Abstract
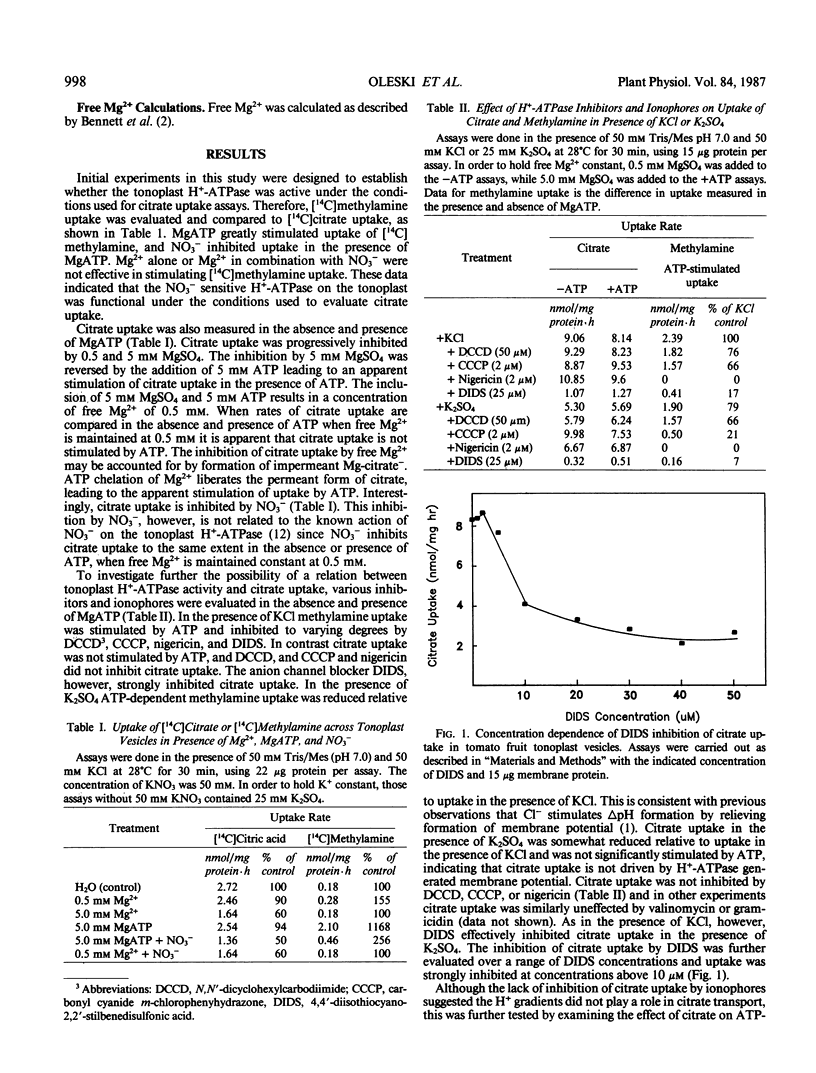
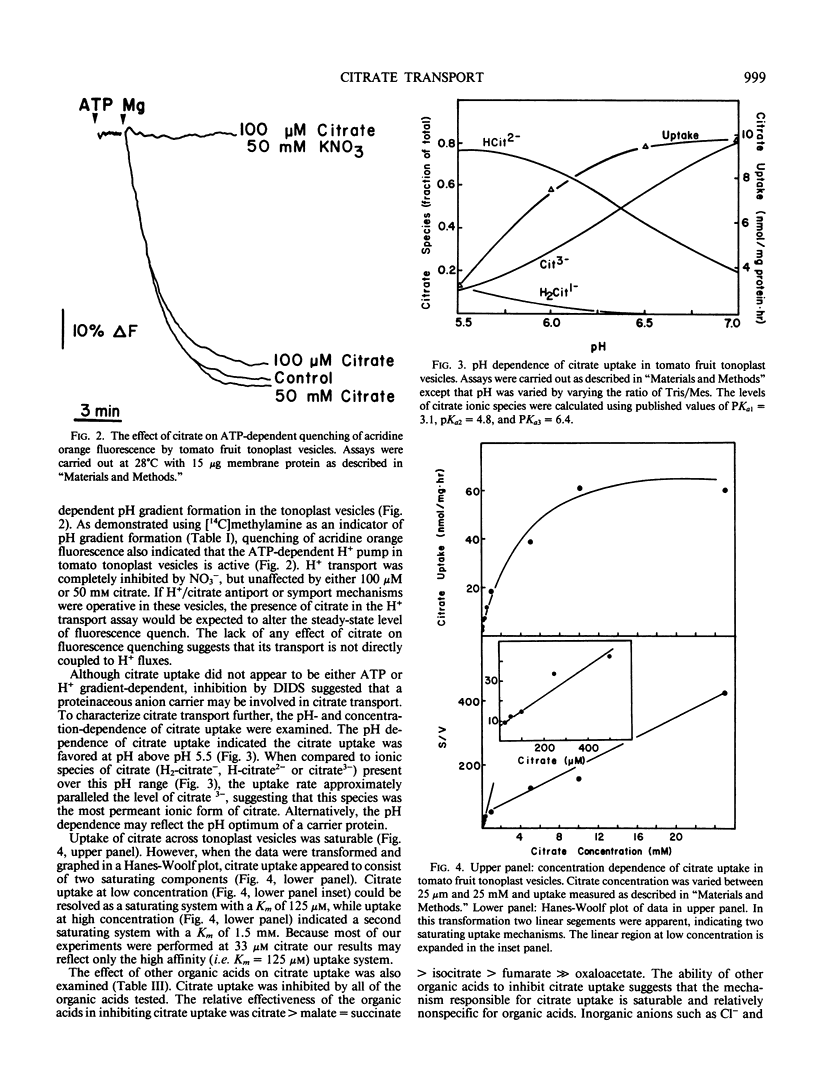
Citrate transport across the membrane of tomato fruit tonoplast vesicles was investigated. In the tonoplast vesicles, [14C]methylamine uptake was stimulated 10-fold by MgATP and strongly inhibited by NO3−. Under identical experimental conditions, [14C]citrate uptake was inhibited by 5 millimolar free Mg2+, and this inhibition was reversed in the presence of ATP, presumably by ATP chelation of free Mg2+. No evidence was obtained in support of energy-linked ATP stimulation of citrate uptake. Citrate uptake showed saturation kinetics, and was inhibited by 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid and by other organic acids. The pH-dependence of uptake suggested that citrate3− was the transported species. Our results indicate that citrate transport across the tomato fruit tonoplast occurs by facilitated diffusion of citrate3−. The carrier shares some features in common with anion channels in that it is relatively nonspecific for organic acids and is inhibitable by 4,4′-diisothyocyano-2,2′-stilbenedisulfonic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Eilmann M., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: III. Modulation of ATPase Activity by Reaction Substrates and Products. Plant Physiol. 1985 Jul;78(3):495–499. doi: 10.1104/pp.78.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser-Suter C., Wiemken A., Matile P. A malic Acid permease in isolated vacuoles of a crassulacean Acid metabolism plant. Plant Physiol. 1982 Feb;69(2):456–459. doi: 10.1104/pp.69.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. N., Hobson G. E. The constituents of tomato fruit--the influence of environment, nutrition, and genotype. Crit Rev Food Sci Nutr. 1981;15(3):205–280. doi: 10.1080/10408398109527317. [DOI] [PubMed] [Google Scholar]
- O'neill S. D., Bennett A. B., Spanswick R. M. Characterization of a NO(3)-Sensitive H-ATPase from Corn Roots. Plant Physiol. 1983 Jul;72(3):837–846. doi: 10.1104/pp.72.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleski N., Mahdavi P., Peiser G., Bennett A. B. Transport Properties of the Tomato Fruit Tonoplast : I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1987 Aug;84(4):993–996. doi: 10.1104/pp.84.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]


