Abstract
Phosphoenolpyruvate carboxylase in Crassulacean acid metabolism plants during the day exists in dimeric form the activity of which is strongly inhibited by malate. Enzyme purified from Crassula leaves collected during the day and stored at −70°C for 49 days shows a steady progression of change from dimer to tetramer, and this change in oligomeric state is accompanied by a decrease in the sensitivity of the enzyme to inhibition by malate. At 10 minutes preincubation of enzyme after 11 days storage—which is composed of an equilibrium mixture of dimer and tetramer—with malate causes most of the enzyme to be converted to dimer and increases the sensitivity of the enzyme to malate inhibition during assay. Preincubation with phosphoenolpyruvate shifts the equilibrium toward the tetrameric form and reduces the maximal inhibition produced by 5 millimolar malate to less than 20%. However, none of the treatments used resulted in shifting the oligomerization equilibrium completely in either direction. Thus the question of whether some covalent modification of the enzyme, such as phosphorylation, is required to permit complete changes in equilibrium remains open.
Full text
PDF
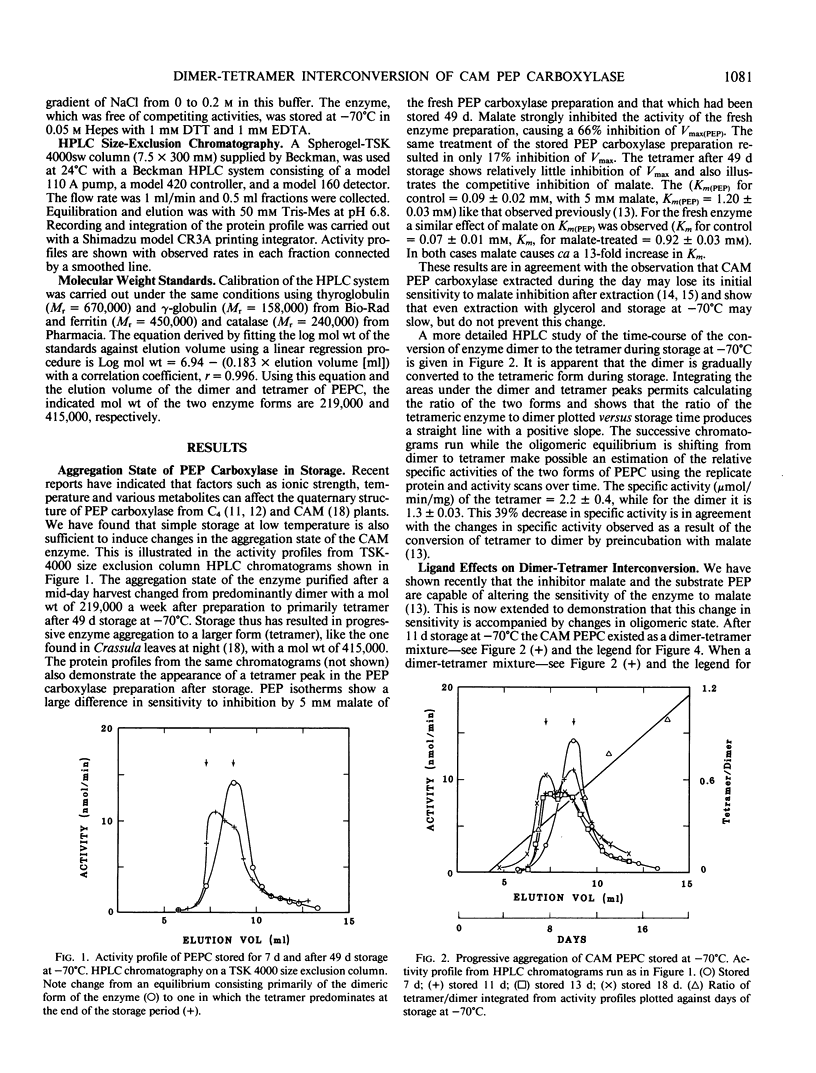

Selected References
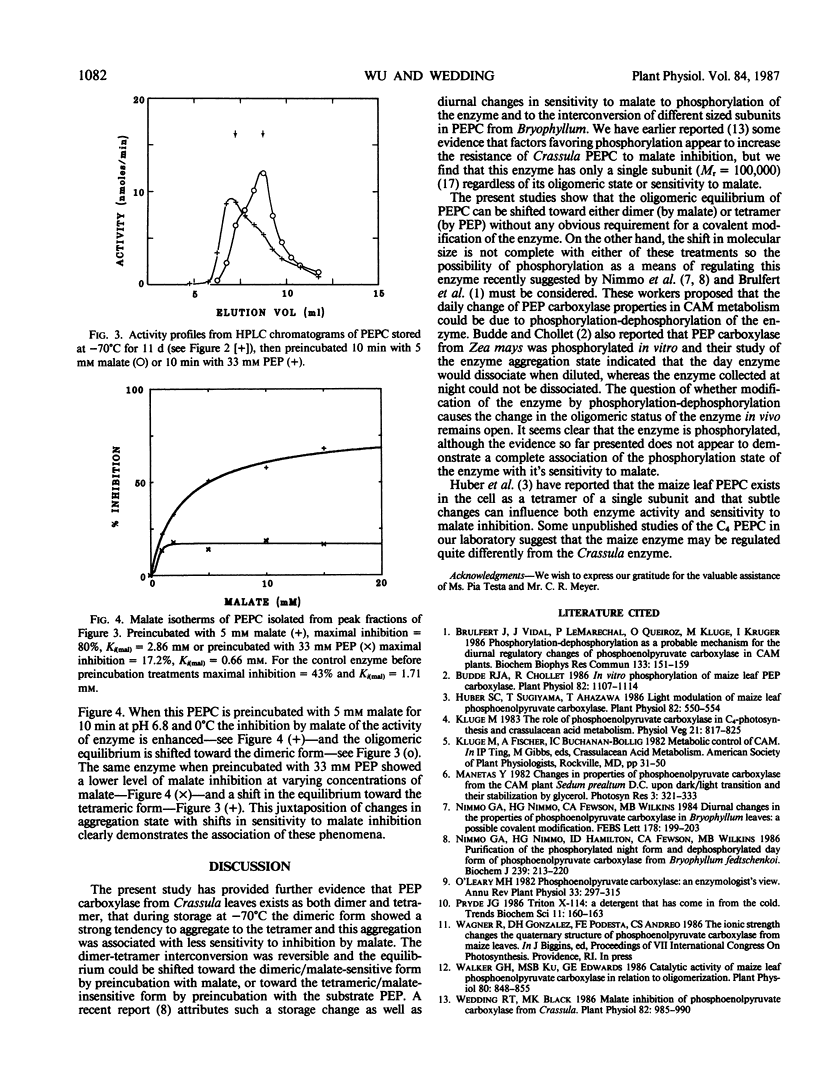
These references are in PubMed. This may not be the complete list of references from this article.
- Brulfert J., Vidal J., Le Marechal P., Gadal P., Queiroz O., Kluge M., Kruger I. Phosphorylation-dephosphorylation process as a probable mechanism for the diurnal regulatory changes of phosphoenolpyruvate carboxylase in CAM plants. Biochem Biophys Res Commun. 1986 Apr 14;136(1):151–159. doi: 10.1016/0006-291x(86)90889-2. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Chollet R. In vitro phosphorylation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986 Dec;82(4):1107–1114. doi: 10.1104/pp.82.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T., Akazawa T. Light modulation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986 Oct;82(2):550–554. doi: 10.1104/pp.82.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G., Hamilton I. D., Fewson C. A., Wilkins M. B. Purification of the phosphorylated night form and dephosphorylated day form of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochem J. 1986 Oct 1;239(1):213–220. doi: 10.1042/bj2390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Ku M. S., Edwards G. E. Catalytic activity of maize leaf phosphoenolpyruvate carboxylase in relation to oligomerization. Plant Physiol. 1986 Apr;80(4):848–855. doi: 10.1104/pp.80.4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K. Malate inhibition of phosphoenolpyruvate carboxylase from crassula. Plant Physiol. 1986 Dec;82(4):985–990. doi: 10.1104/pp.82.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Diurnal regulation of phosphoenolpyruvate carboxylase from crassula. Plant Physiol. 1985 Mar;77(3):667–675. doi: 10.1104/pp.77.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of phosphoenolpyruvate carboxylase from Crassula by interconversion of oligomeric forms. Arch Biochem Biophys. 1985 Aug 1;240(2):655–662. doi: 10.1016/0003-9861(85)90073-6. [DOI] [PubMed] [Google Scholar]


