Abstract
Background
Tumor-devitalized autografts treated with deep freezing, pasteurization, and irradiation are biological reconstruction methods after tumor excision for aggressive or malignant bone or soft tissue tumors that involve a major long bone. Tumor-devitalized autografts do not require a bone bank, they carry no risk of viral or bacterial disease transmission, they are associated with a smaller immunologic response, and they have a better shape and size match to the site in which they are implanted. However, they are associated with disadvantages as well; it is not possible to assess margins and tumor necrosis, the devitalized bone is not normal and has limited healing potential, and the biomechanical strength is decreased owing to processing and tumor-related bone loss. Because this technique is not used in many countries, there are few reports on the results of this procedure such as complications, graft survival, and limb function.
Questions/purposes
(1) What was the rate of complications such as fracture, nonunion, infection, or recurrence in a tumor-devitalized autograft treated with deep freezing, pasteurization, and irradiation, and what factors were associated with the complication? (2) What were the 5-year and 10-year grafted bone survival (free from graft bone removal) of the three methods used to devitalize a tumor-containing autograft, and what factors were associated with grafted bone survival? (3) What was the proportion of patients with union of the tumor-devitalized autograft and what factors were associated with union of the graft-host bone junction? (4) What was the limb function after the tumor-devitalized autograft, and what factors were related to favorable limb function?
Methods
This was a retrospective, multicenter, observational study that included data from 26 tertiary sarcoma centers affiliated with the Japanese Musculoskeletal Oncology Group. From January 1993 to December 2018, 494 patients with benign or malignant tumors of the long bones were treated with tumor-devitalized autografts (using deep freezing, pasteurization, or irradiation techniques). Patients who were treated with intercalary or composite (an osteoarticular autograft with a total joint arthroplasty) tumor-devitalized autografts and followed for at least 2 years were considered eligible for inclusion. Accordingly, 7% (37 of 494) of the patients were excluded because they died within 2 years; in 19% (96), an osteoarticular graft was used, and another 10% (51) were lost to follow-up or had incomplete datasets. We did not collect information on those who died or were lost to follow-up. Considering this, 63% of the patients (310 of 494) were included in the analysis. The median follow-up was 92 months (range 24 to 348 months), the median age was 27 years (range 4 to 84), and 48% (148 of 310) were female; freezing was performed for 47% (147) of patients, pasteurization for 29% (89), and irradiation for 24% (74). The primary endpoints of this study were the cumulative incidence rate of complications and the cumulative survival of grafted bone, assessed by the Kaplan-Meier method. We used the classification of complications and graft failures proposed by the International Society of Limb Salvage. Factors relating to complications and grafted autograft removal were analyzed. The secondary endpoints were the proportion of bony union and better limb function, evaluated by the Musculoskeletal Tumor Society score. Factors relating to bony union and limb function were also analyzed. Data were investigated in each center by a record review and transferred to Kanazawa University.
Results
The cumulative incidence rate of any complication was 42% at 5 years and 51% at 10 years. The most frequent complications were nonunion in 36 patients and infection in 34 patients. Long resection (≥ 15 cm) was associated with an increased risk of any complication based on the multivariate analyses (RR 1.8 [95% CI 1.3 to 2.5]; p < 0.01). There was no difference in the rate of complications among the three devitalizing methods. The cumulative graft survival rates were 87% at 5 years and 81% at 10 years. After controlling for potential confounding variables including sex, resection length, reconstruction type, procedure type, and chemotherapy, we found that long resection (≥ 15 cm) and composite reconstruction were associated with an increased risk of grafted autograft removal (RR 2.5 [95% CI 1.4 to 4.5]; p < 0.01 and RR 2.3 [95% CI 1.3 to 4.1]; p < 0.01). The pedicle freezing procedure showed better graft survival than the extracorporeal devitalizing procedures (94% versus 85% in 5 years; RR 3.1 [95% CI 1.1 to 9.0]; p = 0.03). No difference was observed in graft survival among the three devitalizing methods. Further, 78% (156 of 200 patients) of patients in the intercalary group and 87% (39 of 45 patients) of those in the composite group achieved primary union within 2 years. Male sex and the use of nonvascularized grafts were associated with an increased risk of nonunion (RR 2.8 [95% CI 1.3 to 6.1]; p < 0.01 and 0.28 [95% CI 0.1 to 1.0]; p = 0.04, respectively) in the intercalary group after controlling for confounding variables, including sex, site, chemotherapy, resection length, graft type, operation time, and fixation type. The median Musculoskeletal Tumor Society score was 83% (range 12% to 100%). After controlling for confounding variables including age, site, resection length, event occurrence, and graft removal, age younger than 40 years (RR 2.0 [95% CI 1.1 to 3.7]; p = 0.03), tibia (RR 6.9 [95% CI 2.7 to 17.5]; p < 0.01), femur (RR 4.8 [95% CI 1.9 to 11.7]; p < 0.01), no event (RR 2.2 [95% CI 1.1 to 4.5]; p = 0.03), and no graft removal (RR 2.9 [95% CI 1.2 to 7.3]; p = 0.03) were associated with an increased limb function. The composite graft was associated with decreased limb function (RR 0.4 [95% CI 0.2 to 0.7]; p < 0.01).
Conclusion
This multicenter study revealed that frozen, irradiated, and pasteurized tumor-bearing autografts had similar rates of complications and graft survival and all resulted in similar limb function. The recurrence rate was 10%; however, no tumor recurred with the devitalized autograft. The pedicle freezing procedure reduces the osteotomy site, which may contribute to better graft survival. Furthermore, tumor-devitalized autografts had reasonable survival and favorable limb function, which are comparable to findings reported for bone allografts. Overall, tumor-devitalized autografts are a useful option for biological reconstruction and are suitable for osteoblastic tumors or osteolytic tumors without severe loss of mechanical bone strength. Tumor-devitalized autografts could be considered when obtaining allografts is difficult and when a patient is unwilling to have a tumor prosthesis and allograft for various reasons such as cost or socioreligious reasons.
Level of Evidence
Level III, therapeutic study.
Introduction
Advances in imaging modalities, multiagent chemotherapy, and surgical procedures have increased the prevalence of limb-sparing surgery in treating malignant bone tumors [5]. Endoprosthesis reconstruction can enable early mobilization and immediate weightbearing; however, they are associated with potential problems, including loosening, infection, and high cost. The survival rate of an endoprosthesis depends on the type of prosthesis and tumor location [29]. Biological reconstruction is a potentially longer-lasting alternative if the graft regenerates with new bone formation and revascularization. Vascularized autografts are a good choice for large intercalary bone defects in pediatric patients; however, this reconstruction is technically demanding and is associated with donor site morbidity [47]. Moreover, osteoarthritic changes, infection, fracture, and nonunion might occur in osteoarticular allografts, all of which can lead to further surgical procedures [41].
Several techniques are used for tumor-devitalized autografts, including extracorporeal radiation [9, 31], pasteurization [23, 24], autoclaving [21], and freezing [43, 46]. The advantages of recycling autografts include availability, no requirement for a bone bank, biological reconstruction, no viral or bacterial disease transmission, reattachment of preserved soft tissue and ligament, and use of available host bone stock [43]. When used in the osteoarticular setting, autograft-prosthesis composites have been used to avoid subchondral bone collapse and osteoarthritis, which may occur after devitalized osteoarticular autografts and might improve functional outcomes. Nonunion, fracture, infection, and loosening are the primary complications [14]. How these autografts survive in the longer term has not been determined in large numbers. Considering that this technique is not used in many countries, there are few reports on the outcomes of this procedure, such as complications, graft survival, and limb function. To address this gap, we reviewed our experience with a group of patients treated with different devitalization techniques.
We asked: (1) What was the rate of complications such as fracture, nonunion, infection, or recurrence in a tumor-devitalized autograft treated with deep freezing, pasteurization, and irradiation, and what factors were associated with the complication? (2) What were the 5-year and 10-year grafted bone survival (free from graft bone removal) of the three methods used to devitalize a tumor-containing autograft, and what factors were associated with grafted bone survival? (3) What was the proportion of patients with union of the tumor-devitalized autograft and what factors were associated with union of the graft-host bone junction? (4) What was the limb function after the tumor-devitalized autograft, and what factors were related to favorable limb function?
Patients and Methods
Study Design and Setting
This was a retrospective, multicenter, observational study that included data from 26 sarcoma centers affiliated with the Japanese Musculoskeletal Oncology Group. The data were investigated by chart reviews in each center and transferred to the department of orthopaedic surgery, Kanazawa University Graduate School of Medical Sciences. All analyses were performed there.
Participants
From January 1993 to December 2018, 494 patients with benign or malignant tumors that affected the long bones were treated with tumor-devitalized autografts (deep freezing, pasteurization, or irradiation). Patients who were treated with intercalary or composite (an osteoarticular autograft with a total joint arthroplasty) tumor-devitalized autografts and were followed for at least 2 years were considered eligible for inclusion. Accordingly, 7% (37 of 434) of patients were excluded because they died within 2 years, 19% (96) had an osteoarticular graft, and another 10% (51) were lost to follow-up or had incomplete datasets. The data were collected from July 2018 to September 2021. A questionnaire was distributed to each sarcoma center; it included age, sex, tumor location, histology, chemotherapy, tumor resection size, devitalization method (extracorporeal irradiation, pasteurization, or freezing [free or pedicle]), fixation methods of tumor-devitalized autografts, complications (modified Henderson classification) [17], the number and types of additional surgical procedures, time of graft union, Musculoskeletal Tumor Society (MSTS) functional score [13], and follow-up time. Because the questionnaire did not ask about patients who were lost to follow-up, we had no other information on these patients. Considering this, 63% (310) of patients were included in this study.
Descriptive Data
Overall, 52% (162 of 310) of patients were male with a median age of 27 years (range 4 to 84 years); patients were followed for a median of 92 months (range 24 to 348 months). Further, 72% (222 of 310) of tumors were primary malignant bone tumors and the most common histology was osteosarcoma (48% [150 of 310]), followed by chondrosarcoma (8% [25 of 310]), and Ewing sarcoma (5% [15 of 310]). Moreover, 22% (67 of 310) of tumors were soft tissue sarcomas, 6% (20 of 310) were metastatic tumors, and 2% (five of 310) were benign tumors. Regarding the types of devitalization, 47% (147 of 310) of patients underwent freezing, 29% (89 of 310) had pasteurization, and 24% (74 of 310) underwent the irradiation technique. According to the demographic characteristic analysis, the patients treated with each type of graft were similar in age, gender, resection length, and operative time, but follow-up was less in the freezing group, and these patients had more composite grafts than the other two groups. The tumor histology and use of chemotherapy also varied somewhat between the groups (Table 1).
Table 1.
Patient demographics and clinical data
| Demographics | Freezing (n = 147)a | Pasteurization (n = 89) | Irradiation (n = 74) | p value | |
| Exclusion Died within 2 years Lost to follow-up or incomplete datasets Osteoarticular graft |
8 (18) 12 (27) 15 (34) |
10 (13) 10 (13) 14 (18) |
4 (6) 8 (11) 33 (44) |
0.66b | |
| Median patient age in years | 24 (6-78) | 33 (4-78) | 37 (9-84) | 0.84c | |
| Median follow-up in months | 79 (25-264) | 106 (24-285) | 155 (28-348) | < 0.01c | |
| Median resection length in mm | 14 (5-35) | 13 (2-35) | 13 (3-25) | 0.10c | |
| Median operation time in hours | 6.6 (2.9-15.2) | 6.7 (2.8 -15.5) | 7.1 (3.1-14.5) | 0.17c | |
| Male | 56 (82) | 48 (43) | 50 (37) | 0.48b | |
| Histology | |||||
| Primary Benign Osteosarcoma Chondrosarcoma Ewing sarcoma Other malignant bone tumor Soft tissue sarcoma Metastatic |
1 (2) 56 (83) 8 (12) 3 (4) 10 (14) 12 (17) 10 (15) |
3 (3) 44 (39) 6 (5) 7 (6) 8 (7) 29 (26) 3 (3) |
0 38 (28) 11 (8) 7 (5) 9 (7) 32 (24) 3 (2) |
< 0.01b |
|
| Graft type Intercalary Composite |
65 (96) (5 hemicortical) 35 (51) |
82 (73) 18 (16) |
78 (58) (11 hemicortical) 22 (16) |
0.01b | |
| Site Humerus Radius Ulna Femur Tibia |
13 (19) 3 (4) 1(1) 54 (79) 30(44) |
13 (12) 1 (1) 0 39 (35) 46 (41) |
7 (5) 1 (1) 0 49 (36) 43 (32) |
0.14b | |
| Did not receive chemotherapy | 32 (47) | 55 (49) | 47 (35) | < 0.01b | |
| Bone graft No Nonvascularized graft Vascularized graft |
63 (93) 30 (44) 7 (10) |
63 (56) 27 (24) 10 (9) |
73 (54) 18 (13) 9 (7) |
0.33b |
Data presented as median (range) or % (n).
Patients who underwent freezing were further broken down into free (n = 93) and pedicle (n = 54) autografts.
Fisher exact test.
One-way ANOVA test.
Surgical Technique
Extracorporeal Irradiated Autograft
Any muscle around the tumor was removed after wide tumor resection, and the tumor tissues were curetted. Subsequently, the resected specimen was soaked in a sterile plastic container with antibiotic-containing saline and packaged into three sterile drapes to ensure sterilization during irradiation. The irradiation dose was 50 Gy in one fraction using a linac with 6‐ or 10‐MeV photons. After irradiation, the treated bone was rewashed with antibiotic-containing saline, reimplanted, and fixed with some implants [27].
Pasteurized Autograft
After resection, the surgical specimen was cleared of soft tissue, gross tumor, and the intraosseous macroscopic portion of the tumor. The bone was kept in preoperatively heated saline at 60°C for 30 minutes and subsequently retrieved, and then kept in saline at room temperature for approximately 10 minutes. The treated bone was placed in the original site and fixed with implants, with or without bone cement [24].
Frozen Autograft
Frozen autografts were devitalized using liquid nitrogen. Two types of freezing procedure techniques are available, depending on the location of the tumors.
Free-freezing Technique (Extracorporeal Procedure)
Under fluoroscopy, a K-wire was inserted through the planned osteotomy line after the tumor-containing tissue was exposed with an adequate margin. Subsequently, en bloc tumor excision was performed using a microsurgical saw. The soft tissues of the specimen were peeled, and the tumor’s bony lesion was curetted before freezing. Subsequently, the specimen was treated for 20 minutes in liquid nitrogen that was stored in a sterilized flask immediately before freezing, thawed at room temperature for 15 minutes, and placed in distilled water containing 1% iodine for another 15 minutes. The frozen autograft was used for intercalary reconstruction with double-locking or triple-locking plates, and two or three screws were inserted into the epiphysis for stabilization [39]. In patients with proximal tibial osteosarcomas, the patellar tendon was reattached to the frozen autograft using screw and spike washers [38]. When the frozen bone was combined with a prosthesis for composite reconstruction, a cemented long stem and double-plating fixation was used [35].
Pedicle Freezing Technique
Osteotomy (joint preservation surgery) or joint dislocation (composite) was performed after the proximal part of the tumor was exposed to an adequate surgical margin (Supplemental Fig. 1; http://links.lww.com/CORR/B139). The surrounding basal soft tissues were carefully protected with surgical sheets, and the proximal part of the tumor was elevated after isolation using cast padding soft rolls, an Esmarch bandage, and layers of surgical sheets to prevent tumor contamination and frostbite of the surrounding normal tissue during freezing. Subsequently, the intramedullary canal was curetted to remove the bone marrow containing the tumor tissue and prevent graft fracture caused by water expansion during freezing. Next, the isolated tumor-containing bony specimen was carefully rotated and placed in a container filled with liquid nitrogen for 20 minutes [42]. After thawing, reconstruction was performed using the same procedure as for the free-freezing technique.
Primary and Secondary Study Outcomes
The primary endpoints of this study were the cumulative incidence rate of complications and the cumulative survival of grafted bone, assessed with the Kaplan-Meier method. Any complications such as fracture, nonunion, infection, and recurrence that resulted in additional surgery and any cause of graft removal were recorded based on the modified Henderson criteria proposed by the International Society of Limb Salvage [17]. Complications were categorized as soft tissue complications (attached ligament tear or aseptic wound dehiscence), nonunion, structural complications (implant breakage, implant loosening, or grafted autograft fracture), infection, recurrence (soft tissue or host bone), and pediatric complications (growth arrest resulting in limb-length discrepancy). When implant breakage occurred at the osteotomy site (graft-host junction) before union or delayed union, it was considered implant breakage or nonunion. Graft bone fracture was defined as a fracture far from the junction. Factors relating to complications and removal of the grafted autograft were analyzed. The secondary endpoints were the proportion of bony union and limb function, evaluated with the MSTS score. Graft union was confirmed when the osteotomy line disappeared at the metaphysis or epiphysis and more than 75% of the cortices were bridged with new bone at the diaphysis on two radiographic views (AP or lateral view) or a central slice of the junction in any CT plane [46]. Furthermore, when it was challenging to evaluate bone union because of the shadow of the implants, union was confirmed using only one view or a CT image. Considering that osteotomy was not performed after joint dislocation, graft union was not evaluated in patients who underwent pedicle freezing for proximal femoral or humeral tumors with composite prosthetic reconstruction. Nonunion was defined as additional surgery performed to facilitate the union of the graft-host junction [7]. Union after 2 years was defined as a delayed union [23]. We evaluated bony union in only 245 of the patients (200 intercalary and 45 composite) because data for the other patients were unavailable. Furthermore, the graft-host junction union was evaluated in each center using radiographs obtained at no standardized timepoints. Therefore, we could evaluate the population of patients who experienced union within 2 years; we could not perform a survival analysis to determine the cumulative incidence rate of union. Finally, limb function was evaluated in each center using the MSTS score at the latest follow-up interval [13]. The questionnaire did not record who performed the evaluations. MSTS scores were available for 252 patients. Additionally, factors relating to bony union and limb function were analyzed. Data were investigated in each center by chart review and transferred to Kanazawa University.
Ethical Approval
The institutional review board of Kanazawa University approved the human protocol for this investigation (number 2017-209), and each author certifies that all investigations were conducted in conformity with the ethical principles of research.
Statistical Analysis
The statistical analysis was performed using SPSS version 25.0 (IBM Corp). Bony union, MSTS score (> 80% or < 80%), and each factor were evaluated using the chi-square test and logistic regression analysis. We used the t-test to correlate the MSTS score with each factor. In addition, we analyzed the association between each factor and subsequent complications and graft survival using the log-rank test and Cox proportional hazard regression analysis. In Cox proportional hazards models, factors with p < 0.1 in a univariate analysis were included. The analysis was performed using EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for the R software program (The R Foundation for Statistical Computing) [20] to create a survival curve. A post hoc analysis was performed to examine the power of the statistical results in multiple comparisons. One-way ANOVA and Fisher exact tests were performed to evaluate the demographic characteristic in three devitalizing methods.
Results
Cumulative Incidence of Complications After Devitalized Autografts
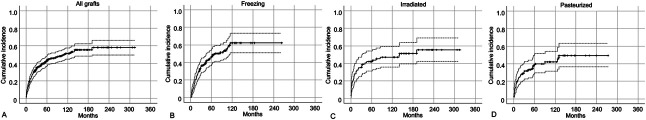
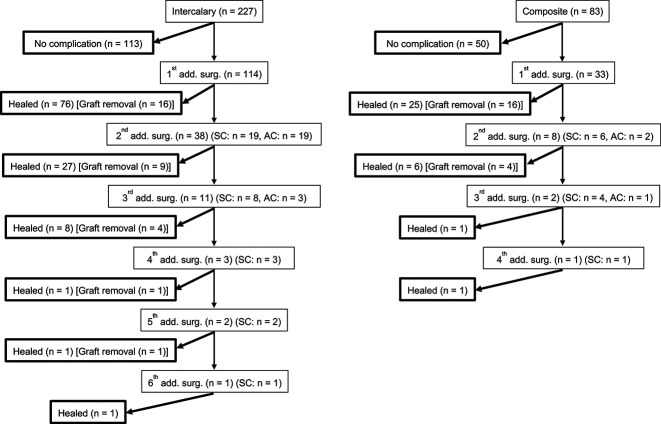
The cumulative incidence of major complications after using devitalized allografts was 42% (95% CI 0.37 to 0.48) at 5 years (Fig. 1A). There was no difference in the rate of complications among the three devitalizing methods (Fig. 1B-D). However, the post hoc analysis showed the power was smaller than 0.8 (Supplemental Table 1; http://links.lww.com/CORR/B140), indicating that the sample size was not enough to conclude there was not a difference. After controlling for confounding variables such as male sex, femur, long resection length (≥ 15 cm), intercalary graft, vascularized graft, and long operation time (≥ 6 hours), long resection length (≥ 15 cm) was associated with an increased risk of complications (RR 1.8 [95% CI 1.3 to 2.5]; p < 0.01) (Table 2). Overall, 174 complications were treated with additional surgery in 147 patients. Further, 46 patients experienced multiple adverse complications (Fig. 2). According to the modified Henderson classification, the most frequent complications were nonunion (21% [36 of 174]), infection (20% [34 of 174]), and implant loosening or breakage (14% [24 of 174]). Additionally, 10% (31 of 310) of patients had local recurrence in the surrounding soft tissue (7% [22 of 310]) and or retained host bone (3% [nine of 310]); however, no recurrence was found from the devitalized autograft.
Fig. 1.
(A) The cumulative incidence rate of complications was 42% at 5 years and 51% at 10 years. (B-D) The cumulative incidence rate of complications showed no difference among the (B) freezing, (C) irradiated, and (D) pasteurized grafts. Dashed lines indicate 95% confidence intervals.
Table 2.
Multivariate analysis for the risk of complication and graft survival
| Factor | Relative risk (95% CI) | p value | ||
| Event | ||||
| Resection length (≥ 15 cm) | 1.80 (1.29-2.52) | < 0.01 | ||
| Graft survival | ||||
| Resection length (≥ 15 cm) | 2.53 (1.41-4.54) | < 0.01 | ||
| Composite | 2.30 (1.30-4.10) | < 0.01 | ||
| Extracorporealb | 3.12 (1.13-8.98) | 0.03 | ||
aComposite means an osteoarticular autograft with a total joint replacement.
bExtracorporeal is the procedure of devitalizing comparison to the pedicle technique (only available in freezing).
Fig. 2.
This flowchart demonstrates the events and results in intercalary and composite-prosthesis grafts. SC = same complication; AC = another complication.
In 150 patients with tumors in their femurs, 61% (91 of 150) of the patients experienced complications, among whom 25% (23 of 91) of the patients had nonunion, 18% (16 of 91) had implant loosening or breakage, and 16% (15 of 91) experienced local recurrence (Table 3). Furthermore, 57% (67 of 117) of patients with tibial tumors had complications; the most prevalent complications were infection (in 31% [21 of 67]), soft tissue complications (in 18% [12 of 67]; 10 attached ligament tears and two aseptic wound dehiscence), and local recurrence (in 18% [12 of 67]; nine with soft tissue and three with host bone recurrences). Additionally, 37% (16 of 43) of the patients with upper limb tumors experienced complications; the most frequent complications were local recurrence (in 25% [four of 16]; three in soft tissue and one in host bone), nonunion, graft fracture, and infection in 19% (three patients each) (Table 3).
Table 3.
Detail of all complications and cause of graft removal
| All complications (n = 174) | Femur (n = 91) | Tibia (n = 67) | Upper (n = 16) |
| Soft tissue complications (attached ligament tear or aseptic wound dehiscence) | 9 (8) | 18 (12) | 13 (2) |
| Nonunion | 25 (23) | 15 (10) | 19 (3) |
| Implant failure (implant breakage or aseptic loosening) | 18 (16) | 10 (7) | 6 (1) |
| Fracture | 13 (12) | 8 (5) | 19 (3) |
| Infection | 11 (10) | 31 (21) | 19 (3) |
| Recurrence | 16 (15) | 18 (12) | 25 (4) |
| Limb-length discrepancy | 8 (7) | 0 | 0 |
| Cause of graft removal (n = 50) | Femur (n = 27) | Tibia (n = 19) | Upper (n = 4) |
| Nonunion | 19 (5) | 5 (1) | 0 |
| Implant failure (implant breakage or aseptic loosening) | 19 (5) | 0 | 0 |
| Fracture | 4 (1) | 0 | 25 (1) |
| Infection | 22 (6) | 68 (13) | 25 (1) |
| Recurrence | 37 (10) | 26 (5) | 50 (2) |
Data presented as % (n).
Grafted Bone Survival of the Three Types of Devitalized Autograft
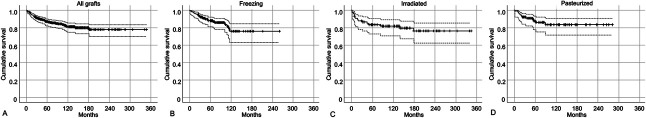
The cumulative grafted bone survival from surgery to the latest follow-up was 87% (95% CI 0.82 to 0.90) at 5 years (Fig. 3A), and we found no difference among the three types of devitalized autografts (freezing, pasteurization, and irradiation) at 5 years (freezing: 88% [95% CI 0.81 to 0.92] [Fig. 3B]; irradiation: 83% [95% CI 0.73 to 0.91] [Fig. 3C], and pasteurization: 88% [95% CI 0.78 to 0.93] [Fig. 3D]). However, the number of patients was not sufficient to confirm this observation because the power was low (< 0.8) in the post hoc analysis (Supplemental Table 1; http://links.lww.com/CORR/B140). After controlling for confounding variables including resection length, reconstruction type, procedure type, and chemotherapy, several factors were independently associated with differences in survival; long resection length (≥ 15 cm) and composite reconstruction were associated with an increased risk of grafted autograft removal (RR 2.5 [95% CI 1.4 to 4.5]; p < 0.01 and RR 2.3 [95% CI 1.3 to 4.1]; p < 0.01). The pedicle freezing procedure resulted in better graft survival at 5 years (94%) than the extracorporeal devitalizing procedures (85%; RR 3.1 [95% CI 1.1 to 9.0]; p = 0.03) (Table 2). Further, 50 patients underwent autograft removal. The most frequent causes of graft removal were infection (in 40% [20 of 50]) and soft tissue recurrence (in 22% [11 of 50]). Moreover, 12% (six of 50) of the other patients experienced host bone recurrence (Table 3). The most common causes in the femur were infection and soft tissue recurrence (in 22% [six of 27]) of the patients each) and nonunion and implant loosening or breakage (in 19% [five of 27] each). In the tibia, the most common causes were infection (68% [13 of 19]), soft tissue recurrence (16% [3 of 19]), and host bone recurrence (11% [two of 19]). In the upper limb, the most common causes of removal were soft tissue recurrence (in two of four patients) and fracture of the grafted autograft and infection (in one of four each).
Fig. 3.
(A) The cumulative graft survival was 87% at 5 years and 81% at 10 years. (B-D) The cumulative graft survival showed no difference among the (B) freezing, (C) irradiated, and (D) pasteurized grafts. Dashed lines indicate 95% confidence intervals.
Proportion of Patients With Union After Tumor-devitalized Autografts
Overall, 78% (156 of 200) of patients in the intercalary group and 87% (39 of 45) of those in the composite group achieved primary union within 2 years. Further, 9% (28 of 310) of patients underwent additional procedures. Subsequently, we performed a factor analysis about nonunion or delayed union after the use of an intercalary graft. There was no difference in the proportion of union events among the three devitalizing methods. However, the post hoc analysis showed the power was smaller than 0.8 (Supplemental Table 2; http://links.lww.com/CORR/B141), indicating the sample was not sufficient to conclude there was not a difference. After accounting for other variables, we found that male sex (RR 2.8 [95% CI 1.3 to 6.1]; p < 0.01) and receiving nonvascularized grafts (RR 3.6 [95% CI 1.0 to 12.2]; p = 0.04) were independent risk factors for nonunion (Table 4). Male sex (RR 2.5 [95% CI 1.2 to 5.6]; p = 0.02) and undergoing intramedullary nail fixation (RR 2.6 [95% CI 1.0 to 6.9]; p = 0.048) were the only independent risk factors for nonunion in lower extremities with intercalary grafts (Table 4).
Table 4.
Multivariate analysis for graft union of the intercalary graft
| Factor | Relative risk (95% CI) | p value |
| All sites | ||
| Male | 2.83 (1.32 to 6.06) | < 0.01 |
| Nonvascularized graft | 3.56 (1.03 to 12.23) | 0.04 |
| Lower extremity | ||
| Male | 2.54 (1.16 to 5.59) | 0.02 |
| Fixation (IM nail only) | 2.64 (1.01 to 6.92) | 0.048 |
IM = intramedullary nail.
MSTS Function of Patients With Tumor-devitalized Autografts
There were no differences in MSTS scores among patients who were treated with any of the three devitalizing methods. However, the post hoc analysis showed the power was smaller than 0.8 (Supplemental Table 3; http://links.lww.com/CORR/B142), indicating the sample size was not sufficient to conclude there was not a difference. After controlling for confounding variables including age, site, resection length, event occurrence, and graft removal, we found that age younger than 40 years (RR 2.0 [95% CI 1.1 to 3.7]; p = 0.03), tibia location (RR 6.9 [95% CI 2.7 to 17.5]; p < 0.01), femur location (RR 4.8 [95% CI 1.9 to 11.7]; p < 0.01), no complications (RR 2.2 [95% CI 1.1 to 4.5]; p = 0.03), and no graft removal (RR 2.9 [95% CI 1.2 to 7.3]; p = 0.03) were associated with increased limb function. The composite graft (RR 0.4 [95% CI 0.2 to 0.7]; p < 0.01) was associated with a decreased limb function (Table 5).
Table 5.
Multivariate analysis for MSTS score
| Factor | Relative risk (95% CI) | p value |
| Age (younger than 40 years) | 1.97 (1.06 to 3.67) | 0.03 |
| Femur | 4.75 (1.93 to 11.69) | < 0.01 |
| Tibia | 6.85 (2.68 to 17.52) | < 0.01 |
| Compositea | 0.36 (0.19 to 0.72) | < 0.01 |
| No event | 2.23 (1.09 to 4.54) | 0.03 |
| No graft removal | 2.89 (1.15 to 7.30) | 0.03 |
aComposite means an osteoarticular autograft with a total joint replacement.
Discussion
Cryosurgery for bone tumors was first introduced by Marcove et al. [25] as a palliative procedure. Subsequently, bone freezing [42, 43] and pasteurized [24] autografts to preserve bone with tumor involvement were developed in Japan. Spira and Lubin [34] first reported an irradiated autograft that is widely used in many countries, including Japan [3, 32]. Three methods (freezing, pasteurization, and irradiation) were approved by Japanese regulations in 2020. However, clinical outcomes in a large population are yet to be reported. This multicenter, nationwide study reported the outcomes of more than 300 patients who were treated with tumor-devitalized autografts. These three techniques appear to have similar rates of complications (51% in 10 years), graft survival (81% at 10 years), and limb function (mean MSTS score 83%) to those reported after bone allografts are used [1, 2, 7, 11, 18, 30, 33, 44] (Table 6). Endoprosthesis reconstruction is widely used worldwide, and in a systemic review analyzing 2721 patients, Thornley et al. [40] reported the primary endoprosthesis survival rate was 63% at a mean follow-up interval of 79 months. The most frequent causes of allograft removal were structural failure (16%), aseptic loosening (12%), and deep infection (9%), which were similar to the results of this study, except for the economic burden. These findings imply that tumor-devitalized autografts are useful for biological reconstruction in countries where it is challenging to obtain allografts because of the cost or lack of a bone bank system. Moreover, tumor-devitalized autografts are preferred over allografts for religious, social, and cultural reasons in some Asian countries [21].
Table 6.
Complication, graft survival, and limb function reported in other studies
| Author | n | Type of graft | Complication | Graft removal | Limb function | |||||
| Rate | Detail | Risk | Rate | Detail | Risk | MSTS | Factor | |||
| Bus et al. [7] | 87 | AL (IC) | 76% | NU (40%), FX (29%), INF (14%) | Tibia and IM nail for NU | 17% | FX (6%), INF (5%), NU (5%), R (2%) | N/A | N/A | N/A |
| Hornicek et al. [18] | 945 | AL (OA, IC, AD, and C) | N/A | INF (18.7%), NU (17.3%), FX (11.9%) | age (> 25 y) with CTX for NU | N/A | N/A | N/A | N/A | N/A |
| Aponte-Tinao et al. [2] | 198 | AL (OA and IC) | 58% | FX (15%), INF (14%), NU (12%), R (7%) | N/A | 40% (10 y) | FX (13%), INF (11%), R (7%) | OA and IC (tibia) | 87% (47%-100%) | N/A |
| Sanders et al. [33] | 131 | AL (IC) | 63% | Soft tissue (5%), NU (16%), FX (19%), IF (6%), INF (6%), R (11%) | IM nail only, nonbridging plate for NU | N/A | Soft tissue, NU, FX: 14% (10 y), INF: 5% (15 y) | N/A | N/A | N/A |
| Puerta-GarciaSandoval et al. [30] | 45 | AL (C) | 23% | INF (4%), R (16%), soft tissue failure (7%), AL (9%), IF (2%), NU (2%) | N/A | 20% | N/A | Stages IIA to IIIa | Femur (79%), tibia (76%) | N/A |
| Donati et al. [11] | 62 | AL (C) | 52% | INF (24%), extensor mechanism (15%), delayed union (13%) | N/A | 27% | INF (19%), local R (5%), AL (3%) | N/A | Score > 65% (90%) | N/A |
| Abdeen et al. [1] | 36 | AL (C) | 11% | Superficial INF (3%), AL (8%) | N/A | 8% | AL (6%), NU (3%) | N/A | 87% | Intra-articular resection |
| Van de Sande et al. [44] | 38 | AL (OA and C) | 32% | INF (8%), FX (8%), NU (5%), R (8%) | N/A | 42% | N/A | N/A | OA (77%), C (72%) | Preserving the adductor mechanism |
| Wu et al. [46] | 164 | IR, FZ (OA, IC, AD, HC, C) | IR (44%), FZ (40%) | IR: R (16%), NU (10%), INF (8%), soft tissue failure (8%); FZ: NU (13%), R (11%), FX (6%) | N/A | IR:83% (5 y), FZ: 84% (5 y) | IR: IF (1%), FX (1%), INF (4%), R (9%); FZ: FX (1%), INF (2%), R (7%) | N/A | N/A | N/A |
| Moran et al. [27] | 11 | IR (C) | 36% | NU (18%), R (18%) | N/A | 0% | N/A | 66% | N/A | |
| Outani et al. [28] | 87 | IR (OA, IC, AD, C) | 65% | Soft tissue (9%), NU (7%), structural failure (16%), INF (15%), R (2%) | N/A | 16% | INF (5%), R (2%), NU (2%), FX (1%) | N/A | UE (73%), LE (83%) | N/A |
| Sugiura et al. [36] | 46 | PZ (OA, IC, C) | 45% | INF (13%), FX (15%), NU (17%) | VFG for NU and absorption | 7% | INF (4%), R (2%) | no | 83% | HC |
| Lee et al. [23] | 278 | PZ (OA, IC, AD, HC, C) | N/A | N/A | N/A | 38% | INF (13%), NU (7%), FX (6%), R (4%) | N/A | N/A | N/A |
| Ikuta et al. [19] | 24 | PZ (IC) | 75% | NU (58%), INF (13%), absorption (21%), FX (4%) | N/A | 25% | INF (8%), NU (4%), R (13%) | N/A | 76% | N/A |
| This study | 310 | IR, PZ, FZ (IC, C) | 42% (5 y) | NU (21%), INF (20%), IF (14%), R (10%) | Long resection (≥ 15 cm) for all complication, male and non-VG for NU | 16% | INF (6%), R (5%), NU (2%), IF (2%), FX (1%) | N/A | 88% | Age (< 40 y), femur, tibia, no complication, no graft removal |
aEnneking staging. AL = allograft; IR = irradiated; FZ = freezing; PZ = pasteurized; IC = intercalary; OA = osteoarticular; C = composite; AD = arthrodesis; HC = hemicortical; N/A = not available; NU = nonunion; FX = fracture; INF = infection; R = recurrence; IF = implant failure; AL = aseptic loosening; IM = intramedullary nail; CTX = chemotherapy; VFG = vascularized fibular graft; VG = vascularized graft.
Limitations
First, there is possible selection bias. We did not evaluate the total number of patients treated for a malignant bone tumor of the long bones in the same period. Therefore, any difference in background between patients treated with tumor-devitalized grafts and those treated with other methods was not evaluated. The devitalizing method was determined according to the preference of each center. In this study, one center changed the devitalized method from one to another; however, no centers used different methods simultaneously. Tumor-devitalized autografting is generally indicated for osteoblastic or osteolytic tumors without severe loss of mechanical bone strength; therefore, we thought selection bias regarding the three devitalized methods was minimum. However, some factors differed among the three methods (including follow-up time, histology, graft type, chemotherapy, and combined bone graft) (Table 1), which might have affected the analysis.
Second, the incidence of complications, the rate of graft removal, rate of union, and mean MSTS score were not different among the three types of devitalized methods; however, the post hoc analysis showed the statistical power did not confirm there was truly no difference. This might have led to an assessment bias. Larger patient numbers are necessary to confirm these results. The graft-host junction union was evaluated in each center by the orthopaedic surgeon using radiographs that were not obtained at standardized timepoints. The number of reviewers in each center varied. We did not evaluate confounders such as pre-existing comorbidities, BMI, or graft quality. There was no group to compare our findings with the results of prostheses, allografts, or other reconstructions. Quality of life was evaluated using only the MSTS score. Patient-reported outcomes, including the Toronto Extremity Salvage Score [10] and SF-36 [6], can be used to evaluate health-related quality of life more accurately. Furthermore, sports activities were not evaluated in this study.
Third, this study might have suffered from transfer bias; we excluded patients in whom osteoarticular grafting was performed, those who died within 2 years, and those who were lost to follow-up or had incomplete datasets. We did not collect information on those who died or were lost to follow-up. Although the rate of exclusion except for osteoarticular grafting was not different among the three devitalization methods, these exclusions might have decreased the incidence rate of complications or increased the graft survival rate. Some patients lacked union times and MSTS scores, which might have decreased the rate of union and mean MSTS score. However, our results are considered a best-case analysis because of the retrospective, multicenter nature of this study.
Finally, there might have been cotreatment bias in this study. This study group comprised tertiary sarcoma centers; however, experience with performing tumor-devitalized autografting might have been different. The implant choice (plate or intramedullary nail) and number or size of implants were determined at each center. The indication for combined bone grafts (nonvascularized or vascularized grafts) varied at each center. The use of chemotherapy including second-line regimens and other adjuvants might also have varied at each center, which might influence the complications and grafted-bone survival. Additionally, the postoperative rehabilitation and follow-up protocols were not standardized. Although the devitalized methods differed among those three grafts, the reconstruction technique is similar to that of allografting. Therefore, we believe this study provided the real current treatment results of tumor-devitalized autografts for biological reconstruction in the largest number of patients.
Cumulative Incidence Rate of Complications After Devitalized Autografts
The cumulative incidence rate of any complications gradually increased to 51% at 10 years; nonunion and infection were the most common. Further, long resection (≥ 15 cm) contributed to an increased risk of complications. No difference was found in the incidence rate of complications among the three types of devitalizing methods. The incidence of complications in patients receiving allografts was reported to be between 11% and 76%, with infection, nonunion, and fracture being the most common complications [1, 2, 7, 11, 30, 33, 44], which is comparable to the findings of the current study. Bus et al. [7] reported that tibial site and intramedullary nail fixation were independent risk factors for nonunion. Hornicek et al. [18] reported that chemotherapy in patients aged < 25 years was an independent risk factor for nonunion. The incidence of complication in tumor-devitalized autografts was reported to be 33% to 73% [19, 26, 27, 36, 46]. Wu et al. [46] compared the outcomes of irradiated and frozen autografts and concluded there was no difference in incidence between the two types of devitalization. Sugiura et al. [36] analyzed the clinical outcomes of pasteurized autografts and found that the addition of a vascularized fibular graft reduced the risk of nonunion and bone absorption (Table 6). In our study, the failure mode appeared to be different between the femur and the tibia. Implant breakage or loosening was the most predominant in the femur. In contrast, infection was the most common complication in the tibia. Therefore, a more rigid and longer fixation might be better in femoral reconstruction. A combination of vascularized fibular grafts is also an option [8], although no benefit was found in this study, possibly because of the heterogeneous study population. Soft tissue coverage is considered a useful option to reduce the risk of infection [15]; however, its exact effect remains controversial [11]. Local recurrence is a serious complication that should be reduced to the extent possible with proper preoperative planning and surgical technique [37]. With this technique, there is a concern that a complete histologic evaluation of resected tumors might be impossible, considering that the tumor is curetted out and not examined in its entirety in an intact specimen. Therefore, we could not evaluate the margin status and response to chemotherapy in our patients. Miwa et al. [26] reported that histologic analysis of resected soft tissue or tissue samples correlated with clinical outcome, implying that a partial histologic evaluation of the tumor can be used to evaluate the chemotherapeutic response. Careful planning of the resection margin is also important.
Grafted Bone Survival of the Three Types of Devitalized Autograft
No difference was observed in grafted autograft survival among the three types of devitalizing methods, with a 10-year cumulative graft survival rate of 81%. The rate of graft removal was reported to be between 8% and 42% [1, 2, 7, 11, 30, 44] (Table 6). Aponte-Tinao et al. [2] reported that osteoarticular tibial and tibial intercalary grafts are associated with a risk of graft removal. Among early complications, infection was a risk factor for graft removal, which was more frequent in the tibia; however, in the longer term, fracture was a risk factor for removal, which was more common in the femur. Puerta-GarciaSandoval et al. [30] mentioned that Enneking Stages IIA to III [12] were correlated with graft removal. The rate of graft removal in tumor-devitalized autografts was reported to be between 0% and 38% [19, 23, 26, 27, 36, 46]. Lee et al. [23] reported the major causes of graft removal of pasteurized grafts were infection (30%) for composite grafts; the major causes were infection, nonunion, and local recurrence (29%) in intercalary grafts. In our study, male sex, long resection length (≥ 15 cm), and composite reconstruction were correlated with graft removal, and the pedicle freezing procedure decreased the risk of graft removal. Pedicle freezing omitted the distal osteotomy site in joint preservation surgery and composite reconstruction (Supplemental Fig. 1; http://links.lww.com/CORR/B139), negating the potential for nonunion at the distal part of the grafted bone. Araki et al. [4] analyzed the viability of frozen autografts using 99mTc-MDP scintigraphy. Notably, the time to tracer uptake normalization in pedicle-frozen bone was earlier than that in free-frozen bone.
Proportion of Patients With Union in Tumor-devitalized Autografts
In this study, primary union within 2 years was achieved in 78% of patients in the intercalary group and in 87% of patients in the composite group, with no difference among the three types of devitalized methods. Male sex and intramedullary nail fixation were the only independent risk factors for nonunion of the lower extremity. Additional procedures were performed in 9% of patients (29). The incidence of nonunion in allografts was reported to be between 2% and 40%, although the definition of bone union has varied in different studies [2, 7, 18, 30, 33, 44] (Table 6). Bus et al. [7] defined union of allograft-host junctions as a lack of continuity in three cortices at 1 year postoperatively and found that intramedullary nail-only fixation and longer grafts (> 10 cm) were associated with the risk of nonunion. Sanders et al. [33] defined nonunion as the need for surgical intervention and reported that 16% of patients experienced nonunion. They also reported that intramedullary nail-only fixation and fixation with a nonbridging plate were risk factors for nonunion (Table 6). Wu et al. [46] defined union as fusion of more than 75% of the cortical thickness of the graft-host bone junction and found no difference between irradiated and freezing grafts. Lee et al. [23] defined union as a callus formation bridging the host-graft junction on AP and lateral-plane radiographs within 2 years, and union after 2 years was defined as delayed union. They reported a nonunion rate of 31% and a delayed union rate of 13% in pasteurized grafts. In our study, the rate of nonunion was comparable to those of allografts and tumor-devitalized grafts. Combined bone grafting can shorten the union time; however, the indications for bone grafting of the osteotomy sites and type of graft used (vascularized or neovascularized) varied in this study. We could not estimate the appropriate time to consider grafting; however, generally, we recommend additional grafting if no callus formation is observed within 1 year to prevent implant loosening or breakage. Careful consideration to durable rigid fixation is advised, but we cannot recommend a specific fixation method because rods or plates were used in our patients. Some studies have reported that dual locking provides the most rigid and strong fixation, using finite-element analysis [16, 45].
MSTS Function of Patients With Tumor-devitalized Autografts
In this study, the MSTS score of the upper extremity was lower than those of lower extremities, with no difference among the three types of devitalized methods. We found that age younger than 40 years, tibia location, femur location, no complication, and no graft removal were associated with an increased limb function, whereas the composite graft was associated with decreased limb function. The MSTS score of allografts was reported to be between 65% and 87% [1, 2, 11, 30, 44] (Table 6). Van de Sande et al. [44] compared the clinical outcomes of proximal humerus reconstruction using an osteochondral allograft, allograft composite, and modular tumor endoprosthesis and concluded that function depends on preserving the abductor mechanism [32]. Lazerges et al. [22] introduced composite allograft-reverse shoulder arthroplasty. Their results showed a favorable MSTS score (73%). This appears to be a useful option for composite reconstruction at this site. The MSTS score of this study was comparable to those of tumor-devitalized grafts (irradiated and pasteurized) [19, 23, 26, 27, 36, 46].
Conclusion
This multicenter study reported a similar incidence of complications, limb function, and graft survival in frozen, irradiated, and pasteurized tumor-bearing autografts. The local tumor recurrence rate was 10%; however, no tumor recurred with the devitalized autograft. The pedicle freezing procedure reduces the osteotomy site, which may contribute to better graft survival. Tumor-devitalized autografts provided durable graft survival and favorable limb function, which appears to be comparable to the results of others using bone allografts. Therefore, we believe this is a good reconstruction option when other alternatives such as allografts or endoprostheses are not available, costly, or there are socioreligious reasons to not use them. Further analysis is necessary to establish the ideal fixation method and indications for combining vascularized autografts with devitalized autografts. In addition, more long-term and prospective analyses are necessary to evaluate the benefits of tumor-devitalized autografts compared with alternative methods of reconstruction.
Supplementary Material
Acknowledgments
We thank Dr. Makoto Endo MD, PhD; Keisuke Ae MD, PhD; Shintaro Iwata MD, PhD; Nasa Fujiwara MD, PhD; Naomasa Fukase MD, PhD; Toshihide Hirai MD, PhD; Yasutaka Murahashi MD, PhD; Takaaki Nakai MD, PhD; Nitta Tomohisa MD, PhD; Hiromichi Oshiro MD, PhD; Naoki Oike MD, PhD; Norio Yamamoto MD, PhD; Katsuhiro Hayashi MD, PhD; Shinji Miwa MD, PhD; and Kentaro Igarashi MD, PhD for their great contributions to this study. We thank Yoshitomo Saiki MSc for his assistance with the statistical analysis. We also thank Editage (www.editage.com) for their English-language editing.
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the institutional review board of Kanazawa University, Kanazawa, Japan (No. 2017-209).
This work was performed at Kanazawa University Hospital, Kanazawa, Japan.
Contributor Information
Hiroyuki Tsuchiya, Email: tsuchi@med.kanazawa-u.ac.jp.
Nokitaka Setsu, Email: sets0rockandsnow@gmail.com.
Tabu Gokita, Email: tabu.gokita@saitama-pho.jp.
Yasunori Tome, Email: yash_toume@hotmail.com.
Naofumi Asano, Email: asanonaofumi@gmail.com.
Yusuke Minami, Email: yusuke.m0811@gmail.com.
Hiroyuki Kawashima, Email: inskawa@med.niigata-u.ac.jp.
Suguru Fukushima, Email: sufukush@ncc.go.jp.
Satoshi Takenaka, Email: s.takenaka.0816@gmail.com.
Hidetatsu Outani, Email: h-otani@ort.med.osaka-u.ac.jp.
Tomoki Nakamura, Email: tomoki66@clin.medic.mie-u.ac.jp.
Satoshi Tsukushi, Email: s-tsuku@aichi-cc.jp.
Teruya Kawamoto, Email: trykwmt@gmail.com.
Teruki Kidani, Email: teruteru@m.ehime-u.ac.jp.
Munehisa Kito, Email: mune0527@yahoo.co.jp.
Hiroshi Kobayashi, Email: hkobayashi-tky@umin.ac.jp.
Takeshi Morii, Email: t-morii@ks.kyorin-u.ac.jp.
Toru Akiyama, Email: toruakiyama827@jichi.ac.jp.
Tomoaki Torigoe, Email: ttorigoe@saitama-med.ac.jp.
Koji Hiraoka, Email: khiraoka@med.kurume-u.ac.jp.
Akihito Nagano, Email: anagano@gifu-u.ac.jp.
Shigeki Kakunaga, Email: shigeki.kakunaga@oici.jp.
Kazuhiko Hashimoto, Email: hazzhiko@med.kindai.ac.jp.
Makoto Emori, Email: memori@sapmed.ac.jp.
Hisaki Aiba, Email: hisakiaiba@yahoo.co.jp.
Yoshikazu Tanzawa, Email: tangiyama@yahoo.co.jp.
Takafumi Ueda, Email: s.uedat@soreiyu.net.
Hirotaka Kawano, Email: hkawanotky@gmail.com.
References
- 1.Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am . 2009;91:2406-2415. [DOI] [PubMed] [Google Scholar]
- 2.Aponte-Tinao LA, Ayerza MA, Albergo JI, Farfalli GL. Do massive allograft reconstructions for tumors of the femur and tibia survive 10 or more years after implantation? Clin Orthop Relat Res . 2020;478:517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki N, Myoui A, Kuratsu S, et al. Intraoperative extracorporeal autogenous irradiated bone grafts in tumor surgery. Clin Orthop Relat Res . 1999;368:196-206. [PubMed] [Google Scholar]
- 4.Araki Y, Yamamoto N, Hayashi K, et al. A viability analysis of tumor-bearing frozen autograft for the reconstruction after resection of malignant bone tumors using 99m Tc-MDP scintigraphy. Clin Nucl Med . 2023;48:25-34. [DOI] [PubMed] [Google Scholar]
- 5.Ayerza MA, Farfalli GL, Aponte-Tinao L, Luis Muscolo D. Does increased rate of limb-sparing surgery affect survival in osteosarcoma? Clin Orthop Relat Res . 2010;468:2854-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaton DE, Schemitsch E. Measures of health-related quality of life and physical function. Clin Orthop Relat Res . 2003;413:90-105. [DOI] [PubMed] [Google Scholar]
- 7.Bus MPA, Dijkstra PDS, van de Sande MAJ, et al. Intercalary allograft reconstructions following resection of primary bone tumors: a nationwide multicenter study. J Bone Joint Surg Am . 2014;96:e26. [DOI] [PubMed] [Google Scholar]
- 8.Campanacci DA, Totti F, Puccini S, et al. Intercalary reconstruction of femur after tumour resection: is a vascularized fibular autograft plus allograft a long-lasting solution? Bone Joint J . 2018;100:378-386. [DOI] [PubMed] [Google Scholar]
- 9.Davidson AW, Hong A, McCarthy SW, Stalley PD. En-bloc resection, extracorporeal irradiation, and re-implantation in limb salvage for bony malignancies. J Bone Joint Surg Br . 2005;87:851-857. [DOI] [PubMed] [Google Scholar]
- 10.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508-516. [DOI] [PubMed] [Google Scholar]
- 11.Donati D, Colangeli M, Colangeli S, Di Bella C, Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin Orthop Relat Res . 2008;466:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res . 1986:204:9-24. [PubMed] [Google Scholar]
- 13.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res . 1993;286:241-246. [PubMed] [Google Scholar]
- 14.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res . 2006;442:223-229. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert NF, Yasko AW, Oates SD, Lewis VO, Cannon CP, Lin PP. Allograft-prosthetic composite reconstruction of the proximal part of the tibia. An analysis of the early results. J Bone Joint Surg Am . 2009;91:1646-1656. [DOI] [PubMed] [Google Scholar]
- 16.He Z, Huang S, Ji T, Tang X, Yang R, Guo W. Plate configuration for biological reconstructions of femoral intercalary defect - a finite element evaluation. Comput Methods Programs Biomed . 2022;224:107006. [DOI] [PubMed] [Google Scholar]
- 17.Henderson ER, O’Connor MI, Ruggieri P, et al. Classification of failure of limb salvage after reconstructive surgery for bone tumours: a modified system Including biological and expandable reconstructions. Bone Joint J . 2014;96:1436-1440. [DOI] [PubMed] [Google Scholar]
- 18.Hornicek FJ, Gebhardt MC, Tomford WW, et al. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res . 2001;382:87-98. [DOI] [PubMed] [Google Scholar]
- 19.Ikuta K, Nishida Y, Sugiura H, et al. Predictors of complications in heat-treated autograft reconstruction after intercalary resection for malignant musculoskeletal tumors of the extremity. J Surg Oncol . 2018;117:1469-1478. [DOI] [PubMed] [Google Scholar]
- 20.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant . 2013;48:452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khattak MJ, Umer M, Haroon-ur R, Umar M. Autoclaved tumor bone for reconstruction: an alternative in developing countries. Clin Orthop Relat Res . 2006;447:138-144. [DOI] [PubMed] [Google Scholar]
- 22.Lazerges C, Dagneaux L, Degeorge B, Tardy N, Coulet B, Chammas M. Composite reverse shoulder arthroplasty can provide good function and quality of life in cases of malignant tumour of the proximal humerus. Int Orthop . 2017;41:2619-2625. [DOI] [PubMed] [Google Scholar]
- 23.Lee SY, Jeon DG, Cho WH, Song WS, Kim BS. Are pasteurized autografts durable for reconstructions after bone tumor resections? Clin Orthop Relat Res . 2018;476:1728-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manabe J, Ahmed AR, Kawaguchi N, Matsumoto S, Kuroda H. Pasteurized autologous bone graft in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res . 2004;419:258-266. [DOI] [PubMed] [Google Scholar]
- 25.Marcove RC, Miller TR, Cahan WC. The treatment of primary and metastatic bone tumors by repetitive freezing. Bull N Y Acad Med . 1968;44:532-544. [PMC free article] [PubMed] [Google Scholar]
- 26.Miwa S, Takeuchi A, Ikeda H, et al. Prognostic value of histological response to chemotherapy in osteosarcoma patients receiving tumor-bearing frozen autograft. PLoS One . 2013;8:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran M, Stalley PD. Reconstruction of the proximal humerus with a composite of extracorporeally irradiated bone and endoprosthesis following excision of high grade primary bone sarcomas. Arch Orthop Trauma Surg . 2009;129:1339-1345. [DOI] [PubMed] [Google Scholar]
- 28.Outani H, Takenaka S, Hamada K, et al. A long-term follow-up study of extracorporeal irradiated autografts in limb salvage surgery for malignant bone and soft tissue tumors: a minimum follow-up of 10 years after surgery. J Surg Oncol . 2020;121:1276-1282. [DOI] [PubMed] [Google Scholar]
- 29.Pala E, Henderson ER, Calabrò T, et al. Survival of current production tumor endoprostheses: complications, functional results, and a comparative statistical analysis. J Surg Oncol . 2013;108:403-408. [DOI] [PubMed] [Google Scholar]
- 30.Puerta-GarciaSandoval P, Lizaur-Utrilla A, Trigueros-Rentero MA, Lopez-Prats FA. Mid- to long-term results of allograft-prosthesis composite reconstruction after removal of a distal femoral malignant tumor are comparable to those of the proximal tibia. Knee Surg Sports Traumatol Arthrosc . 2019;27:2218-2225. [DOI] [PubMed] [Google Scholar]
- 31.Puri A, Byregowda S, Gulia A, Patil V, Crasto S, Laskar S. Reconstructing diaphyseal tumors using radiated (50 Gy) autogenous tumor bone graft. J Surg Oncol . 2018;118:138-143. [DOI] [PubMed] [Google Scholar]
- 32.Puri A, Gulia A, Jambhekar N, Laskar S. The outcome of the treatment of diaphyseal primary bone sarcoma by resection, irradiation and re-implantation of the host bone. J Bone Joint Surg Br. 2012;94:982-988. [DOI] [PubMed] [Google Scholar]
- 33.Sanders PTJ, Spierings JF, Albergo JI, et al. Long-term clinical outcomes of intercalary allograft reconstruction for lower-extremity bone tumors. J Bone Joint Surg Am . 2020;102:1042-1049. [DOI] [PubMed] [Google Scholar]
- 34.Spira E, Lubin E. Extracorporeal irradiation of bone tumors. A preliminary report. Isr J Med Sci . 1968;4:1015-1019. [PubMed] [Google Scholar]
- 35.Subhadrabandhu S, Takeuchi A, Yamamoto N, et al. Frozen autograft-prosthesis composite reconstruction in malignant bone tumors. Orthopedics . 2015;38:e911-e918. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura H, Nishida Y, Nakashima H, Yamada Y, Tsukushi S, Yamada K. Evaluation of long-term outcomes of pasteurized autografts in limb salvage surgeries for bone and soft tissue sarcomas. Arch Orthop Trauma Surg . 2012;132:1685-1695. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi A, Lewis VO, Satcher RL, Moon BS, Lin PP. What are the factors that affect survival and relapse after local recurrence of osteosarcoma? Clin Orthop Relat Res . 2014;472:3188-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeuchi A, Yamamoto N, Hayashi K, et al. Growth of epiphysis after epiphyseal-preservation surgery for childhood osteosarcoma around the knee joint. BMC Musculoskelet Disord . 2018;19:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi A, Yamamoto N, Hayashi K, et al. Joint-preservation surgery for pediatric osteosarcoma of the knee joint. Cancer Metastasis Rev . 2019;38:709-722. [DOI] [PubMed] [Google Scholar]
- 40.Thornley P, Vicente M, MacDonald A, Evaniew N, Ghert M, Velez R. Causes and frequencies of reoperations after endoprosthetic reconstructions for extremity tumor surgery: a systematic review. Clin Orthop Relat Res . 2019;477:894-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toy PC, White JR, Scarborough MT, Enneking WF, Gibbs CP. Distal femoral osteoarticular allografts: long-term survival, but frequent complications. Clin Orthop Relat Res . 2010;468:2914-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuchiya H, Nishida H, Srisawat P, et al. Pedicle frozen autograft reconstruction in malignant bone tumors. J Orthop Sci . 2010;15:340-349. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchiya H, Wan SL, Sakayama K, Yamamoto N, Nishida H, Tomita K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J Bone Joint Surg Br . 2005;87:218-225. [DOI] [PubMed] [Google Scholar]
- 44.Van De Sande MAJ, Sander Dijkstra PD, Taminiau AHM. Proximal humerus reconstruction after tumour resection: biological versus endoprosthetic reconstruction. Int Orthop . 2011;35:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisanuyotin T, Sirichativapee W, Paholpak P, Kosuwon W, Kasai Y. Optimal configuration of a dual locking plate for femoral allograft or recycled autograft bone fixation: a finite element and biomechanical analysis. Clin Biomech (Bristol, Avon) . 2020;80:105156. [DOI] [PubMed] [Google Scholar]
- 46.Wu P, Chen C-F, Chen C-M, et al. Intraoperative extracorporeal irradiation and frozen treatment on tumor-bearing autografts show equivalent outcomes for biologic reconstruction. Clin Orthop Relat Res . 2018;476:877-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zelenski N, Brigman BE, Levin LS, Erdmann D, Eward WC. The vascularized fibular graft in the pediatric upper extremity: a durable, biological solution to large oncologic defects. Sarcoma . 2013;2013:321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.