. A systematic review of surgical treatments of idiopathic intracranial hypertension (IIH). Neurosurg Rev. 2021;44:773–792. [DOI] [PubMed] [Google Scholar]
- 35. Mollan SP, Aguiar M, Evison F, et al. The expanding burden of idiopathic intracranial hypertension. Eye. 2019;33:478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blagojevic M, Jinks C, Jeffery A, et al. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. [DOI] [PubMed] [Google Scholar]
- 37. Gadalla TM. Association of obesity with mood and anxiety disorders in the adult general population. Chronic Dis Can. 2009;30:29–36. [PubMed] [Google Scholar]
- 38. Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev. 2001;2:219–229. [DOI] [PubMed] [Google Scholar]
- 39. Kushner RF, Foster GD. Obesity and quality of life. Nutrition. 2000;16:947–952. [DOI] [PubMed] [Google Scholar]
- 40. Mannucci E, Petroni ML, Villanova N, et al. Clinical and psychological correlates of health-related quality of life in obese patients. Health Qual Life Outcomes. 2010;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riazi A, Shakoor S, Dundas I, et al. Health-related quality of life in a clinical sample of obese children and adolescents. Health Qual Life Outcomes. 2010;8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Angrisani L, Santonicola A, Iovino P, et al. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:2279–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. [DOI] [PubMed] [Google Scholar]
- 44. Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66 (suppl 2):7–12. [DOI] [PubMed] [Google Scholar]
- 45. Reynolds CL, Byrne SM, Hamdorf JM. Treatment success: investigating clinically significant change in quality of life following bariatric surgery. Obes Surg. 2017;27:1842–1848. [DOI] [PubMed] [Google Scholar]
- 46. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. [DOI] [PubMed] [Google Scholar]
- 47. Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: cosponsored by american association of clinical endocrinologists/american college of endocrinology, the obesity society, american society for metabolic & bariatric surgery, obesity medicine association, and american society of anesthesiologists—executive summary. Endocr Pract. 2019;25:1346–1359. [DOI] [PubMed] [Google Scholar]
- 48. National Institute for Health and Care-Excellence . NICE CG189 Obesity: identification, assessment and management of overweight and obesity in children, young people and adults; 2014. Accessed July 26, 2023. http://www.nice.org.uk/guidance/cg189 .
- 49. Mechanick JI, Apovian C, Brethauer S, et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity (Silver Spring). 2020;28:O1–O58. [DOI] [PubMed] [Google Scholar]
- 50. Aarts MA, Sivapalan N, Nikzad SE, et al. Optimizing bariatric surgery multidisciplinary follow-up: a focus on patient-centered care. Obes Surg. 2017;27:730–736. [DOI] [PubMed] [Google Scholar]
- 51. Fletcher PC, Kenny PJ. Food addiction: a valid concept? Neuropsychopharmacology. 2018;43:2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heinberg LJ, Ashton K, Coughlin J. Alcohol and bariatric surgery: review and suggested recommendations for assessment and management. Surg Obes Relat Dis. 2012;8:357–363. [DOI] [PubMed] [Google Scholar]
- 53. Bordignon S, Aparício MJG, Bertoletti J, et al. Personality characteristics and bariatric surgery outcomes: a systematic review. Trends Psychiatry Psychother. 2017;39:124–134. [DOI] [PubMed] [Google Scholar]
- 54. Kirk S, Ramos Salas X, Alberga, et al. Canadian Adult Obesity Clinical Practice Guidelines: Reducing weight bias, stigma and discrimination in obesity management, practice and policy; 2021. Accessed December 18, 2023. https://obesitycanada.ca/guidelines/weightbias/.
- 55. Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875–E891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring). 2009;17:941–964. [DOI] [PubMed] [Google Scholar]
- 57. Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: is anyone immune? Int J Obes Relat Metab Disord. 2001;25:1525–1531. [DOI] [PubMed] [Google Scholar]
- 58. Vallis MT, Currie B, Lawlor D, et al. Healthcare professional bias against the obese: how do we know if we have a problem? Can J Diab. 2007;31:365–370. [Google Scholar]
- 59. Parrott J, Frank L, Rabena R, et al. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis. 2017;13:727–741. [DOI] [PubMed] [Google Scholar]
- 60. Poitou Bernert C, Ciangura C, Coupaye M, et al. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33:13–24. [DOI] [PubMed] [Google Scholar]
- 61. Stroh C, Manger T, Benedix F. Metabolic surgery and nutritional deficiencies. Minerva Chir. 2017;72:432–441. [DOI] [PubMed] [Google Scholar]
- 62. Ziegler O, Sirveaux MA, Brunaud L, et al. Medical follow up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab. 2009;35:544–557. [DOI] [PubMed] [Google Scholar]
- 63. O’Kane M, Parretti HM, Pinkney J, et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery—2020 update. Obes Rev. 2020;21:e13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parrott JM, Craggs-Dino L, Faria SL, et al. The optimal nutritional programme for bariatric and metabolic surgery. Curr Obes Rep. 2020;9:326–338. [DOI] [PubMed] [Google Scholar]
- 65. Quilliot D, Coupaye M, Ciangura C, et al. Recommendations for nutritional care after bariatric surgery: recommendations for best practice and SOFFCO-MM/AFERO/SFNCM/expert consensus. J Visc Surg. 2021;158:51–61. [DOI] [PubMed] [Google Scholar]
- 66. Sherf-Dagan S, Sinai T, Goldenshluger A, et al. Nutritional assessment and preparation for adult bariatric surgery candidates: clinical practice. Adv Nutr. 2021;12:1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hassannejad A, Khalaj A, Mansournia MA, et al. The effect of aerobic or aerobic-strength exercise on body composition and functional capacity in patients with BMI ≥35 after bariatric surgery: a randomized control trial. Obes Surg. 2017;27:2792–2801. [DOI] [PubMed] [Google Scholar]
- 68. Jakicic JM, Davis KK. Obesity and physical activity. Psychiatr Clin North Am. 2011;34:829–840. [DOI] [PubMed] [Google Scholar]
- 69. Gusso G, Lopes JMC. Tratado de medicina de família e comunidade: princípios, formação e prática v I/Treaty of Family and Community Medicine: Principles, Practice and Training v I. Artmed; 2012:1417–1427. [Google Scholar]
- 70. Lau DC, Douketis JD, Morrison KM, et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ. 2007;176:S1–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Parikh M, Liu J, Vieira D, et al. Preoperative endoscopy prior to bariatric surgery: a systematic review and meta-analysis of the literature. Obes Surg. 2016;26:2961–2966. [DOI] [PubMed] [Google Scholar]
- 72. Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105:dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sattar N, Ho FK, Gill JM, et al. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: preliminary findings from UK biobank. Diabetes Metab Syndr. 2020;14:1149–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goel R, Nasta AM, Goel M, et al. Complications after bariatric surgery: a multicentric study of 11,568 patients from Indian bariatric surgery outcomes reporting group. J Minim Access Surg. 2021;17:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93:S89–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Marczuk P, Kubisa MJ, Święch M, et al. Effectiveness and safety of Roux-en-Y gastric bypass in elderly patients-systematic review and meta-analysis. Obes Surg. 2019;29:361–368. [DOI] [PubMed] [Google Scholar]
- 80. Giordano S, Victorzon M. Bariatric surgery in elderly patients: a systematic review. Clin Interv Aging. 2015;10:1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38:10–47. [DOI] [PubMed] [Google Scholar]
- 82. Chow A, Switzer NJ, Gill RS, et al. Roux-en-Y gastric bypass in the elderly: a systematic review. Obes Surg. 2016;26:626–630. [DOI] [PubMed] [Google Scholar]
- 83. Domienik-Karłowicz J, Pruszczyk P, Lisik W. Bariatric Surgery in the elderly patient: safety and short-time outcome. a case match analysis: Letter to the Editor. Obes Surg. 2019;29:1658. [DOI] [PubMed] [Google Scholar]
- 84. Gray KD, Moore MD, Bellorin O, et al. Increased metabolic benefit for obese, elderly patients undergoing Roux-en-Y gastric bypass vs sleeve gastrectomy. Obes Surg. 2018;28:636–642. [DOI] [PubMed] [Google Scholar]
- 85. Shenoy SS, Gilliam A, Mehanna A, et al. Laparoscopic sleeve gastrectomy versus laparoscopic roux-en-y gastric bypass in elderly bariatric patients: safety and efficacy—a systematic review and meta-analysis. Obes Surg. 2020;30:4467–4473. [DOI] [PubMed] [Google Scholar]
- 86. Kaplan U, Penner S, Farrokhyar F, et al. Bariatric surgery in the elderly is associated with similar surgical risks and significant long-term health benefits. Obes Surg. 2018;28:2165–2170. [DOI] [PubMed] [Google Scholar]
- 87. Balasundaram P, Krishna S. StatPearls [Internet] . Obesity effects on child health. Treasure Island (FL): StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- 88. Gurnani M, Birken C, Hamilton J. Childhood obesity: causes, consequences, and management. Pediatr Clin North Am. 2015;62:821–840. [DOI] [PubMed] [Google Scholar]
- 89. Sahoo K, Sahoo B, Choudhury AK, et al. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gordon-Larsen P, Adair LS, Nelson MC, et al. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. Am J Clin Nutr. 2004;80:569–575. [DOI] [PubMed] [Google Scholar]
- 91. Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289:187–193. [DOI] [PubMed] [Google Scholar]
- 92. Poliakin L, Roberts A, Thompson KJ, et al. Outcomes of adolescents compared with young adults after bariatric surgery: an analysis of 227,671 patients using the MBSAQIP data registry. Surg Obes Relat Dis. 2020;16:1463–1473. [DOI] [PubMed] [Google Scholar]
- 93. ASMBS Clinical Issues Committee . Peri-operative management of obstructive sleep apnea. Surg Obes Relat Dis. 2012;8:e27–e32. [DOI] [PubMed] [Google Scholar]
- 94. Pinhas-Hamiel O, Hamiel U, Bendor CD, et al. The global spread of severe obesity in toddlers, children, and adolescents: a systematic review and meta-analysis. Obes Facts. 2022;15:118–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Malhotra S, Czepiel KS, Akam EY, et al. Bariatric surgery in the treatment of adolescent obesity: current perspectives in the United States. Expert Rev Endocrinol Metab. 2021;16:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jirapinyo P, Thompson CC. Endoscopic bariatric and metabolic therapies: surgical analogues and mechanisms of action. Clin Gastroenterol Hepatol. 2017;15:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic bariatric and metabolic therapies: new and emerging technologies. Gastroenterology. 2017;152:1791–1801. [DOI] [PubMed] [Google Scholar]
- 98. Sharaiha RZ, Hajifathalian K, Kumar R, et al. Five-year outcomes of endoscopic sleeve gastroplasty for the treatment of obesity. Clin Gastroenterol Hepatol. 2021;19:1051.e2–1057.e2. [DOI] [PubMed] [Google Scholar]
- 99. Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, et al. Efficacy and safety of endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18:1043.e4–1053.e4. [DOI] [PubMed] [Google Scholar]
- 100. Jirapinyo P, Thompson CC. Obesity primer for the practicing gastroenterologist. Am J Gastroenterol. 2021;116:918–934. [DOI] [PubMed] [Google Scholar]
- 101. Alsabah S, Al Haddad E, Ekrouf S, et al. The safety and efficacy of the procedureless intragastric balloon. Surg Obes Relat Dis. 2018;14:311–317. [DOI] [PubMed] [Google Scholar]
- 102. Bazerbachi F, Haffar S, Sawas T, et al. Fluid-filled versus gas-filled intragastric balloons as obesity interventions: a network meta-analysis of randomized trials. Obes Surg. 2018;28:2617–2625. [DOI] [PubMed] [Google Scholar]
- 103. Ienca R, Al Jarallah M, Caballero A, et al. The procedureless elipse gastric balloon program: multicenter experience in 1770 consecutive patients. Obes Surg. 2020;30:3354–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jamal MH, Almutairi R, Elabd R, et al. The safety and efficacy of procedureless gastric balloon: a study examining the effect of elipse intragastric balloon safety, short and medium term effects on weight loss with 1-year follow-up post-removal. Obes Surg. 2019;29:1236–1241. [DOI] [PubMed] [Google Scholar]
- 105. Kumar N, Bazerbachi F, Rustagi T, et al. The influence of the Orbera intragastric balloon filling volumes on weight loss, tolerability, and adverse events: a systematic review and meta-analysis. Obes Surg. 2017;27:2272–2278. [DOI] [PubMed] [Google Scholar]
- 106. Stavrou G, Shrewsbury A, Kotzampassi K. Six intragastric balloons: which to choose? World J Gastrointest Endosc. 2021;13:238–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Trang J, Lee SS, Miller A, et al. Incidence of nausea and vomiting after intragastric balloon placement in bariatric patients—a systematic review and meta-analysis. Int J Surg. 2018;57:22–29. [DOI] [PubMed] [Google Scholar]
- 108. Jalal MA, Cheng Q, Edye MB. Systematic review and meta-analysis of endoscopic sleeve gastroplasty with comparison to laparoscopic sleeve gastrectomy. Obes Surg. 2020;30:2754–2762. [DOI] [PubMed] [Google Scholar]
- 109. Marincola G, Gallo C, Hassan C, et al. Laparoscopic sleeve gastrectomy versus endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Endosc Int Open. 2021;9:E87–E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yoon JY, Arau RT. The efficacy and safety of endoscopic sleeve gastroplasty as an alternative to laparoscopic sleeve gastrectomy. Clin Endosc. 2021;54:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Carlsson LMS, Sjöholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rutledge R. The mini-gastric bypass: experience with the first 1274 cases. Obes Surg. 2001;11:276–280. [DOI] [PubMed] [Google Scholar]
- 113. Mejía-Rivas MA, Herrera-López A, Hernández-Calleros J, et al. Gastroesophageal reflux disease in morbid obesity: the effect of Roux-en-Y gastric bypass. Obes Surg. 2008;18:1217–1224. [DOI] [PubMed] [Google Scholar]
- 114. Qumseya BJ, Qumsiyeh Y, Ponniah SA, et al. Barrett’s esophagus after sleeve gastrectomy: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:343.e2–352.e2. [DOI] [PubMed] [Google Scholar]
- 115. Sebastianelli L, Benois M, Vanbiervliet G, et al. Systematic endoscopy 5 years after sleeve gastrectomy results in a high rate of barrett’s esophagus: results of a multicenter study. Obes Surg. 2019;29:1462–1469. [DOI] [PubMed] [Google Scholar]
- 116. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015;21:10348–10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Morais M, Faria G, Preto J, et al. Gallstones and bariatric surgery: to treat or not to treat? World J Surg. 2016;40:2904–2910. [DOI] [PubMed] [Google Scholar]
- 118. Mulliri A, Menahem B, Alves A, et al. Ursodeoxycholic acid for the prevention of gallstones and subsequent cholecystectomy after bariatric surgery: a meta-analysis of randomized controlled trials. J Gastroenterol. 2022;57:529–539. [DOI] [PubMed] [Google Scholar]
- 119. Sneineh MA, Harel L, Elnasasra A, et al. Increased incidence of symptomatic cholelithiasis after bariatric Roux-En-Y gastric bypass and previous bariatric surgery: a single center experience. Obes Surg. 2020;30:846–850. [DOI] [PubMed] [Google Scholar]
- 120. Talha A, Abdelbaki T, Farouk A, et al. Cholelithiasis after bariatric surgery, incidence, and prophylaxis: randomized controlled trial. Surg Endosc. 2020;34:5331–5337. [DOI] [PubMed] [Google Scholar]
- 121. Antozzi P, Soto F, Arias F, et al. Development of acute gouty attack in the morbidly obese population after bariatric surgery. Obes Surg. 2005;15:405–407. [DOI] [PubMed] [Google Scholar]
- 122. Friedman JE, Dallal RM, Lord JL. Gouty attacks occur frequently in postoperative gastric bypass patients. Surg Obes Relat Dis. 2008;4:11–13. [DOI] [PubMed] [Google Scholar]
- 123. Romero-Talamás H, Daigle CR, Aminian A, et al. The effect of bariatric surgery on gout: a comparative study. Surg Obes Relat Dis. 2014;10:1161–1165. [DOI] [PubMed] [Google Scholar]
- 124. Tana C, Busetto L, Di Vincenzo A, et al. Management of hyperuricemia and gout in obese patients undergoing bariatric surgery. Postgrad Med. 2018;130:523–535. [DOI] [PubMed] [Google Scholar]
- 125. Duffey BG, Pedro RN, Makhlouf A, et al. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg. 2008;206:1145–1153. [DOI] [PubMed] [Google Scholar]
- 126. Lieske JC, Mehta RA, Milliner DS, et al. Kidney stones are common after bariatric surgery. Kidney Int. 2015;87:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Thongprayoon C, Cheungpasitporn W, Vijayvargiya P, et al. The risk of kidney stones following bariatric surgery: a systematic review and meta-analysis. Ren Fail. 2016;38:424–430. [DOI] [PubMed] [Google Scholar]
- 128. Upala S, Jaruvongvanich V, Sanguankeo A. Risk of nephrolithiasis, hyperoxaluria, and calcium oxalate supersaturation increased after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. Surg Obes Relat Dis. 2016;12:1513–1521. [DOI] [PubMed] [Google Scholar]
- 129. Algahtani HA, Khan AS, Khan MA, et al. Neurological complications of bariatric surgery. Neurosciences (Riyadh). 2016;21:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Alligier M, Borel AL, Savey V, et al. A series of severe neurologic complications after bariatric surgery in France: the NEUROBAR Study. Surg Obes Relat Dis. 2020;16:1429–1435. [DOI] [PubMed] [Google Scholar]
- 131. Bal BS, Finelli FC, Shope TR, et al. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544–556. [DOI] [PubMed] [Google Scholar]
- 132. Hadi YB, Mann R, Sohail AH, et al. Metabolic bone disease and fracture risk after gastric bypass and sleeve gastrectomy: comparative analysis of a multi-institutional research network. Surg Obes Relat Dis. 2022;18:604–609. [DOI] [PubMed] [Google Scholar]
- 133. Krez AN, Stein EM. The skeletal consequences of bariatric surgery. Curr Osteoporos Rep. 2020;18:262–272. [DOI] [PubMed] [Google Scholar]
- 134. Paccou J, Caiazzo R, Lespessailles E, et al. Bariatric surgery and osteoporosis. Calcif Tissue Int. 2022;110:576–591. [DOI] [PubMed] [Google Scholar]
- 135. Paccou J, Tsourdi E, Meier C, et al. Bariatric surgery and skeletal health: a narrative review and position statement for management by the European Calcified Tissue Society (ECTS). Bone. 2022;154:116236. [DOI] [PubMed] [Google Scholar]
- 136. Ben-Porat T, Elazary R, Goldenshluger A, et al. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy-are supplements required for a lifetime? Surg Obes Relat Dis. 2017;13:1138–1144. [DOI] [PubMed] [Google Scholar]
- 137. Madhok BM, Mahawar KK, Hadfield JN, et al. Haematological indices and haematinic levels after mini gastric bypass: a matched comparison with Roux-en-Y gastric bypass. Clin Obes. 2018;8:43–49. [DOI] [PubMed] [Google Scholar]
- 138. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lombardo M, Franchi A, Padua E, et al. Potential nutritional deficiencies in obese subjects 5 years after bariatric surgery. Bariatr Surg Pract Patient Care. 2019;14:125–130. [Google Scholar]
- 140. Nicoletti A, Ponziani FR, Biolato M, et al. Intestinal permeability in the pathogenesis of liver damage: from non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol. 2019;25:4814–4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shiau J, Biertho L. Canadian Adult Obesity Clinical Practice Guidelines: Bariatric Surgery: Postoperative Management; 2020. Accessed December 18, 2023. https://obesitycanada.ca/guidelines/postop/ .
- 142. Trainer S, Benjamin T. Elective surgery to save my life: rethinking the “choice” in bariatric surgery. J Adv Nurs. 2017;73:894–904. [DOI] [PubMed] [Google Scholar]
- 143. Vallis MT, Macklin D, Russell-Mayhew S. Canadian Adult Obesity Clinical Practice Guidelines: Effective psychological and behavioural interventions in obesity management; 2020.
- 144. Cornejo-Pareja I, Molina-Vega M, Gómez-Pérez AM, et al. Factors related to weight loss maintenance in the medium-long term after bariatric surgery: a review. J Clin Med. 2021;10:1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Eisenberg D, Noria S, Grover B, et al. ASMBS position statement on weight bias and stigma. Surg Obes Relat Dis. 2019;15:814–821. [DOI] [PubMed] [Google Scholar]
- 146. Soricelli E, Casella G, Baglio G, et al. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surg Obes Relat Dis. 2018;14:751–756. [DOI] [PubMed] [Google Scholar]


