Abstract
Moxifloxacin is a new 8-methoxyquinolone with high activity against gram-positive bacteria, including penicillin-resistant pneumococci. In an experimental meningitis model, we studied the pharmacokinetics of moxifloxacin in infected and uninfected rabbits and evaluated the antibiotic efficacies of moxifloxacin, ceftriaxone, and vancomycin against a penicillin-resistant Streptococcus pneumoniae strain (penicillin, ceftriaxone, vancomycin, and moxifloxacin MICs were 1, 0.5, 0.5, and 0.125 μg/ml, respectively). Moxifloxacin entered cerebrospinal fluid (CSF) readily, with peak values within 15 to 30 min after bolus intravenous infusion and with a mean percent penetration into normal and purulent CSF of approximately 50 and 80%, respectively. The bactericidal effect of moxifloxacin was concentration dependent, and regrowth was seen only when the concentration of moxifloxacin in CSF was below the minimal bactericidal concentration. All antibiotic-treated groups (moxifloxacin given in two doses of 40 mg/kg of body weight, moxifloxacin in two 20-mg/kg doses, ceftriaxone in one 125-mg/kg dose, and vancomycin in two 20-mg/kg doses) had significantly higher reductions in CSF bacterial concentration than the untreated group (P < 0.05). Moxifloxacin was as effective as vancomycin and ceftriaxone in reducing bacterial counts at all time points tested (3, 5, 10, and 24 h). Moreover, moxifloxacin given in two 40-mg/kg doses resulted in a significantly higher reduction in CSF bacterial concentration (in log10 CFU per milliliter) than vancomycin within 3 h after the start of antibiotic treatment (3.49 [2.94 to 4.78] versus 2.50 [0.30 to 3.05]; P < 0.05). These results indicate that moxifloxacin could be useful in the treatment of meningitis, including penicillin-resistant pneumococcal meningitis.
Penicillin-resistant pneumococci (PRP) have become an increasing problem worldwide. High prevalences of PRP have been reported in the United States, Spain, South Africa, South Korea, and many other countries (31). Penicillin resistance is followed by reduced susceptibility to other β-lactams (1). Indeed, failure has been reported in the treatment of meningitis with extended-spectrum cephalosporins, which are usually recommended for treatment of meningitis with unknown etiology (12).
New quinolones (e.g., levofloxacin, temafloxacin, trovafloxacin, Win 57273, clinafloxacin) have increased activity against gram-positive bacteria. These drugs enter the cerebrospinal fluid (CSF) readily because of their lipophilicity (23) and have been shown to be effective in the treatment of experimental meningitis caused by pneumococcal strains, independently of the strains’ penicillin susceptibilities (8, 13, 18, 21).
Moxifloxacin is a new quinolone {1-cyclopropyl-7-[(S,S)-2,8- diazabicyclo[4.3.0]non-8-yl]-6-fluoro-8-methoxy-1,4-dihydro-4- oxo-3-quinoline} with high activity against gram-positive bacteria, including pneumococci (MIC at which 50% of isolates are inhibited [MIC50] and MIC90, 0.12 and 0.25 μg/ml, respectively) (4, 33). In this study, we evaluated moxifloxacin pharmacokinetics, including penetration of CSF, with normal as well as inflamed meninges in rabbits. Furthermore, we studied the efficacies of different dosing regimens of moxifloxacin in the treatment of experimental meningitis in rabbits caused by PRP, and compared moxifloxacin with ceftriaxone and vancomycin, which are considered the treatment of choice for this type of meningitis.
MATERIALS AND METHODS
Test organisms.
Strain 1 (1395) was a penicillin-resistant Streptococcus pneumoniae type 9V strain originally isolated from the CSF of a 78-year-old female. Strain 2 (3058) was a penicillin-susceptible S. pneumoniae type 9V strain, kindly provided by Terry O’Reilly, Novartis, Basel, Switzerland. Enhancement of virulence was performed by inoculation of the two strains into mouse peritoneum. Infected peritoneal fluid was cultured for 24 h on 5% blood agar plates; then the bacteria were suspended in sterile beef broth with 10% glycerol (Statens Serum Institut), and aliquots were kept at −80°C. For in vivo and in vitro experiments, the frozen organisms were thawed and then grown on blood agar plates for 24 h, and then colonies were suspended in beef broth to an optical density of 0.35 at 540 nm and incubated for 1 h. The test organism was diluted in sterile beef broth to a final concentration of 1 × 106 to 2 × 106 CFU/ml, as confirmed by quantitative cultures, and 0.2 ml was used for intracisternal inoculation. Strain 2 was a CO2-dependent strain, so if not mentioned otherwise, incubation was performed in 5% CO2.
Antimicrobial agents.
Moxifloxacin was provided by Bayer AG, Wuppertal, Germany. Stock solutions were made by dissolving the drug in sterile water to a final concentration of 5 mg/ml. Ceftriaxone (catalog no. C-5793) and vancomycin (catalog no. V-2002), (both from Sigma Chemical Co., St. Louis, Mo.) were dissolved in sterile water to final concentrations of 40 and 5 mg/ml, respectively. Antibiotics were administered intravenously as infusions over 15 to 30 min.
Susceptibility testing.
MICs and minimal bactericidal concentrations (MBCs) of moxifloxacin, ceftriaxone, vancomycin, and penicillin were determined by a standard microdilution method (17). Briefly, the MIC of each antibiotic was determined in Mueller-Hinton broth supplemented with 5% sheep blood with an inoculum of 105 CFU/ml, and the antibiotic was added in twofold dilutions from 16 to 0.0313 μg/ml. Microtiter plates (microwell plates 262170; Nunc, Roskilde, Denmark) were incubated in 5% CO2 at 37°C for 20 h. The MICs were the lowest concentrations where no visible growth was observed. The MBCs were determined by plating 50 μl from each microtiter well without visible growth and 50 μl from controls on 5% blood agar for another 24 h of incubation. The MBC was defined as the lowest concentration that reduced the CFU of the inoculum by at least 99.9%.
Time-kill experiments.
The test organisms were diluted to ∼1 × 105 CFU/ml in 20 ml of sterile beef broth and were placed in 50-ml flasks. After 1 h of incubation to ensure the presence of the growth phase, the organisms were challenged with antibiotics at twofold dilutions from 32 to 0.5 times the MIC and were placed on a shaker at 37°C in ambient air or 5% CO2 (strain 1 or 2, respectively). Bacterial titers were determined after −1, 0, 1/2, 1, 2, and 4 h by plating 10-fold serial dilutions on 5% blood agar. Samples of 20 μl were plated to detect the lower detection level (50 CFU/ml). Experiments with all combinations of strains and drugs were performed in triplicate.
Determination of antibiotic concentrations in blood and CSF.
Antibiotic concentrations in CSF and serum were determined by a disc diffusion bioassay using Klebsiella pneumoniae (ATCC 10031) for measuring the concentrations of moxifloxacin and ceftriaxone. A cup-plate bioassay method using Micrococcus luteus (ATCC 9341) was used for the bioassay of vancomycin. Lower detection limits were 0.1, 0.2, and 0.6 μg/ml for moxifloxacin, ceftriaxone, and vancomycin, respectively. The inter- and intra-assay variation coefficients for the standard concentrations tested were less than 10% for all antibiotics.
Protein binding.
The protein binding of moxifloxacin was determined for a range of concentrations by an ultrafiltration method as previously described (10). Briefly, the fluids (human serum, rabbit serum, sterile beef broth, and sterile water) were incubated in ambient air at 37°C for 2 h. After the pH was adjusted to 7.0 to 7.5 with HCl, moxifloxacin was added in concentrations of 5, 10, 20, and 40 μg/ml, and the mixtures were incubated for 2 h. Half of the samples were centrifuged at 3,000 × g for 20 min in tubes with filters with a cutoff at approximately 30 kDa (Centricon 30, catalog no. 4208; Amicon, Beverly, Mass.), with subsequent determination of concentrations of moxifloxacin in broth, serum, water, or ultrafiltrates.
Meningitis model.
The experimental protocols were approved by the Danish committee for inspection of animal experiments. A modification of the model originally described by Dacey and Sande was used (3). New Zealand White rabbits weighing approximately 2.5 kg were anesthetized with 0.5 mg of midazolam (F. Hoffmann-La Roche AG, Basel, Switzerland)/kg of body weight given subcutaneously and a combination of fentanyl and fluanisone (0.35 ml/kg given intramuscularly; Janssen Pharmaceutica N.V., Beerse, Belgium), and a dental acrylic helmet containing a half turnbuckle was attached to the skull of each rabbit. Ten hours later, the rabbits were reanesthetized with ethyl carbamate (1.75 g/kg given subcutaneously; Fluka Kemi AG, Buchs, Switzerland) and pentobarbital (10 mg/kg; Nykomed, Roskilde, Denmark) and were immobilized in a stereotaxic frame. A 25-gauge spinal needle was introduced into the cisterna magna for the inoculation of 0.2 ml of bacterial suspension and repetitive CSF sampling. The rate of removal of CSF did not exceed the rate of CSF formation, which is approximately 0.4 ml/h (26). Blood samples were taken from a central ear artery. After tests for bacterial concentration, CSF and blood samples were centrifuged, and supernatants were immediately stored at −80°C for subsequent analysis. The lowest detectable bacterial concentration was 50 CFU/ml.
Antibiotic treatment. (i) Treatment.
Antibiotics were given intravenously into a central ear vein, and therapy was started approximately 10 h after inoculation.
(ii) Pharmacokinetics of moxifloxacin.
Three different doses (10, 20, and 40 mg/kg) were tested in uninfected (n = 2, 2, and 2, respectively) and infected (n = 3, 8, and 9, respectively) rabbits. CSF and blood samples were taken from uninfected rabbits at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, and 5 h after dosing. In infected rabbits CSF and blood samples were taken at 0, 0.5, 1.5, 3, and 5 h after antibiotic challenge.
(iii) Pharmacodynamics of moxifloxacin in penicillin-resistant pneumococcal meningitis.
Three different dosing intervals with the same total dose of moxifloxacin were compared: one dose of 40 mg/kg (n = 3), two doses of 20 mg/kg given 5 h apart (n = 8), and four doses of 10 mg/kg given 2 1/2 h apart (n = 3).
Two different total doses of moxifloxacin administered with the same dosing intervals were compared: two doses of 40 mg/kg given 5 h apart (n = 6) and two doses of 20 mg/kg given 5 h apart (n = 8). CSF and blood samples were taken at 0, 0.5, 1.5, 3, 5, 6, 10, and 24 h after the start of antibiotic treatment. Additional CSF and blood samples were taken from rabbits given four doses at 2.5, 7.5, and 8.5 h.
(iv) Efficacy of moxifloxacin, ceftriaxone, and vancomycin in penicillin-resistant pneumococcal meningitis.
Two moxifloxacin dosing regimens, consisting of two doses of 40 mg/kg 5 h apart (n = 6) and two doses of 20 mg/kg 5 h apart (n = 8), were compared to ceftriaxone (one dose of 125 mg/kg [n = 5]) and vancomycin (two doses of 20 mg/kg 5 h apart [n = 5]). Six untreated rabbits were reserved as a control group. CSF and blood samples were taken at 0, 0.5, 1.5, 3, 5, 6, 10, and 24 h after the start of antibiotic treatment.
Pharmacokinetic and pharmacodynamic parameters.
The time course of moxifloxacin concentrations in CSF and serum was evaluated for each rabbit. The concentration curves followed one-compartment distribution. The following parameters were determined: Cmax, the maximum concentration measured in CSF or blood, usually within 30 min after injection; t1/2, the elimination half-life in CSF or serum, estimated by the expression −log10 2/β, where β is the slope of the elimination regression line (log10 concentration versus time); AUC, the area under the concentration-time curve, calculated by the trapezoidal rule; and T>MBC, the time the concentration curve exceeded the MBC, estimated by linear regression. For pharmacodynamic considerations, Cmax, AUC, and T>MBC were correlated to colony counts in CSF conducted during the same time span.
Efficacy of moxifloxacin in meningitis caused by a penicillin-susceptible strain.
Rabbits with meningitis caused by a penicillin-susceptible strain (strain 2) were treated with two doses of 40 mg of moxifloxacin/kg (n = 3).
Statistical analysis.
All results are provided as means ± standard deviations, except bacterial concentrations, which are provided as medians (between minimum and maximum). Comparisons between two groups were performed by the nonparametric Mann-Whitney test. Comparisons among three or more groups were performed by the nonparametric Kruskal-Wallis test. When groups tested significantly different, a subsequent Dunn’s multiple-comparisons test was performed among groups. Correlations were calculated by the nonparametric Spearman rank correlation test. A value of P <0.05 was considered significant.
RESULTS
In vitro susceptibility.
The MICs and MBCs of moxifloxacin, ceftriaxone, vancomycin, and penicillin for strains 1 and 2 are provided in Table 1.
TABLE 1.
MICs and MBCs for the two S. pneumoniae type 9V strains used in the meningitis model
| Agent | Strain 1 (1395)
|
Strain 2 (3058)
|
||
|---|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | |
| Penicillin | 1 | 1 | ≤0.031 | ≤0.031 |
| Ceftriaxone | 0.5 | 1 | ≤0.031 | ≤0.031 |
| Vancomycin | 0.5 | 0.5 | 0.5 | 0.5 |
| Moxifloxacin | 0.125 | 0.25 | 0.125 | 0.25 |
Protein binding of moxifloxacin.
The protein binding of moxifloxacin in human and rabbit sera was 54% ± 14% and 24% ± 5%, respectively; in beef broth it was 33%, and in water it was 0%. We found no correlation between degree of protein binding and antibiotic concentration.
Time-kill experiments.
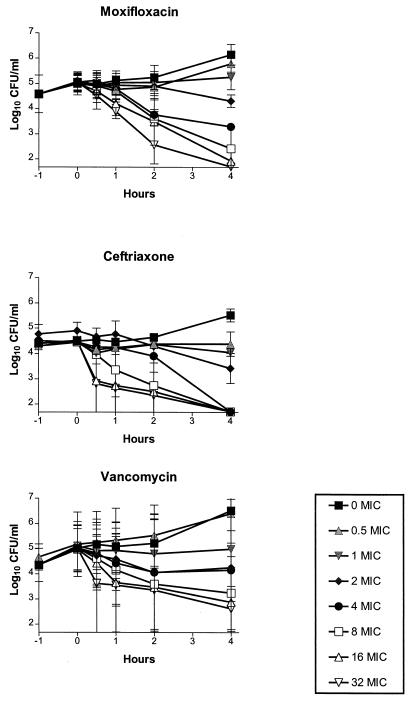
Figure 1 shows the time-kill curves of moxifloxacin, vancomycin, and ceftriaxone for strain 1. The data are expressed as log10 CFU per milliliter. The kill rates were dependent on concentrations when moxifloxacin was studied, in contrast to vancomycin and ceftriaxone, which showed no additional efficacy at concentrations above 16 times the MIC. The time-kill curves for strain 2 (data not shown) were comparable with the time-kill curves for strain 1.
FIG. 1.
In vitro time-kill curves with a penicillin-resistant S. pneumoniae strain. Antibiotic concentrations are shown as multiples of the MIC for the strain. The lower limit of detection of bacteria was 1.7 log10 CFU/ml.
Pharmacokinetics of moxifloxacin.
Table 2 shows pharmacokinetic parameters of moxifloxacin in the CSF of rabbits with and without meningitis. Moxifloxacin entered the CSF readily in rabbits with and without meningitis, reaching Cmax within 30 min after the start of therapy. Cmax values in CSF, measured after 30 min, were approximately twice as high in infected rabbits as in uninfected rabbits for low doses (10 mg/kg), but Cmax values were similar for higher doses (20 and 40 mg/kg). Cmax values (in micrograms per milliliter) in sera of infected versus uninfected animals were as follows: 1.70 ± 0.37 versus 1.99 ± 0.08 with 10 mg/kg, 5.05 ± 1.02 versus 4.92 ± 0.69 with 20 mg/kg, and 10.28 ± 1.03 versus 10.26 ± 0.42 with 40 mg/kg. The t1/2 in CSF was approximately twice as long in infected rabbits as in uninfected rabbits given high doses (20 and 40 mg/kg), but t1/2 values were similar with doses of 10 mg/kg. The t1/2 in serum (in hours) was longer for infected than for uninfected animals (1.50 ± 0.30 versus 1.20 ± 0.07 with 10 mg/kg, 2.21 ± 0.24 versus 1.66 ± 0.11 with 20 mg/kg, and 2.66 ± 0.50 versus 1.70 ± 0.04 with 40 mg/kg, respectively). Accumulation of moxifloxacin in CSF occurred with a higher Cmax after the second dose than after the first dose. The mean percent penetration into CSF, calculated as the AUC in CSF relative to the AUC in serum, was 78% ± 9% and 50% ± 2% for rabbits with and without meningitis, respectively. The mean concentration in CSF relative to the concentration in serum was 81% ± 29% and 51% ± 12% for rabbits with and without meningitis, respectively.
TABLE 2.
Pharmacokinetics and pharmacodynamics of moxifloxacin in CSF of rabbits with and without penicillin-resistant pneumococcal meningitisa
| Dose of moxifloxacin | t1/2 (h) | 0–3 h
|
0–5 h
|
0–10 h
|
0–24 h
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmaxb | AUCc | Cmaxb | AUCc | Cmaxb | AUCc | Cmaxb | AUCc | T>MBC (h) | ||
| With meningitis | ||||||||||
| 10 mg/kg × 4 | 1.14 ± 0.20 | 2.46 ± 0.33d | 3.02 ± 1.03 | 3.04 ± 1.70e | 7.67 ± 2.61f | 4.17 ± 1.15 | 20.76 ± 2.22 | 4.17 ± 1.15 | 39.87 ± 1.81 | 22.75 ± 0.35 |
| 20 mg/kg × 2 | 2.59 ± 0.18 | 2.87 ± 0.43 | 5.33 ± 0.94 | 2.87 ± 0.43 | 7.68 ± 1.40 | 6.28 ± 1.51 | 21.67 ± 1.24 | 6.28 ± 1.51 | 34.92 ± 3.34 | 22.34 ± 1.57 |
| 40 mg/kg × 1 | 2.62 ± 0.18 | 7.06 ± 1.39 | 12.55 ± 1.16 | 7.06 ± 1.39 | 18.02 ± 1.78 | 7.06 ± 1.39 | 22.48 ± 1.99 | 7.06 ± 1.39 | 28.47 ± 2.55 | 18.56 ± 0.38 |
| 40 mg/kg × 2 | 8.29 ± 0.93 | 33.09 ± 2.32 | 8.29 ± 0.93 | 62.36 ± 9.51 | 24 | |||||
| No meningitis | ||||||||||
| 10 mg/kg | 1.10 ± 0.07 | 1.28 ± 0.07 | 1.88 ± 0.16 | 1.28 ± 0.07 | 2.25 ± 0.23 | ND | ND | ND | ND | ND |
| 20 mg/kg | 1.37 ± 0.07 | 3.50 ± 0.36 | 5.14 ± 0.24 | 3.50 ± 0.36 | 6.21 ± 0.20 | ND | ND | ND | ND | ND |
| 40 mg/kg | 1.35 ± 0.07 | 7.06 ± 0.96 | 9.79 ± 0.15 | 7.06 ± 0.96 | 11.94 ± 0.03 | ND | ND | ND | ND | ND |
All data are means ± standard deviations. Concentrations were above the MBC during the first 10 h after the start of antibiotic therapy. ND, not detected.
Measured in micrograms per milliliter.
Expressed as micrograms times hours per milliliter.
Peak after 0.5 h.
Peak after 3 h.
After two doses of 10 mg/kg.
Pharmacokinetics of vancomycin and ceftriaxone.
The Cmax (expressed in micrograms per milliliter) of ceftriaxone in CSF was 9.79 ± 2.24, and that of vancomycin was 1.96 ± 0.76 after the first dose and 2.62 ± 1.54 after the second dose. The Cmax of ceftriaxone in blood was 218.0 ± 2.24, and that of vancomycin was 36.5 ± 8.28 after the first dose and 62.73 ± 77.62 after the second dose. t1/2 values in blood and CSF were 1.8 and 6.9 h, respectively, for ceftriaxone and 0.92 and 5.7 h, respectively, for vancomycin. The concentrations of both drugs in the CSF were above their respective MBCs during the whole study. The mean penetration into CSF, calculated as the AUC in CSF relative to the AUC in serum, was 30% ± 11% and 19% ± 4% for ceftriaxone and vancomycin, respectively.
Pharmacodynamics of moxifloxacin in animals with meningitis caused by a penicillin-resistant pneumococcal strain.
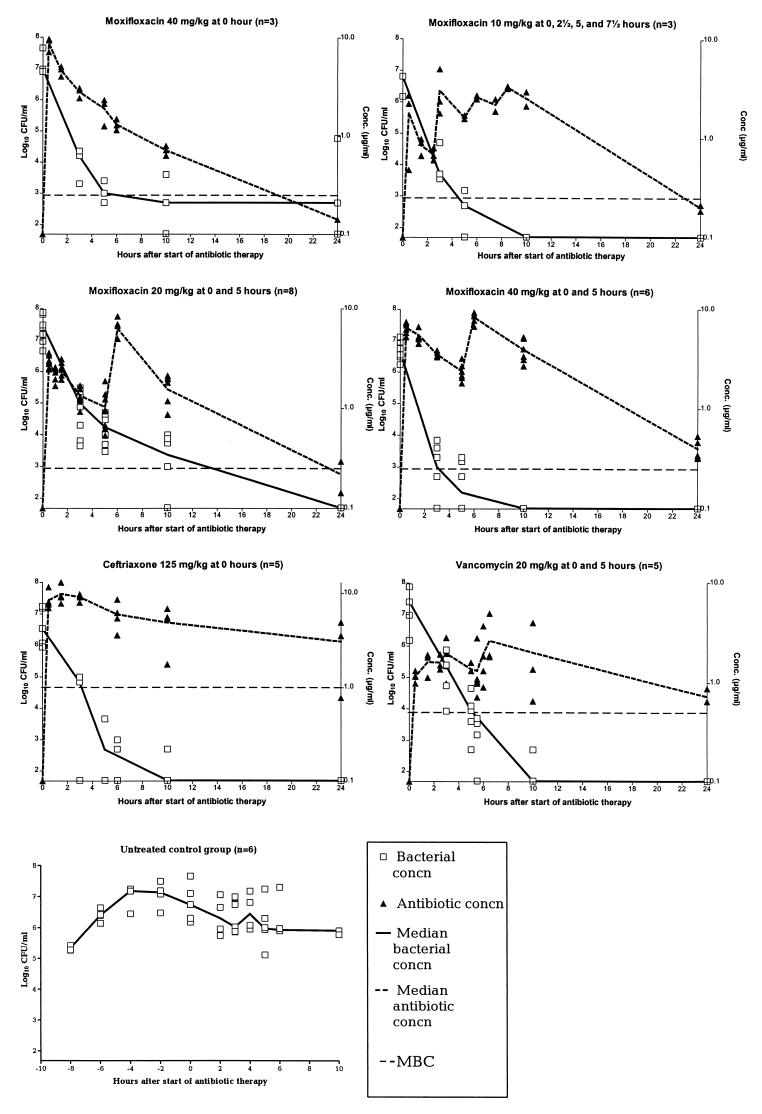
Figure 2 shows the concentration of moxifloxacin in CSF, as well as the concentration of bacteria in CSF, for different dosing regimens.
FIG. 2.
Median concentrations of bacteria in CSF and mean concentrations of antibiotics in CSF after the start of antibiotic therapy for experimental meningitis caused by a penicillin-resistant S. pneumoniae strain. Antibiotics were given as intravenous bolus infusions. The following differences were significant (P < 0.05): two 40-mg/kg doses of moxifloxacin versus vancomycin at 3 h; two 40-mg/kg doses of moxifloxacin versus two 20-mg/kg doses of moxifloxacin at 3, 5, and 10 h; all antibiotic groups versus the untreated control group. The lower limit of detection of bacteria was 1.7 log10 CFU/ml.
(i) Influence of different dosing intervals for the same total dose.
When 40 mg/kg was given as a single dose, the median concentration of bacteria in CSF was never under the detection limit, and indeed, regrowth was seen between 10 and 24 h, whereas all measured concentrations of bacteria in CSF remained under the detection limit when two 20-mg/kg doses or four 10-mg/kg doses were given.
(ii) Influence of different total doses administered with the same dosing intervals.
Two 40-mg/kg doses resulted in a significantly higher reduction in the median concentration of bacteria in CSF (expressed as log10 CFU per milliliter) than two 20-mg/kg doses at 3 h (3.49 [2.94 to 4.78] versus 2.65 [2.00 to 3.14]; P = 0.0027 by the Mann-Whitney test), at 5 h (4.29 [3.78 to 4.85] versus 3.28 [2.70 to 3.48]; P = 0.0007), and at 10 h (4.94 [4.54 to 5.42] versus 4.09 [3.21 to 4.96]; P = 0.0152) after the start of antibiotic therapy. This indicates that bacterial killing in vivo is dependent on the concentration of moxifloxacin as detected in vitro.
(iii) Pharmacodynamic parameters.
Table 2 shows pharmacodynamic parameters of moxifloxacin in CSF. Changes in log10 CFU per milliliter (n = 20) in CSF correlated significantly with the AUC from 0 to 3 h (AUC0–3) (P = 0.0002), AUC0–5 (P = 0.009), and Cmax at 3 and 5 h (P = 0.002 and P = 0.02, respectively). AUC0–3, AUC0–5, and AUC0–10 (n = 17) correlated significantly with Cmax at 3, 5 and 10 h (P < 0.0001, P = 0.0002, and P = 0.046, respectively), and AUC0–24 (n = 15) correlated significantly with T>MBC at 24 h (P < 0.0001).
Antibiotic efficacy in meningitis caused by a penicillin-resistant pneumococcal strain.
CSF bacterial concentrations (log10 CFU per milliliter) are shown in Fig. 2. No significant differences in median bacterial concentrations were observed among groups at the start of antibiotic therapy (P > 0.05 by the Kruskal-Wallis test). Groups treated with antibiotics (two 20-mg/kg doses of moxifloxacin, two 40-mg/kg doses of moxifloxacin, one 125-mg/kg dose of ceftriaxone, or two 20-mg/kg doses of vancomycin) had significantly higher reductions in median bacterial concentration than the untreated group at 3 and 5 h after the start of antibiotic therapy (P < 0.05 by the Mann-Whitney test). At 10 h most of the rabbits in the untreated group were either dead or euthanized. Moxifloxacin (two 20-mg/kg doses or two 40-mg/kg doses) was as effective in reducing CSF bacterial concentrations as vancomycin and ceftriaxone at 3, 5, 10, and 24 h. Furthermore, two 40-mg/kg doses of moxifloxacin initially resulted in a significantly higher reduction in median CSF bacterial concentration (measured in log10 CFU per milliliter) than vancomycin at 3 h (3.49 [2.94 to 4.78] versus 2.50 [0.30 to 3.05]; P = 0.02 by the Kruskal-Wallis test; P < 0.05 by Dunn’s multiple-comparison test). Indeed, this was the only group with CSF bacterial concentrations under the detection limit for all rabbits after 10 h (Table 3). No regrowth was seen in any of these antibiotic-treated groups, and all had sterile CSF after 24 h of therapy.
TABLE 3.
Number of rabbits with CSF bacterial concentrations under the detection limit at various time points
| Agent and dose | No. of rabbits with sterile (obtained) CSF (n) at:
|
|||
|---|---|---|---|---|
| 3 h | 5 h | 10 h | 24 h | |
| None (untreated) | 0 (5) | 0 (4) | 0 (1) | NDa |
| Ceftriaxone, 125 mg/kg × 1 | 2 (5) | 3 (5) | 4 (5) | 4 (4) |
| Vancomycin, 20 mg/kg × 2 | 0 (5) | 0 (5) | 2 (3) | 3 (3) |
| Moxifloxacin | ||||
| 20 mg/kg × 2 | 0 (8) | 0 (8) | 1 (6) | 5 (5) |
| 40 mg/kg × 2 | 1 (9) | 3 (9) | 6 (6) | 5 (5) |
ND, not detected. All rabbits were either dead or euthanized.
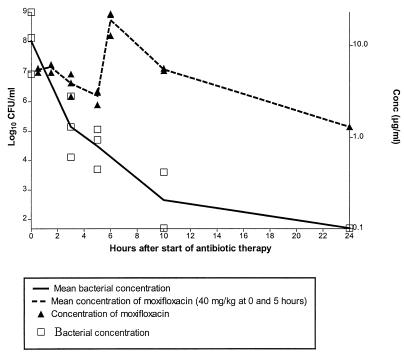
Efficacy of moxifloxacin in meningitis caused by a penicillin-susceptible pneumococcal strain.
The concentrations of bacteria and of moxifloxacin in CSF for rabbits with meningitis caused by a penicillin-susceptible pneumococcal strain, who were treated with two 40-mg/kg doses of moxifloxacin, 5 h apart, are shown in Fig. 3. As expected, no significant differences were observed when the efficacies of moxifloxacin for treatment of strains 1 and 2 were compared, as confirmed both by time-kill experiments and in the meningitis model (P > 0.05).
FIG. 3.
Median concentration of bacteria and mean concentration of moxifloxacin in CSF in a model of experimental meningitis (n = 3) caused by a penicillin-susceptible S. pneumoniae strain. The lower limit of detection of bacteria was 1.7 log10 CFU/ml.
DISCUSSION
Bacterial meningitis remains a serious disease associated with high mortality and morbidity. In pneumococcal meningitis, the mortality rate reaches as high as 20 to 30%, with neurological sequelae in up to 50% of survivors (7, 24, 32). In addition, reduced susceptibility of pneumococcal strains to penicillin and other β-lactams (e.g., extended-spectrum cephalosporins) has become an increasing problem, which requires new strategies for antibiotic treatment of pneumococcal meningitis.
In the present study, we tested moxifloxacin, a new 8-methoxyquinolone, in a model of experimental pneumococcal meningitis caused by a penicillin-resistant strain. The drug enters CSF readily through both inflamed and noninflamed meninges, with similar Cmax values within 15 to 30 min, in contrast to vancomycin, which enters uninfected CSF poorly. The penetration of moxifloxacin into purulent CSF relative to serum was approximately 80%, which is comparable with that of fleroxacin (5) but two- to fourfold better than those of other quinolones (ciprofloxacin, ofloxacin, levofloxacin, temafloxacin, trovafloxacin, and clinafloxacin), studied for treatment of experimental meningitis in rabbits (18, 21, 23).
The protein binding of moxifloxacin in human serum was approximately 54%, which is higher than previously reported (approximately 30%) (33). This may be due to differences in methods.
An intravenous bolus infusion of 10 mg of moxifloxacin/kg in uninfected rabbits equals an oral intake of 400 to 600 mg in humans, as determined by Cmax values in serum (15, 27). With higher doses, the CSF t1/2 was twice as long in rabbits with meningitis as in uninfected rabbits. Previous studies suggest an active efflux transport system of quinolones across the blood-brain barrier (19, 20). Our results indicate that an impairment of this active transport system could occur during meningitis. Several factors are involved in drug transportation through the blood-brain barrier (e.g., protein binding, lipophilicity, organic anion and cation transport) (25). P-glycoprotein has been shown to be an active pump for lipophilic drugs present at the luminal surface of the brain capillary epithelial cells (6). Furthermore, studies on transintestinal (2) or renal (11) elimination suggest that P-glycoprotein is partially involved in the transport of quinolones. But whether moxifloxacin is a substrate for P-glycoprotein is, to our knowledge, not known at present.
Therapy with moxifloxacin, given in two 20-mg/kg doses or two 40-mg/kg doses, was as effective as therapy with ceftriaxone or vancomycin in reducing CSF bacterial concentrations in our study. Moreover, moxifloxacin reduced CSF bacterial concentrations to a significantly greater extent within 3 h than vancomycin. This is in accordance with the findings of experiments with clinafloxacin (8) and CP-99,219, which also provide evidence of the superiority of quinolones, when combined with dexamethasone, over vancomycin (21).
Previous studies of the bactericidal effects of different dosing regimens of β-lactams in experimental pneumococcal meningitis have only in part investigated the influence of the various pharmacodynamic parameters (e.g., AUC, Cmax, T>MBC) (22, 28–30). It has been concluded that the bactericidal effect of β-lactams is dependent on the duration of therapy and concentrations above 8 to 10 times the MBC, whereas different dosing intervals with the same total dose showed no difference. A recent study of the pharmacodynamics of ceftriaxone in cephalosporin-resistant pneumococcal meningitis (16) confirms our previous findings in the mouse peritonitis model that the bactericidal effect of β-lactams is concentration independent and that the most important parameter is T>MBC (MIC) (9). Here, we found that moxifloxacin exhibits rapid concentration-dependent killing of PRP in vivo, and, as previously reported, in vitro (4, 14), even at concentrations above 16 times the MBC.
We found no correlation between changes in log10 CFU per milliliter in CSF and pharmacodynamic parameters after 24 h of therapy, because nearly all the animals at that time had CSF bacterial concentrations under the detection limit. But when we dosed two or four times, a total dose of 40 mg/kg was enough to eradicate bacteria from the CSF (at least to reduce them to below the detection limit) after 24 h of therapy, whereas regrowth was seen after a single dose. Indeed, this was the group with the lowest AUC and T>MBC, indicating that not only the Cmax in CSF, but also the T>MBC or the AUC is of importance in therapy with quinolones. In addition, no regrowth was seen when the concentration of moxifloxacin exceeded the MBC. Assuming that the penetration of the CSF by moxifloxacin is similar in humans and rabbits and that the t1/2 is 5 to 10 times longer in humans than in rabbits (27), we suggest that an initial high loading dose of moxifloxacin, with a dosing regimen of one to two times a day to keep the AUC high and the drug concentration above the MBC, would be an appropriate strategy in the treatment of pneumococcal meningitis. This has to be confirmed in pharmacokinetic studies of humans with meningitis.
In conclusion, the present study showed that moxifloxacin was highly effective in the treatment of both penicillin-resistant and -susceptible pneumococcal meningitis in rabbits. The penetration into infected and uninfected CSF was rapid and very high. The bactericidal effect of moxifloxacin was concentration dependent, and no regrowth was seen when the concentration was above the MBC. These results indicate that moxifloxacin could be useful in the treatment of bacterial meningitis in humans and that it merits clinical trials.
REFERENCES
- 1.Bradley J S, Scheld W M. The challenge of penicillin-resistant Streptococcus pneumoniae meningitis: current antibiotic therapy in the 1990s. Clin Infect Dis. 1997;24:S213–S221. doi: 10.1093/clinids/24.supplement_2.s213. [DOI] [PubMed] [Google Scholar]
- 2.Cavet M E, West M, Simmons N L. Fluoroquinolone (ciprofloxacin) secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 1997;121:1567–1578. doi: 10.1038/sj.bjp.0701302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy (Basel) 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 5.Decazes J M, Mohler J, Bure A, Vallois J M, Meulemans A, Modai J. Pharmacokinetics of fleroxacin and its metabolites in serum, cerebrospinal fluid, and brain of rabbits with and without experimental Escherichia coli meningitis. Rev Infect Dis. 1989;11:1208–1209. [Google Scholar]
- 6.Drion N, Lemaire M, Lefauconnier J M, Scherrmann J M. Role of P-glycoprotein in the blood-brain transport of colchicine and vinblastine. J Neurochem. 1996;67:1688–1693. doi: 10.1046/j.1471-4159.1996.67041688.x. [DOI] [PubMed] [Google Scholar]
- 7.Durand M L, Calderwood S B, Weber D J. Acute bacterial meningitis in adults: a review of 493 episodes. N Engl J Med. 1993;328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 8.Friedland I R, Paris M, Ehrett S, Hickey S, Olsen K, McCracken G H. Evaluation of antimicrobial regimens for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1993;37:1630–1636. doi: 10.1128/aac.37.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frimodt-Moller N. Correlation of in vitro activity and pharmacokinetic parameters with effect in vivo for antibiotics. Observations from experimental pneumococcus infection in mice. Dan Med Bull. 1988;35:422–437. [PubMed] [Google Scholar]
- 10.Frimodt-Moller N, Bentzon M W, Thomsen V F. Experimental pneumococcus infection in mice: comparative in vitro and in vivo effect of cefuroxime, cefotaxime and ceftriaxone. Acta Pathol Microbiol Immunol Scand Sect B. 1987;95:261–267. doi: 10.1111/j.1699-0463.1987.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Yano I, Tanaka K, Inui K I. Transport of quinolone antibacterial drugs by human P-glycoprotein expressed in a kidney epithelial cell line, LLC-PK1. J Pharmacol Exp Ther. 1997;282:955–960. [PubMed] [Google Scholar]
- 12.John C C. Treatment failure with use of a third-generation cephalosporin for penicillin resistant pneumococcal meningitis: case report and review. Clin Infect Dis. 1997;18:188–193. doi: 10.1093/clinids/18.2.188. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y S, Liu Q X, Chow L L, Tauber M G. Trovafloxacin in treatment of rabbits with experimental meningitis caused by high-level penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:1186–1189. doi: 10.1128/aac.41.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klugman K P, Capper T. Concentration-dependent killing of antibiotic-resistant pneumococci by the methoxyquinolone moxifloxacin. J Antimicrob Chemother. 1997;40:797–802. doi: 10.1093/jac/40.6.797. [DOI] [PubMed] [Google Scholar]
- 15.Kubitza D, Stass H H, Wingender W, Kuhlmann J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. BAY 12-8039, a new 8-methoxyquinolone: safety, tolerability and steady state pharmacokinetics in healthy male volunteers, abstr. F25; p. 104. [Google Scholar]
- 16.Lutsar I, Ahmed A, Friedland I R, Trujillo M, Wubbel L, Olsen K, McCracken G H., Jr Pharmacodynamics and bactericidal activity of ceftriaxone therapy in experimental cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1997;41:2414–2417. doi: 10.1128/aac.41.11.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. 1997. pp. 10–13. . Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 18.Nau R, Schmidt T, Kaye K, Froula J L, Tauber M G. Quinolone antibiotics in therapy of experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1995;39:593–597. doi: 10.1128/AAC.39.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooie T, Suzuki H, Terasaki T, Sugiyama Y. Comparative distribution of quinolone antibiotics in cerebrospinal fluid and brain in rats and dogs. J Pharmacol Exp Ther. 1996;278:590–596. [PubMed] [Google Scholar]
- 20.Ooie T, Terasaki T, Suzuki H, Sugiyama Y. Quantitative brain microdialysis study on the mechanism of quinolones distribution in the central nervous system. Drug Metab Dispos. 1997;25:784–789. [PubMed] [Google Scholar]
- 21.Paris M M, Hickey S M, Trujillo M, Shelton S, McCracken G H. Evaluation of CP-99,219, a new fluoroquinolone, for treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1995;39:1243–1246. doi: 10.1128/aac.39.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sande M A, Korzeniowski O M, Allegro G M, Brennan R O, Zak O, Scheld W M. Intermittent or continuous therapy of experimental meningitis due to Streptococcus pneumoniae in rabbits: preliminary observations on the post-antibiotic effect in vivo. Rev Infect Dis. 1981;3:98–109. doi: 10.1093/clinids/3.1.98. [DOI] [PubMed] [Google Scholar]
- 23.Scheld, W. M. 1989. Quinolone therapy for infections of the central nervous system. Rev. Infect. Dis. 11(Suppl. 5):S1194–S1202. [DOI] [PubMed]
- 24.Schlech W F, III, Ward J I, Band J D, Hightower A, Fraser D W, Broome C V. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA. 1985;253:1749–1754. [PubMed] [Google Scholar]
- 25.Schmidt, T., and M. G. Tauber. 1993. Pharmacodynamics of antibiotics in the therapy of meningitis: infection model observations. J. Antimicrob. Chemother. 31(Suppl. D):61–70. [DOI] [PubMed]
- 26.Spector R, Lorenzo A V. Inhibition of penicillin transport from the cerebrospinal fluid after intracisternal inoculation of bacteria. J Clin Invest. 1974;54:316–325. doi: 10.1172/JCI107767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stass H, Schühly U, Wingender W. Abstracts of the 8th European Congress of Clinical Microbiology and Infectious Diseases. 1997. Pharmacokinetics, safety and tolerability of 600 mg BAY 12-8039 administered once daily over 10 days, abstr. 387; p. 87. [Google Scholar]
- 28.Tauber M G, Doroshow C A, Hackbarth C J, Rusnak M G, Drake T A, Sande M A. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J Infect Dis. 1984;149:568–574. doi: 10.1093/infdis/149.4.568. [DOI] [PubMed] [Google Scholar]
- 29.Tauber M G, Kunz S, Zak O, Sande M A. Influence of antibiotic dose, dosing interval, and duration of therapy on outcome in experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1989;33:418–423. doi: 10.1128/aac.33.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tauber M G, Zak O, Scheld W M, Hengstler B, Sande M A. The postantibiotic effect in the treatment of experimental meningitis caused by Streptococcus pneumoniae in rabbits. J Infect Dis. 1984;149:575–583. doi: 10.1093/infdis/149.4.575. [DOI] [PubMed] [Google Scholar]
- 31.Tomasz A. Antibiotic resistance in Streptococcus pneumoniae. Clin Infect Dis. 1997;24:S85–S88. doi: 10.1093/clinids/24.supplement_1.s85. [DOI] [PubMed] [Google Scholar]
- 32.Wenger J D, Hightower A W, Facklam R R, Gaventa S, Broome C V. Bacterial meningitis in the United States, 1986: report of a multistate surveillance study. J Infect Dis. 1990;162:1316–1323. doi: 10.1093/infdis/162.6.1316. [DOI] [PubMed] [Google Scholar]
- 33.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]