Abstract
In vitro and in vivo efficacies of NS-718, a lipid nanosphere-encapsulated amphotericin B (AMPH-B), have been studied. Of the tested AMPH-B formulations, NS-718 had the lowest MIC for Cryptococcus neoformans. In a murine model, low-dose therapy (0.8 mg/kg of body weight) with NS-718 showed higher efficacy than that with AmBisome. High-dose therapy (2.0 mg/kg) with NS-718 was much more effective than those with Fungizone and AmBisome. In mice treated with a high dose of NS-718, only a few yeast cells had grown in lung by 7 days after inoculation. A pharmacokinetic study showed higher concentrations of AMPH-B in lung following administration of NS-718 than after administration of AmBisome. Our results indicated that NS-718, a new AMPH-B formulation, is a promising antifungal agent for treatment of pulmonary cryptococcosis and could be the most effective antifungal agent against C. neoformans infections.
Amphotericin B (AMPH-B) is a widely used, broad-spectrum antifungal agent that shows efficacy against Cryptococcus neoformans, and it is fungicidal at high concentrations; however, a significant toxicity profile limits its clinical usefulness. To improve the therapeutic efficacy and to reduce the toxicity of AMPH-B even at high doses, several strategies including combination therapy, modification of the AMPH-B molecule, and modification of the physical state of AMPH-B or changes in the drug delivery system have been used (1). New drug delivery systems, such as liposomal formulations, lipid complexes, and colloidal dispersions, have been introduced, and research studies and clinical trials are in progress (8, 9, 17). However, the efficacy of these newer formulations against cryptococcosis is as yet undefined.
The carrier potentials of lipid nanosphere are characterized by lower uptake by the reticuloendothelial system and good distribution to sites of inflammation. NS-718 has been prepared by encapsulating AMPH-B (0.5 mg/ml) with lipid nanosphere (15). This study was aimed at evaluation of in vitro and in vivo efficacies of NS-718, a lipid nanosphere-encapsulated new AMPH-B formulation, against C. neoformans in comparison with those of Fungizone (conventional AMPH-B) and AmBisome (liposomal AMPH-B).
MATERIALS AND METHODS
Antifungal agents and yeast strains.
AMPH-B, Fungizone (Bristol-Myers Squibb K.K., Tokyo, Japan), AmBisome (NeXstar Pharmaceuticals Ltd., Cambridge, United Kingdom), and NS-718 (Nippon Shinyaku Co. Ltd., Kyoto, Japan) were used in this study. NS-718 is a lyophilized formulation, composed of 10 mg of AMPH-B, 1 g each of soybean oil and egg-lecithin, and 2 g of maltose in each vial. After reconstitution, the average particle is 25 to 50 nm in diameter. Fungizone was dissolved in 5% dextrose, and AmBisome and NS-718 were dissolved in sterile distilled water. They were diluted with 5% dextrose to desired concentrations. Purified AMPH-B was dissolved in dimethyl sulfoxide. Eighteen strains of C. neoformans, isolated from patients at Nagasaki University Hospital and the affiliated hospitals, were used in this study. Identification and serotyping of the C. neoformans strains were done as described in our previous report (18).
Measurement of MIC.
The MICs of antifungal agents were determined by the microdilution method using a flat-bottom 96-well plate, modified from the macrodilution method of the National Committee for Clinical Laboratory Standards (19). In brief, 103 cells/ml were inoculated into plates containing RPMI 1640 with MOPS (morpholinepropanesulfonic acid). The plates were incubated at 35°C for 72 h in the presence of the antifungal agents, and the end point was defined as the point of no visible growth.
In vivo efficacy in treatment of murine pulmonary cryptococcosis.
The guidelines for animal experimentation of the Nagasaki University Laboratory Animal Center for Biomedical Research were followed. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Nagasaki University School of Medicine.
Animal inoculation was done according to the procedure described in our previous report (11). Briefly, while under general anesthesia (pentobarbital sodium [Nembutal] intraperitoneally administered), 6-week-old BALB/c male mice purchased from Charles River Inc. (Yokohama, Japan) were inoculated intratracheally with 50 μl of cell suspension containing 105 cells of C. neoformans (strain YC-11, serotype A) in normal saline. The final concentrations of the AMPH-B formulations were adjusted to 0.8 or 2.0 mg/kg of body weight in 5% dextrose. For each mouse, Fungizone, AmBisome, NS-718, or 5% dextrose (as untreated control) was injected via the lateral tail vein once daily for 5 days beginning 2 h after the inoculation (day 0). The mice were observed for survival daily for 60 days. The animals were sacrificed 7 days after inoculation, and the lungs were removed, suspended in sterile saline, and homogenized. A volume of 50 μl of 10-fold serially diluted suspension was inoculated on Sabouraud dextrose agar (Becton Dickinson & Co., Cockeysville, Md.) and incubated at 35°C for 48 h, and the colonies were counted.
Pharmacokinetic study.
Blood and lung of mice inoculated with C. neoformans were collected at time points of 10 min and 2, 4, 6, 12, and 24 h after intravenous injections of Fungizone, AmBisome, or NS-718 at 0.8- and 2.0-mg/kg doses. While the mice were under general anesthesia, whole blood was collected from axillary vessels. A thoracotomy was performed, and the lungs were perfused with normal saline and then removed surgically. Lung was homogenized with methanol containing 1-amino 4-nitronaphthalene. Serum and the supernatants of lung homogenates were preserved at −20°C until analysis. The concentration of AMPH-B was determined by high-performance liquid chromatography (HPLC) according to the method of Granich et al. with some modifications (6). Briefly, serum samples (0.1 ml) were combined with 1.0 ml of methanol containing 1.0 μg of an internal standard, 1-amino 4-nitronaphthalene (Aldrich, Milwaukee, Wis.), per ml and mixed by vortexing. After centrifugation at 1,500 × g for 10 min, the supernatant was dried under reduced pressure followed by redissolving with 0.2 ml of methanol for injection into an HPLC. Weighted wet tissue samples were homogenized in 10 volumes of methanol containing 5.0 μg of the internal standard per ml with a glass homogenizer. After centrifugation at 1,500 × g for 10 min, the supernatant was saved for HPLC analysis. The HPLC system consisted of an SLC-10A system controller, an LC-10AD pump, an SIL-10A auto sampler with a 20-μl sampler loop, an SCL-10A UV-visible detector at 408 nm, a CTO-10AC column oven set at 40°C, and a C-R5A Chromatopac data station (Shimadzu, Tokyo, Japan). Analysis was performed with an octyldecylsilane L-column (4.6 by 150 mm; Chemicals Inspection and Testing Institute, Tokyo, Japan) equipped with a LiChroCART guard cartridge (E. Merck, Darmstadt, Germany). The mobile phase was a mixture of acetonitrile and 10 mM sodium acetate buffer (pH 4.0; 11:17 [vol/vol]), and the flow rate was 1.0 ml/min. The concentration of AMPH-B was determined by the ratio of the peak height of AMPH-B to that of the internal standard.
Statistical analysis.
Each experiment was repeated at least twice to ascertain the reproducibility. Data were expressed as means ± standard deviations. Tests for differences in survival distributions were based on a generalized Wilcoxon test from survival rates calculated by the Kaplan-Meier method. The mean numbers of CFU per gram of lung tissue from the mycological study were compared by Scheffe’s multiple-comparison test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Susceptibility of C. neoformans strains to NS-718.
The MICs of AMPH-B, conventional AMPH-B (Fungizone), liposomal AMPH-B (AmBisome), and NS-718 against 18 strains of C. neoformans are summarized in Table 1. The MIC of NS-718 was the lowest, and the MIC at which 90% of the strains were inhibited by NS-718 was 16-fold less than that of AMPH-B.
TABLE 1.
MICs of antifungal agents for 18 strains of C. neoformans
| Drug | MIC (μg/ml)a
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| AMPH-B | 0.5–2 | 1 | 2 |
| Fungizone | 0.5 | 0.5 | 0.5 |
| AmBisome | 0.25–1 | 0.5 | 1 |
| NS-718 | 0.0313–0.125 | 0.0625 | 0.125 |
The MICs of antifungal agents were determined by the microdilution method modified from the macrodilution method of the National Committee for Clinical Laboratory Standards (19). The 50% and 90% MICs are the MICs at which 50 and 90% of the isolates were inhibited, respectively.
In vivo efficacy of NS-718 against murine pulmonary cryptococcosis.
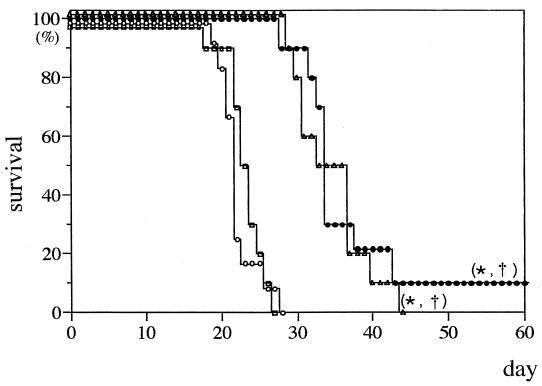
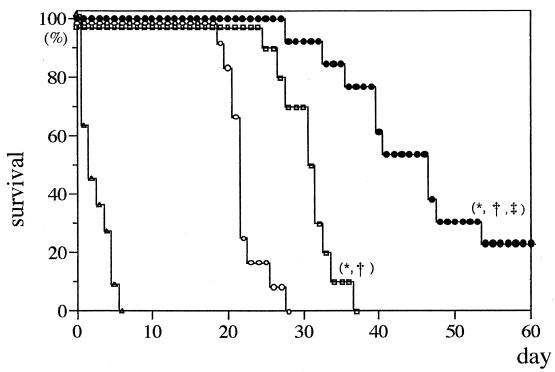
All the untreated control mice injected with 5% dextrose died within 28 days after inoculation of C. neoformans. Following low-dose therapy (0.8 mg/kg) with Fungizone or NS-718, survival was prolonged compared to that of the control, and Fungizone and NS-718 were found to be more effective than AmBisome (Fig. 1). Moreover, following high-dose therapy (2.0 mg/kg), 30% of mice injected with NS-718 survived for more than 60 days of observation. The efficacy of NS-718 was significant compared with that of the control or Fungizone- or AmBisome-treated mice. However, AmBisome also showed significant efficacy compared with that of the control, and all Fungizone-treated mice died within 6 days after inoculation (Fig. 2).
FIG. 1.
Survival rate of mice with experimental pulmonary cryptococcosis treated with an intravenous injection of 5% dextrose (○), NS-718 (•), conventional AMPH-B (Fungizone) (▵), or liposomal AMPH-B (AmBisome) □ (dose, 0.8 mg of AMPH-B per kg of body weight). Ten mice were used in each group. ∗ and †, P < 0.05, compared with results for 5% dextrose and AmBisome, respectively, by generalized Wilcoxon test. Each experiment was repeated at least twice to ascertain the reproducibility.
FIG. 2.
Survival rate of mice with pulmonary cryptococcosis treated with an intravenous injection of 5% dextrose (○), NS-718 (•), conventional AMPH-B (Fungizone) (▵), or liposomal AMPH-B (AmBisome) □ (dose, 2.0 mg of AMPH-B per kg of body weight). Ten mice were used in each group. ∗, ‡, and †, P < 0.05, compared with results for 5% dextrose, Fungizone, and AmBisome, respectively, by generalized Wilcoxon test.
Table 2 indicates the numbers (log10 CFU per gram) of yeast cells in mouse lung 7 days after inoculation. Low-dose therapy with NS-718 or Fungizone inhibited the growth of the cells in lung. The cell count following high-dose therapy was the lowest in the NS-718-treated group.
TABLE 2.
C. neoformans cell count in murine lung with pulmonary cryptococcosis after drug treatmenta
| Treatment | No. of cells (log10 CFU/g) after dose of:
|
|
|---|---|---|
| 0.8 mg/kg | 2.0 mg/kg | |
| Control (5% dextrose) | 7.4 ± 0.04 | 7.4 ± 0.04 |
| NS-718 | 6.7 ± 0.38b,c | <3.0b,c |
| AmBisome | 7.3 ± 0.38 | 7.6 ± 0.09 |
| Fungizone | 5.7 ± 0.51b,c,d | NDe |
Drug or dextrose was injected intravenously into 10 mice in each group, and the number of C. neoformans cells was counted 7 days after infection. The values shown are the means ± standard deviations.
P < 0.05, compared to the respective control value (Scheffe multiple-comparison test).
P < 0.05, compared to the respective AmBisome value (Scheffe multiple-comparison test).
P < 0.05, compared to the respective NS-718 value (Schiffe multiple-comparison test).
ND, not done.
Pharmacokinetic study.
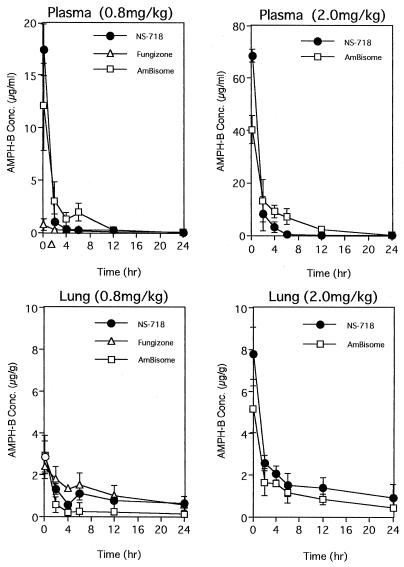
The concentration of AMPH-B in serum at 10 min was the highest (17.4 μg/g) after administration of 0.8 mg of NS-718 per kg; at 2 h, the concentration after NS-718 administration was lower (1.04 μg/g) than that after AmBisome administration (3.03 μg/g) but higher than that after Fungizone administration (0.29 μg/g). After administration of 2.0 mg of NS-718 per kg, the concentration of AMPH-B in serum at 10 min was higher (68.5 μg/g) than that after AmBisome administration (40.3 μg/g), but at 2 h onward the concentration was lower. The concentration of AMPH-B in lung at 10 min was the same after administration of 0.8 mg of NS-718 or AmBisome (per kg; from 2 to 12 h, the concentration after NS-718 administration was higher (1.33 μg/g) than that after AmBisome administration (0.59 μg/g) but lower than that after Fungizone administration; at 24 h, the concentrations did not differ. The concentration of AMPH-B in lung at 10 min was slightly higher after administration of 2.0 mg of NS-718 per kg (7.82 μg/g) than after administration of AmBisome (5.19 μg/ml) (P < 0.069, t test) (Fig. 3).
FIG. 3.
Concentrations of AMPH-B in serum and lung after a single administration in mice with pulmonary cryptococcosis of three forms of AMPH-B, NS-718, Fungizone, and AmBisome. Each value plotted was the mean ± standard deviation of the concentrations in three mice.
DISCUSSION
NS-718, a novel lipid nanosphere-encapsulated AMPH-B, was found to be the most efficacious of three AMPH-B formulations against C. neoformans isolates in vitro. While the underlying mechanism of such difference in potency needs to be elucidated by basic studies, we speculate that facilitation of AMPH-B release and yeast cell surface contact might improve the MIC and potency of lipid formulations, although the vehicle does not have any antifungal activity. In our previous study, NS-718 was found to be more effective than Fungizone or AmBisome against clinical isolates of Candida albicans and Aspergillus fumigatus (13). Thus, NS-718 has a broad spectrum of antifungal activities.
Our results in this study indicate that NS-718, a novel lipid nanosphere-encapsulated amphotericin B, showed efficacy in treatment of murine pulmonary cryptococcosis that correlates with the results of in vitro study. NS-718 in a high dose could completely suppress growth of cells in lung. NS-718 maintained the potent activity of AMPH-B. AmBisome showed weaker antifungal activity than that of NS-718, because the release of AMPH-B from AmBisome was slow and slight. A previous investigation involving a pharmacokinetic study with rats indicated that the AMPH-B level in plasma after administration of NS-718 was higher than those of Fungizone, Amphocil, and Abelcet and similar to that of AmBisome. In a tissue distribution study, the concentration of NS-718 in liver was lower than that of Fungizone. The concentration of AMPH-B in pleural exudate after NS-718 was intravenously injected was higher than that obtained with Fungizone. The results showed that NS-718 easily permeates leaky blood vessels at sites of inflammation (5). In the present study, the concentration of AMPH-B in lung after administration of a high dose of NS-718 was slightly higher than that after administration of AmBisome and this higher concentration was maintained although the differences were not statistically significant. The higher concentration of AMPH-B might have resulted in the greater efficacy of NS-718 in treatment of pulmonary infection.
Comparable therapeutic activities of lipid formulations and Fungizone have been reported when they were used at equivalent doses in murine models of cryptococcosis (7). Since the most devastating complication of cryptococcosis in immunocompromised humans is meningoencephalitis, disseminated cryptococcosis or cryptococcal meningitis has been popularly used as an animal model of cryptococcosis (3, 14). In our model of murine cryptococcosis, the route of inoculation was intratracheal. Since aspiration of the yeast cells results in pulmonary cryptococcosis and the first infectious site target for C. neoformans is the lung, the present animal model is closer to the natural course of infection irrespective of immune status. The reason for selecting the present model is the importance of pulmonary cryptococcosis as a disease entity, since delay in proper diagnosis and treatment may lead to life-threatening cryptococcal disease of the brain and meninges. It has been shown in previous animal studies with the YC-11 strain of C. neoformans that untreated control mice die during the fourth week after intratracheal inoculation (12). Although the mouse strain used previously is different from that used in the present study, we observed that both mouse strains show similar survival patterns.
Several kinds of lipid-based formulations were found to be well tolerated in higher doses but less effective than Fungizone when used at equivalent doses in murine models of cryptococcosis (2, 10). In the present study, NS-718 was the most effective lipid formulation of AMPH-B against cryptococcosis. In a multicenter study, although not well established, amphotericin B lipid complex was found to have apparently better clinical and microbiological activity against cryptococcal meningitis in patients with AIDS and was significantly better tolerated than amphotericin B but was not free of toxicities (16).
Although a study of acute lethal toxicity showed AmB-Poly(ɛ-caprolacton) nanoparticles to be less toxic than Fungizone and more toxic than AmBisome, improvement in the therapeutic index was suggested despite relatively lower stability (4). In our study, all mice died rapidly with a high-dose therapy with Fungizone because of the acute toxicity of AMPH-B, but NS-718 and AmBisome were well tolerated at equivalent doses, indicating a reduction in toxicity by lipid formulations. Reduction of cell growth in lung with prolonged survival suggests that NS-718, a novel lipid nanosphere-encapsulated AMPH-B, is effective in treatment of murine pulmonary cryptococcosis.
In conclusion, the results of the present studies are encouraging and further investigations for evaluation of NS-718 in treatment of meningoencephalitis and comparative toxicity profiles are needed to establish NS-718 as the most effective antifungal agent in human cryptococcal infections.
ACKNOWLEDGMENTS
We are grateful to Y. Tomii and J. Seki (Nippon-Shinyaku Co. Ltd., Kyoto, Japan), who provided us with NS-718, helped with the pharmacokinetic study, and gave useful suggestions for our experiments.
REFERENCES
- 1.Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brajtburg J, Elberg S, Travis S J, Kobayashi G S. Treatment of murine candidiasis and cryptococcosis with amphotericin B incorporated into egg lecithin-bile salt mixed micelles. Antimicrob Agents Chemother. 1994;38:294–299. doi: 10.1128/aac.38.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S C A, Muller M, Zhou J Z, Wright L C, Sorrel T C. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J Infect Dis. 1997;175:414–420. doi: 10.1093/infdis/175.2.414. [DOI] [PubMed] [Google Scholar]
- 4.Espuelas M S, Legrand P, Irache J M, Gamazo C, Orecchioni A M, Devissaguet J P, Ygartua P. Poly(ɛ-caprolacton) nanoparticles as an alternative way to reduce amphotericin B toxicity. Int J Pharm. 1997;158:19–27. [Google Scholar]
- 5.Fukui H, Koike T, Saheki A, Sonoke S, Yoshikawa H, Sasaki H, Tomii Y, Seki J. Proceedings of the 23rd International Symposium on Controlled Release of Bioactive Materials, Kyoto, Japan. 1996. A novel antifungal drug delivery system: lipid nano-sphere incorporating amphotericin B (LNS-AmB), abstr. 5026; p. 655. [Google Scholar]
- 6.Granich G G, Kobayashi G S, Krogstad D J. Sensitive high-pressure liquid chromatographic assay for amphotericin B which incorporates an internal standard. Antimicrob Agents Chemother. 1986;29:584–588. doi: 10.1128/aac.29.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graybill J R, Craven P C, Taylor R L, Williams D M, Magee W E. Treatment of murine cryptococcosis with liposome-associated amphotericin B. J Infect Dis. 1982;145:748–752. doi: 10.1093/infdis/145.2.748. [DOI] [PubMed] [Google Scholar]
- 8.Hay R J. Liposomal amphotericin B, AmBisome. J Infect. 1994;1:35–43. doi: 10.1016/s0163-4453(94)95956-0. [DOI] [PubMed] [Google Scholar]
- 9.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22(Suppl. 2):S133–144. [DOI] [PubMed]
- 10.Hostetler J S, Clemons K V, Hanson L H, Stevens D A. Efficacy and safety of amphotericin B colloidal dispersion compared with those of amphotericin B deoxycholate suspension for treatment of disseminated murine cryptococcosis. Antimicrob Agents Chemother. 1992;36:2556–2560. doi: 10.1128/aac.36.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakeya H, Udono H, Ikuno N, Yamamoto Y, Mitsutake K, Miyazaki T, Tomono K, Koga H, Tashiro T, Nakayama E, Kohno S. A 77-kilodalton protein of Cryptococcus neoformans, a member of the heat shock protein family, is a major antigen detected in the sera of mice with pulmonary cryptococcosis. Infect Immun. 1997;65:1653–1658. doi: 10.1128/iai.65.5.1653-1658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami K, Qifeng X, Tohyama M, Qureshi M H, Saito A. Contribution of tomour necrosis factor-alpha (TNF-α) in host defence mechanism against Cryptococcus neoformans. Clin Exp Immunol. 1996;106:468–474. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohno S, Otsubo T, Hara K, Tomii Y, Seki J. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. A new antifungal drug delivery system, lipid nano-sphere encapsulated amphotericin B (LNS-AmB), its evaluation in the rat model of invasive pulmonary aspergillosis, abstr. F109; p. 131. [Google Scholar]
- 14.Savoy A C, Lupan D M, Manalo P B, Roberts J S, Schlageter A M, Weinhold L C, Kozel T R. Acute lethal toxicity following passive immunization for treatment of murine cryptococcosis. Infect Immun. 1997;65:1800–1807. doi: 10.1128/iai.65.5.1800-1807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seki J, Sasaki H, Doi M, Yoshikawa H, Tanaka Y, Yamane S, Fukui H, Sonoke S, Yamamoto H, Hirose M, Ezure Y, Ando T, Ushimaru K, Sugiyama M. Lipid nano-sphere (LNS), a protein-free analogue of lipoproteins, as a novel drug carrier for parenteral administration IV. J Control Release. 1994;28:352–353. [Google Scholar]
- 16.Sharkey P K, Graybill J R, Johnson E S, Hausrath S G, Pollard R B, Kolokathis A, Mildvan D, Fan-Havard P, Eng R H K, Patterson T F, Pottage J C, Jr, Simberkoff M S, Wolf J, Meyer R D, Gupta R, Lee L W, Gordon D S. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1996;22:315–321. [PubMed] [Google Scholar]
- 17.Stevens D A. Overview of amphotericin B colloidal dispersion (amphocil) J Infect. 1994;1:45–49. doi: 10.1016/s0163-4453(94)95971-4. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Miyazaki T, Maesaki S, Mitsutake K, Kakeya H, Yamamoto Y, Yanagihara K, Hossain M A, Tashiro T, Kohno S. Detection of Cryptococcus neoformans gene in patients with pulmonary cryptococcosis. J Clin Microbiol. 1996;34:2826–2828. doi: 10.1128/jcm.34.11.2826-2828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi H, Uchida K, Kume H, Shinoda T, Watanabe K, Kusunoki T, Hiruma M, Ishizaki H. Report of the committee of clinical laboratory standards. Jpn J Med Mycol. 1994;36:61–86. [Google Scholar]