Abstract
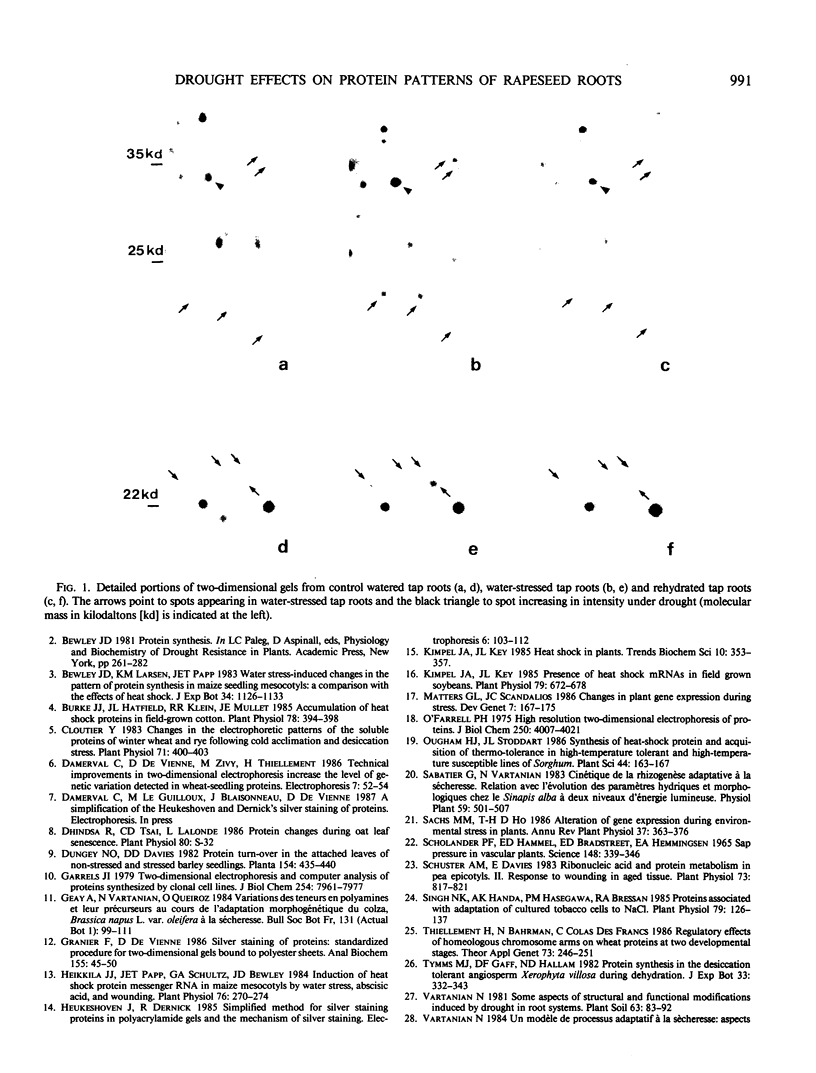
Drought-induced changes in two-dimensional silver stained protein patterns of Brassica napus L. var. oleifera M. root system were detected both at quantitative and qualitative levels. Particularly, 13 new polypeptides of low molecular weight were evidenced in the drought-stressed tap root, 12 of which were also present in the short tuberized roots, a specific drought-induced root type. The reversibility of these modifications, observed after 3 days rehydration, suggests that they might be involved in drought tolerance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. J., Hatfield J. L., Klein R. R., Mullet J. E. Accumulation of heat shock proteins in field-grown cotton. Plant Physiol. 1985 Jun;78(2):394–398. doi: 10.1104/pp.78.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier Y. Changes in the Electrophoretic Patterns of the Soluble Proteins of Winter Wheat and Rye following Cold Acclimation and Desiccation Stress. Plant Physiol. 1983 Feb;71(2):400–403. doi: 10.1104/pp.71.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Granier F., de Vienne D. Silver staining of proteins: standardized procedure for two-dimensional gels bound to polyester sheets. Anal Biochem. 1986 May 15;155(1):45–50. doi: 10.1016/0003-2697(86)90222-8. [DOI] [PubMed] [Google Scholar]
- Heikkila J. J., Papp J. E., Schultz G. A., Bewley J. D. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic Acid, and wounding. Plant Physiol. 1984 Sep;76(1):270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel J. A., Key J. L. Presence of Heat Shock mRNAs in Field Crown Soybeans. Plant Physiol. 1985 Nov;79(3):672–678. doi: 10.1104/pp.79.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matters G. L., Scandalios J. G. Changes in plant gene expression during stress. Dev Genet. 1986;7(4):167–175. doi: 10.1002/dvg.1020070402. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Bradstreet E. D., Hemmingsen E. A., Hammel H. T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science. 1965 Apr 16;148(3668):339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and Protein Metabolism in Pea Epicotyls : II. Response to Wounding in Aged Tissue. Plant Physiol. 1983 Nov;73(3):817–821. doi: 10.1104/pp.73.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Handa A. K., Hasegawa P. M., Bressan R. A. Proteins Associated with Adaptation of Cultured Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]



