Abstract
Antibiotics have previously been shown to have immunomodulatory effects. We examined the effect of the broad-spectrum fluoroquinoline antibiotic trovafloxacin on cytokine synthesis by monocytes obtained from healthy human volunteers and stimulated with either lipopolysaccharide or gram-positive cells (heat-killed Staphylococcus aureus [Pansorbin]). Trovafloxacin levels achievable in humans suppressed in vitro synthesis of each of the cytokines analyzed, viz., interleukin-1α (IL-1α), IL-1β, IL-6, IL-10, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha. This effect was not due to direct effects of the drug on cellular viability; at these concentrations, trovafloxacin did not have demonstrable cytotoxicity for the monocytes, as determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Although similar patterns of suppression of cytokine synthesis were observed in samples obtained from the same volunteers on different days, there were significant day-to-day variations. These results reveal that trovafloxacin possesses significant immunomodulatory activity in vitro and suggest that suppression of acute-phase inflammatory responses may occur in vivo, elicited through trovafloxacin’s effect on cytokine synthesis by human monocytes.
In 1982, we published a review of the then existing literature on the subject of the effects of antibiotics on the immune response (13). During the ensuing years, a large body of literature on this subject has appeared, attesting to the remarkable interest in this area of research and reflecting both new discoveries in antibiotics and the field of immunology and the development of better methods for the study of immune cell function. It has become increasingly apparent that in addition to their antimicrobial activity, many antibiotics have significant effects on human cellular immune function (16, 20, 24, 25, 29–33, 38). Although the effects of the immunomodulatory activity of antibiotics observed in vitro have not been correlated with beneficial or harmful effects in vivo in humans, such activity may be beneficial through modulation of the inflammatory response to the invading pathogen.
The increasing use of fluoroquinolones for treatment of a wide variety of infections and the likelihood that their use as first-line drugs will increase were the impetus for us to study the effect of these drugs on human monocyte cytokine production. Our initial studies focused on the new broad-spectrum antimicrobial fluoroquinolone trovafloxacin: it is active against both gram-positive and gram-negative aerobic and anaerobic bacterial pathogens, and, recently, we have reported its activity against Toxoplasma gondii both in vitro and in vivo in animal models of the infection (18, 19). Our interest in the role of cytokines in the immunopathogenesis of infection (14, 15); the observations that overproduction of proinflammatory cytokines may result in a variety of pathological states, including septic shock; and the relative paucity of literature on the effect of fluoroquinolone antibiotics on production of various cytokines by cells of the immune system led us to study the effects of trovafloxacin on cytokine production by human monocytes.
MATERIALS AND METHODS
Reagents.
Trovafloxacin (trovafloxacin mesylate; lot no. 310750-199-1F) was obtained in powder form from Pfizer, Inc., Groton, Conn. The drug stock solution and further dilutions were made in RPMI 1640 medium. Lipopolysaccharide (LPS) (Escherichia coli O26:B6) was purchased from Difco Laboratories, Detroit, Mich. Pansorbin (heat-killed Staphylococcus aureus Cowan strain I cells) was purchased from Calbiochem-Behring Co., La Jolla, Calif.
Isolation of monocyte-enriched PBMCs.
Blood was obtained by venipuncture from healthy donor volunteers. Peripheral blood mononuclear cells (PBMCs) were separated on Ficoll-Paque (Pharmacia Biotech AB, Uppsala, Sweden) density gradients, washed twice with calcium- and magnesium-free phosphate-buffered saline (Mediatech, Inc., Herndon, Va.), and fractionated by centrifugation over discontinuous Percoll (Pharmacia Biotech AB, Uppsala, Sweden) gradients. The monocyte-enriched cell fraction was collected, washed three times with calcium- and magnesium-free phosphate-buffered saline, and resuspended in RPMI 1640 (with 25 mM HEPES–2 mM l-glutamine; Mediatech, Inc., Herndon, Va.) containing 10% fetal bovine serum (GIBCO BRL Products, Grand Island, N.Y.) at a cell density of 106 cells/ml. At least 90% of the cells thus obtained were monocytes, as determined by esterase staining.
Cytokine assays.
Cells were seeded into 24-well plates (Costar Corporation, Cambridge, Mass.) at a cell density of 106 cells per ml (1 ml per well) and incubated in the presence of LPS (100 ng/ml) or 0.0075% (wt/vol) Pansorbin, with or without various concentrations of trovafloxacin, for 3, 6, or 24 h at 37°C in a 5% CO2 incubator. Cell-free supernatants were recovered by centrifugation and stored at −20°C until assayed. The concentration of each cytokine (interleukin-1α [IL-1α], IL-6, IL-10, granulocyte-macrophage colony-stimulating factor [GM-CSF], and tumor necrosis factor alpha [TNF-α]) was determined by enzyme-linked immunosorbent assay (ELISA) with commercially available reagents (PharMingen, San Diego, Calif.). The concentration of IL-1β was determined by ELISA with matched antibody pairs and supporting reagents from Endogen (Woburn, Mass.). Quantification was performed on the basis of a standard curve derived by linear dilution of the cytokine standards included in the respective kits. The detection limit for IL-1α, IL-10, and GM-CSF was 8 pg/ml; that for IL-6 and TNF-α was 20 pg/ml; and that for IL-1β was 3.9 pg/ml. In reproducibility assays for each cytokine, the coefficient of variation was <12% in replicate assays from the same sample. Cytokine assays were performed in quadruplicate with the supernatant samples or appropriate dilutions of the supernatants, as determined in preliminary studies.
Cellular toxicity assay.
The toxicity of the drug for the purified monocytes was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) cytotoxicity assay with the Cell Titer 96 Kit (Promega Corp., Madison, Wis.). Briefly, cells were plated in quadruplicate wells at 103 cells/well. Following a 4-h incubation at 37°C in a 5% CO2 incubator, trovafloxacin in various concentrations was added. Four hours before the 24-h time point, 16 μl of the dye indicator solution was added. Following an additional 4-h incubation, 100 μl of the solubilization-stop solution was added to each well. One hour later, the plates were read for A570 in an automatic ELISA plate reader. The results are quantified as the optical density at 570 nm (OD570) of cultures exposed to trovafloxacin or exposed to the diluent alone.
Statistics.
All values were expressed as means ± standard deviations. Welch’s test, which does not assume a similar distribution of variances, was utilized to determine statistical differences. A P value of ≤0.05 was considered statistically significant.
RESULTS
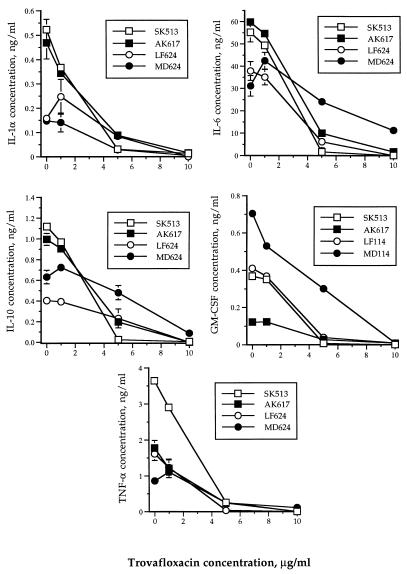
LPS-stimulated monocytes induced production of each of the cytokines measured, viz., IL-1α, IL-6, IL-10, GM-CSF, and TNF-α. The 24-h accumulation of the cytokines described above in supernatants of LPS-stimulated monocyte culture were 0.325 ± 0.199 (mean ± standard deviation), 45.998 ± 13.679, 0.788 ± 0.329, 0.303 ± 0.127, and 1.973 ± 1.184 ng/ml, respectively (Fig. 1). Synthesis and accumulation of each of the cytokines by LPS-stimulated monocytes were suppressed in a concentration-dependent manner following a 24-h exposure to trovafloxacin at 1, 5, or 10 μg/ml (Fig. 1). Mean percentages of inhibition of each cytokine at trovafloxacin concentrations of 1, 5, or 10 μg/ml were as follows: IL-1, 1.2, 75.6, and 97.4% (P values of 0.673, 0.039, and 0.019, respectively); IL-6, −3.9, 63.9, and 90.1% (P values of 0.984, 0.029, and 0.002, respectively); IL-10, 2.68, 61.2, and 96.5% (P values of 0.854, 0.026, and 0.004, respectively); GM-CSF, −0.55, 78.2, and 98.0% (P values of 0.977, 0.035, and 0.017, respectively); and TNF-α, 11.9, 87.4, and 96.9% (P values of 0.640, 0.024, and 0.017, respectively). Although in some individuals (Fig. 1), synthesis of IL-1α, IL-6, IL-10, and TNF-α was increased slightly at 1 μg of trovafloxacin per ml, a suppressive effect was observed at higher concentrations of the drug.
FIG. 1.
Effect of trovafloxacin on cytokine production by LPS-stimulated human monocytes in vitro. The symbols represent four different healthy human volunteers.
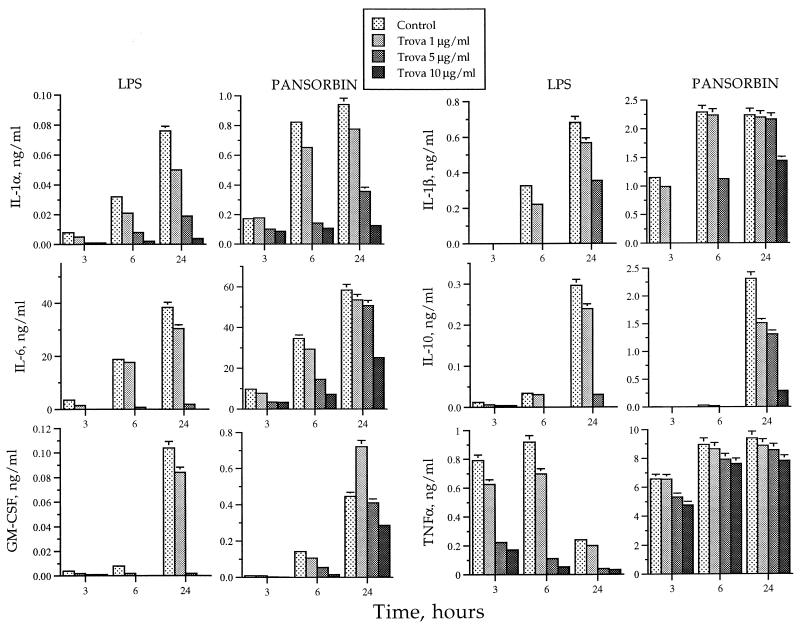
Pansorbin-stimulated monocytes produced amounts of cytokines that were many fold higher than those produced by LPS-stimulated monocytes, as shown in one of the two experiments conducted with similar results (Fig. 2). When monocytes were stimulated with LPS, each of the cytokines, except for TNF-α, achieved a maximal concentration in the supernatants following 24 h of accumulation. TNF-α was induced by 3 h and reached a maximum concentration as early as 6 h. At 24 h, the concentration of TNF-α was approximately 3.5-fold lower than at earlier hours. In contrast, when monocytes were stimulated with Pansorbin, IL-1α, IL-1β, and TNF-α reached maximal accumulation only after 6 h and remained at the same level even after 24 h. IL-6, IL-10, and GM-CSF reached maximal concentration after 24 h. Mean percentages of inhibition of each accumulation of cytokine at trovafloxacin concentrations of 1, 5, or 10 μg/ml following stimulation of monocytes with LPS at 24 h in two samples were as follows: IL-1, 42.4, 77.5, and 92.7% (P values of 0.039, 0.001, and 0.001, respectively); IL-1β, 22.5, 82.5, and 94.7% (P values of 0.302, 0.106, and 0.010, respectively); IL-6, 15.3, 92.7, and 100.0% (P values of 0.452, 0.010, and 0.008, respectively); IL-10, 23.7, 87.0, and 100.0% (P values of 0.571, 0.116, and 0.087, respectively); GM-CSF, 18.9, 97.6, and 100.0% (P values of 0.527, 0.038, and 0.036, respectively); and TNF-α, 31.8, 82.1, and 93.1% (P values of 0.132, 0.002, and 0.006, respectively). Mean percentages of inhibition of each cytokine at trovafloxacin concentrations of 1, 5, or 10 μg/ml following stimulation of monocytes with Pansorbin at 24 h in two samples were as follows: IL-1, 13.2, 56.3, and 85.5% (P values of 0.621, 0.107, and 0.052, respectively); IL-1β, 0.09, 4.0, and 43.9% (P values of 0.968, 0.456, and 0.051, respectively); IL-6, 7.8, 14.2, and 51.6% (P values of 0.001, 0.007, and 0.009, respectively); IL-10, 39.0, 46.5, and 82.5% (P values of 0.423, 0.331, and 0.108, respectively); GM-CSF, −31.8, 4.1, and 34.7% (P values of 0.518, 0.748, 0.100, respectively); and TNF-α, 0.84, 5.47, and 10.1% (P values of 0.859, 0.424, and 0.204, respectively).
FIG. 2.
Effect of trovafloxacin (Trova) on cytokine production by human monocytes in vitro stimulated with LPS or Pansorbin. These results are from one of the two experiments with similar results.
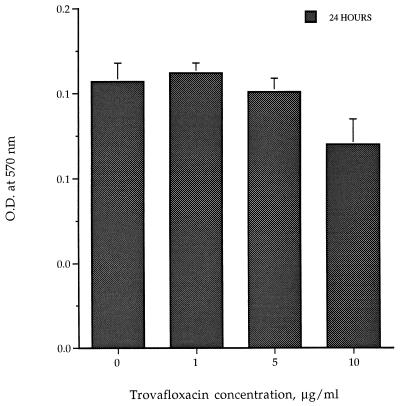
To determine whether this concentration-dependent suppressive effect of trovafloxacin was due to toxicity directly to the cells, monocytes cultured in the absence of LPS were exposed to the same concentrations of trovafloxacin. As determined by the MTT assay in two separate experiments, viability of monocytes was not reduced significantly, except at 10 μg/ml (P = 0.007) (Fig. 3).
FIG. 3.
Effect of trovafloxacin on viability of purified human monocytes as determined by the MTT cytotoxicity assay. The lower the absorbance reading, the more toxic the drug. O.D., optical density.
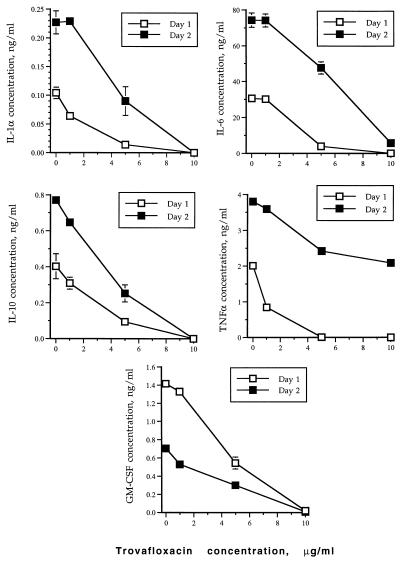
Although there was wide variation in cytokine production by activated monocytes from different volunteers, levels of cytokine production by monocytes from each individual were affected in a similar manner following antibiotic treatment. In multiple samples obtained on different days from a single volunteer, there was a statistically significant difference (P < 0.001) in the production of each cytokine (Fig. 4). However, on each occasion, exposure of the monocytes to trovafloxacin resulted in a similar pattern of cytokine synthesis suppression.
FIG. 4.
Interday variations and effect of trovafloxacin on cytokine production upon LPS stimulation of purified human monocytes obtained on different days from a single healthy human volunteer.
DISCUSSION
The results described above reveal that trovafloxacin can modulate in vitro production of IL-1α, IL-1β, IL-6, IL-10, GM-CSF, and TNF-α by monocytes. This was achieved at concentrations of trovafloxacin that are achievable in humans (7, 34). Peak levels of trovafloxacin in blood (mean ± standard deviation) in adults after an oral dose of 300 or 600 mg or in children or infants after a single 4-mg/kg intravenous dose (the prodrug alatrofloxacin) were 4.4 ± 1.1, 6.6 ± 1.4, and 4.23 + 1.37 μg/ml, respectively (17, 34). This suggests that in addition to its antimicrobial effects, trovafloxacin might have significant regulatory effects on the inflammatory response in vivo. Of particular interest is that for each of the cytokines tested, trovafloxacin inhibited accumulation of the cytokines in the culture supernatants of LPS- or Pansorbin-stimulated monocytes. The suppression of cytokine synthesis occurred in a concentration-dependent manner irrespective of individual variations in the levels of the cytokines produced. We studied monocytes because of their critical role in the early cytokine response to a number of pathogens and/or their products (8, 14).
The cytokines we studied are important in the immune response to infections. IL-1 has as its major source peripheral blood monocytes. Greater than 95% of LPS-activated monocytes produce IL-1α and IL-1β mRNA (4, 35). Transcription is regulated by a variety of signals, including cytokines, immune stimuli, bacterial cell wall components, and mediators of inflammation. Receptor antagonists or inhibitors of production have been shown to reduce inflammatory processes and lethality due to endotoxic shock in a variety of animal models (6). IL-10 is secreted by multiple cell types, including activated monocytes. It can inhibit its own production in an autocrine fashion, down-regulate major histocompatibility complex class II expression, cytokine synthesis and inhibit lymphocyte, NK cell, and monocyte/macrophage effector functions (5, 23). Monocytes are also a prominent source of IL-6, a pleiotropic cytokine that is important in the immune response, in regulation of the acute-phase response, and in hematopoiesis. Gene expression is stimulated by a number of mediators, including bacterial endotoxins, growth factors, cytokines, and neuropeptides. GM-CSF stimulates proliferation and differentiation of progenitor cells for neutrophilic granulocyte and macrophage lineages; induces peripheral monocytosis, eosinophilia, and neutrophilia in humans, and activates monocytes and neutrophils to phagocytose and kill a variety of pathogens (10, 12, 21, 22). TNF is primarily produced by monocytes/macrophages during activation of the immune response by invading pathogens. It can induce its own synthesis and release by monocytes, cause the clinical manifestations of overwhelming infection, and is a pivotal mediator of lethality from infection (36, 37). Principal stimuli for its release from cells are bacterial endotoxin, a variety of molecules of infectious pathogens, and other cytokines (3).
Some earlier quinolones that were evaluated for their effect on cells of the immune system are pefloxacin, ciprofloxacin, and ofloxacin. Each of them was found by Roche et al. to significantly inhibit proliferation of human PBMCs in response to phytohemagglutinin (30). A similar effect of ciprofloxacin on lymphocyte proliferation was reported by Hahn et al. (11). However, Gollapudi et al. reported that ciprofloxacin did not affect proliferation of PBMCs (9). Stunkel et al. demonstrated that ciprofloxacin increased the amount of IL-2 found in the supernatants of phytohemagglutinin-stimulated human PBMCs and also increased the levels of IL-1 in the culture supernatants of adherence-enriched mouse macrophages, but not in freshly isolated human monocytes (32). In the studies by Roche et al., the activity of IL-1 and TNF was reduced in supernatants of LPS-stimulated monocytes cultured in the presence of concentrations of the quinolones that are not achievable in humans (≥50 μg/ml) after the use of the recommended dosages; no reduction was noted at 10 μg/ml (1, 29). Bailly et al. also noted a decrease in release of IL-1 in vitro by LPS-stimulated monocytes when pharmacologic (greater than 25 μg/ml) but not physiologic concentrations of ciprofloxacin were used. At a concentration of 100 μg/ml, the effect of ciprofloxacin was found to be posttranscriptional (2).
Since the cytokines we evaluated are not similarly regulated, a strong and nonspecific suppressive effect of trovafloxacin on LPS-stimulated monocytes suggests the possibility that trovafloxacin may directly interact with LPS or its receptors. However, trovafloxacin also suppressed, although to a lesser extent in some cases, cytokine production when Pansorbin-stimulated monocytes were used, suggesting that trovafloxacin exerts its effect through other mechanisms.
A number of investigators have reported that the quinolones temafloxacin and ciprofloxacin can induce lymphocytes to overproduce IL-2 (26–28). Although lymphocytes rather than monocytes were studied, and despite the fact that different cytokines were examined, their results reveal the remarkable immunomodulatory effect that quinolones can have on lymphocyte function. The results of similar studies with human monocytes have not been published.
In conclusion, our experiments revealed that trovafloxacin has potent concentration-dependent suppressive effects on cytokine production by LPS- or Pansorbin-stimulated monocytes in vitro and thus may have immunomodulatory effects on the host response to infection.
ACKNOWLEDGMENTS
We are grateful to Pfizer, Inc., and the National Institute of Allergy and Infectious Diseases (contract N01-AI-35174) for their support of these studies.
REFERENCES
- 1.Bailly S, Fay M, Roche Y, Gougerot-Pocidalo M A. Effects of quinolones on tumor necrosis factor production by human monocytes. Int J Immunopharmacol. 1990;12:31. doi: 10.1016/0192-0561(90)90065-u. [DOI] [PubMed] [Google Scholar]
- 2.Bailly S, Mahe Y, Ferrua B, Fay M, Tursz T, Wakasugi H, Gougerot-Pocidalo M-A. Quinolone induced differential modification of IL-1α and IL-1β production by LPS-stimulated human monocytes. Cell Immunol. 1990;128:277–288. doi: 10.1016/0008-8749(90)90025-m. [DOI] [PubMed] [Google Scholar]
- 3.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 4.Chin J, Kostura M J. Dissociation of IL-1β synthesis and secretion in human blood monocytes stimulated with bacterial cell wall products. J Immunol. 1993;151:5574–5581. [PubMed] [Google Scholar]
- 5.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello C A. The IL-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 7.Fischman A J, Babich J W, Bonab A A, Alpert N M, Vincent J, Callahan R J, Correia J A, Rubin R H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Tissue pharmacokinetics of F-18 trovafloxacin in healthy human subjects, abstr. A-68; p. 14. [Google Scholar]
- 8.Gazzinelli R T, Amichay D, Sharton-Kersten T, Grunwald E, Farber J M, Sher A. Role of macrophage-derived cytokines in the induction and regulation of cell-mediated immunity to Toxoplasma gondii. Curr Top Microbiol Immunol. 1996;219:127–139. doi: 10.1007/978-3-642-51014-4_12. [DOI] [PubMed] [Google Scholar]
- 9.Gollapudi S V S, Prabhala R H, Thadepalli H. Effect of ciprofloxacin on mitogen-stimulated lymphocyte proliferation. Antimicrob Agents Chemother. 1986;29:337–338. doi: 10.1128/aac.29.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabstein K H, Urdal D L, Tushinski R J, Mochizuki D Y, Price V L, Cantrell M A, Gillis S, Conlon P J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986;232:506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- 11.Hahn H, Wos B, Sperling U. Influence of ciprofloxacin on specific interactions on listeria-specific T cells with antigen in vitro. In: Adam D, Hahn H, Opferkuch W, editors. The influence of antibiotics on the host-parasite relationship. II. Berlin, Germany: Springer-Verlag; 1985. pp. 96–103. [Google Scholar]
- 12.Handman E, Burgess A W. Stimulation by granulocyte-macrophage colony stimulating factor of Leishmania tropica killing by macrophages. J Immunol. 1979;122:1134. [PubMed] [Google Scholar]
- 13.Hauser W E, Jr, Remington J S. Effect of antibiotics on the immune response. Am J Med. 1982;72:711–716. doi: 10.1016/0002-9343(82)90534-4. [DOI] [PubMed] [Google Scholar]
- 14.Hunter C A, Remington J S. Immunopathogenesis of toxoplasmic encephalitis. J Infect Dis. 1994;170:1057–1067. doi: 10.1093/infdis/170.5.1057. [DOI] [PubMed] [Google Scholar]
- 15.Hunter C A, Suzuki Y, Subauste C S, Remington J S. Cells and cytokines in resistance to Toxoplasma gondii. Curr Top Microbiol Immunol. 1996;219:113–125. doi: 10.1007/978-3-642-51014-4_11. [DOI] [PubMed] [Google Scholar]
- 16.Iino Y, Toriyama M, Kudo K, Natori Y, Yuo A. Erythromycin inhibition of lipopolysaccharide-stimulated tumor necrosis factor alpha production by human monocytes in vitro. Ann Otol Rhinol Laryngol. 1992;101:16–20. doi: 10.1177/0003489492101s1005. [DOI] [PubMed] [Google Scholar]
- 17.Kearns G L, Bradley J S, Reed M D, Vincent J. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Trovafloxacin (TROV) pharmacokinetics (PK) in infants and children, abstr. A-104; p. 21. [Google Scholar]
- 18.Khan A A, Slifer T, Araujo F G, Polzer R J, Remington J S. Activity of trovafloxacin in combination with other drugs for treatment of acute murine toxoplasmosis. Antimicrob Agents Chemother. 1997;41:893–897. doi: 10.1128/aac.41.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan A A, Slifer T, Araujo F G, Remington J S. Trovafloxacin is active against Toxoplasma gondii. Antimicrob Agents Chemother. 1996;40:1855–1859. doi: 10.1128/aac.40.8.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korzeniowski O M. Effects of antibiotics on the mammalian immune system. Infect Dis Clin N Am. 1989;3:469–478. [PubMed] [Google Scholar]
- 21.Mayer P, Lam C, Obenaus H, Liehl E, Besemer J. Recombinant human GM-CSF induces leukocytosis and activates peripheral blood polymorphonuclear neutrophils in non-human primates. Blood. 1987;70:206–213. [PubMed] [Google Scholar]
- 22.Metcalf D. Hematopoietic colonies. In vitro cloning of normal and leukemic cells. Berlin, Germany: Springer-Verlag; 1977. [PubMed] [Google Scholar]
- 23.Moore G W, O’Garra A, de Waal-Malefyt R, Vieria P, Mosman T. Interleukin-10. Annu Rev Immunol. 1993;11:167–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 24.Morikawa K, Oseko F, Morikawa S, Iwamoto K. Immunomodulatory effects of three macrolides, midecamycin acetate, josamycin, and clarithromycin, on human T-lymphocyte function in vitro. Antimicrob Agents Chemother. 1994;38:2643–2647. doi: 10.1128/aac.38.11.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riesbeck K, Forsgren A. Increased interleukin 2 transcription in murine lymphocytes by ciprofloxacin. Immunopharmacology. 1994;27:155–164. doi: 10.1016/0162-3109(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 27.Riesbeck K, Forsgren A. Limited effects of temafloxacin compared with ciprofloxacin on T-lymphocyte function. Antimicrob Agents Chemother. 1994;38:879–882. doi: 10.1128/aac.38.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riesbeck K, Sigvardsson M, Leanderson T, Forsgren A. Superinduction of cytokine gene transcription by ciprofloxacin. J Immunol. 1994;153:343–352. [PubMed] [Google Scholar]
- 29.Roche Y, Fay M, Gougerot-Pocidalo M-A. Effects of quinolones on interleukin 1 production in vitro by human monocytes. Immunopharmacology. 1987;13:99–109. doi: 10.1016/0162-3109(87)90046-4. [DOI] [PubMed] [Google Scholar]
- 30.Roche Y, Gougerot-Pocidalo M-A, Fay M, Etienne D, Forest N, Pocidalo J-J. Comparative effects of quinolones on human mononuclear leucocyte functions. J Antimicrob Chemother. 1987;19:781–790. doi: 10.1093/jac/19.6.781. [DOI] [PubMed] [Google Scholar]
- 31.Stevens D L. Immune modulatory effects of antibiotics. Curr Opin Infect Dis. 1996;9:165–169. [Google Scholar]
- 32.Stunkel K G E, Hewlett G, Zeiler H J. Ciprofloxacin enhances T cell function by modulating interleukin activities. Clin Exp Immunol. 1991;86:525–531. doi: 10.1111/j.1365-2249.1991.tb02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeshita K, Yamagishi I, Harada M, Otomo S, Nakagawa T, Mizushima Y. Immunological and anti-inflammatory effects of clarithromycin: inhibition of interleukin 1 production of murine peritoneal macrophages. Drugs Exp Clin Res. 1989;15:527–533. [PubMed] [Google Scholar]
- 34.Teng R, Harris S C, Nix D E, Schentag J J, Foulds G, Liston T E. Pharmacokinetics and safety of trovafloxacin (CP-99,219), a new quinolone antibiotic, following administration of single oral doses to healthy male volunteers. J Antimicrob Chemother. 1995;36:385–394. doi: 10.1093/jac/36.2.385. [DOI] [PubMed] [Google Scholar]
- 35.Tocchi M J, Hutchinson N I, Cameron P M, Kirk K E, Norman D J, Chin J, Rupp E A, Limjuco G A, Bonilla-Argudo V M, Schmidt J A. Expression in Escherichia coli and characterization of recombinant human interleukin-1β: comparison with native IL-1β. J Immunol. 1987;138:1109–1114. [PubMed] [Google Scholar]
- 36.Tracey K J. Tumor necrosis factor. In: Remick D G, Friedland J S, editors. Cytokines in health and disease. 2nd ed. New York, N.Y: Marcel Dekker; 1997. pp. 223–239. [Google Scholar]
- 37.Tracey K J, Fong Y, Hesse D G, Manogue K R, Lee A T, Kuo G C, Lowry S F, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 38.Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]