Abstract
To identify topical antimicrobial preparations which may be effective in preventing the transmission of sexually transmitted diseases, we examined the activity of chlorhexidine gluconate (CHG) against Chlamydia trachomatis. Chlamydial elementary bodies were incubated with dilutions of CHG gel for various times from 0 to 120 min. An aliquot of each dilution was further diluted and was inoculated onto McCoy cell monolayers in individual wells in a 96-well microtiter plate. The cultures were incubated for 48 h, and the chlamydial inclusions were stained and counted. CHG gel diluted fourfold (0.0625% CHG) killed C. trachomatis serovar D, and CHG gel diluted eightfold (0.0313% CHG) killed serovar F immediately upon exposure. CHG gel diluted 16-fold (0.0156% CHG) killed serovar D, and CHG gel diluted 32-fold (0.0078% CHG) killed serovar F after 120 min of exposure. Alteration of the pH over the range of from 4 to 8 did not significantly affect its activity. The addition of 10% whole human blood decreased the CHG gel activity at 0 min but had no significant effect after 120 min of exposure. We conclude that CHG gel may be effective topically against C. trachomatis at concentrations that can be used and under conditions that are found in the female genital tract and that further studies of its antimicrobial efficacy and toxicity in vivo are warranted.
Given the lack of a vaccine or other effective means of preventing most sexually transmitted diseases (STDs), new approaches to prevention are urgently needed. One new approach to reducing the transmission of Chlamydia trachomatis and other STD pathogens would be the topical application of antimicrobial agents intravaginally before sexual contact. The goal would be to kill the STD pathogens, including C. trachomatis, Neisseria gonorrhoeae, human immunodeficiency virus, human papillomavirus, herpes simplex virus, and Trichomonas vaginalis, before infection is initiated and at the same time avoid adverse effects on the normal flora. A successful topical microbicide should also be nontoxic to the vaginal epithelium and should be able to be easily self-administered prior to sexual contact.
A number of topical microbicides, including nonoxynol-9 (2, 6), chlorhexidine (7), defensins, and protegrins (12), have been examined for their in vitro effects on Chlamydia and other STD pathogens (8). Chlorhexidine (1, 9) is a cationic antiseptic that has been used extensively in hospitals, mostly for disinfection of skin and mucous membranes. It is a member of the bis-biguanide family and has broad-spectrum inhibitory activity against all bacteria and against yeast and fungi. The positively charged chlorhexidine molecule is thought to interact initially with the negatively charged cell membrane. This interaction is followed by damage to the cell’s cytoplasmic membrane, resulting in leakage of the cytoplasmic contents. Additionally, chlorhexidine has been examined extensively for host toxicity and has been found to be safe (9). It has no systemic side effects and low carcinogenic and mutagenic activities. Given its low toxicity and broad-spectrum antimicrobial activity, chlorhexidine thus has potential as a vaginal microbicide.
C. trachomatis is an atypical gram-negative bacterium that has evolved a biphasic life cycle that facilitates its efficient transmission. The bacterium infects the columnar epithelial cells of the human genital and respiratory tracts and replicates within these cells in membrane-bound inclusions in a form designated the reticulate body. When the infected host cell has been depleted of its nutrients by the newly replicated bacteria, the bacterium changes its morphology to that of the infectious, metabolically inactive form designated the elementary body (EB). EBs are released from the depleted infected cell and either can infect adjacent cells or can be transmitted to uninfected individuals through sexual contact. The EB surface contains several cysteine-rich proteins that provide rigidity to the cell wall through intra- and intermolecular disulfide bonds (3). Even though the bacterium does not produce peptidoglycan, its cell wall is sufficiently strengthened by these sulfhydryl bonds such that the organism is resistant to environmental degradation. If the organism in its EB form can be killed with a topical microbicide before it infects the target columnar epithelial cell, the chlamydial infectious process would be blocked. In one evaluation, chlamydial EBs were sensitive to chlorhexidine gluconate (CHG) (7), but whether they can be killed by CHG formulated in a topical gel has not been examined. In the studies reported here, we examined the in vitro susceptibility of C. trachomatis to CHG in a gel.
MATERIALS AND METHODS
Chlamydia strains.
Two standard strains of C. trachomatis were used in these studies, including strains of serovars D (strain UW-3/Cx) and F (strain UW-6/Cx). These strains were propagated in McCoy mouse fibroblast cells (ATCC CRL 1696), purified on Renografin density gradients (4), and stored at −70°C in SPG (219 mM sucrose, 3.8 mM KH2PO4, 8.6 mM Na2HPO4, 4.9 mM glutamic acid [pH 7.0]) (5) until needed. Their serotypes were confirmed with monoclonal antibodies by an inclusion typing method prior to use (11).
Antimicrobial agents.
CHG (0.25%) was added to a water-based gel composed of methylparaben, glucono-δ-lactone, sodium hydroxide, propylene glycol, glycerin, and hydroxyethyl cellulose with no other antimicrobial compounds and was provided by the manufacturer (Johnson and Johnson, Raritan, N.J.). Placebo gel containing no CHG was also provided for testing of antichlamydial activity. Penicillin G and polymyxin B sulfate were purchased as powders from Sigma Chemical Co. (St. Louis, Mo.) and were prepared as sterile stock solutions in SPG or tissue culture medium on the day of assay.
Antimicrobial assays.
Three assays were carried out to examine the susceptibility of C. trachomatis to the CHG gel, including assays for determination of the preinoculation minimal cidal concentration (MCC), the preinoculation MCC in the presence of human blood, and the preinoculation MCC at different pH values.
Preinoculation MCC.
One day prior to the assay for the preinoculation MCC, 9 × 104 antibiotic-free McCoy cells were added to individual wells in a 96-well microtiter plate and the plate was incubated for 24 h to permit the cells to form a confluent monolayer. To mimic topical killing action, we exposed the Chlamydia to the antimicrobial prior to inoculation. A total of 106 C. trachomatis serovar D or F inclusion-forming units (IFU) in SPG were added to equal volumes of dilutions of CHG gel or dilutions of polymyxin B-positive or penicillin G-negative control antibiotic solutions for 0, 30, 60, 90, or 120 min. Undiluted CHG gel had an initial concentration of 0.25% CHG. However, since the undiluted gel was too viscous to ensure proper mixing, it was diluted twofold in SPG before the organisms were added, further diluting the gel fourfold. Thus, the first test concentration contained 0.0625% CHG, as indicated in Fig. 1 to 3. The separate polymyxin B-positive control or penicillin G-negative control each had an initial test concentration of 1,000 μg of drug per ml after addition of the organism. After each time period, a 5-μl aliquot of the organism and drug mixture was added to 195 μl of SPG. A 100-μl aliquot of this dilution was then added to the McCoy cell monolayer in a 96-well microtiter plate, and the plate was centrifuged at 1,200 × g for 1 h to inoculate the tissue culture cells. Unbound organisms in drug were removed, and 200 μl of antibiotic-free Eagle’s minimal essential medium (EMEM) plus 10% fetal calf serum and 0.5 μg of cyclohexamide per ml were added. The cultures were incubated for 48 h, the cells were fixed and stained with the genus-specific fluorescein isothiocyanate-labeled monoclonal antibody CF2 (10), and the inclusions were counted and any toxicity was noted. The lowest concentration of CHG gel which showed 100% killing was defined as the MCC. All assays were performed in triplicate, three fields per well were counted, the inclusion counts were averaged, and a polymyxin B-positive control and penicillin G-negative control were run in parallel. Two additional wells inoculated with either the C. trachomatis organisms with no drug (organism control) or SPG only were also included to monitor normal inclusion formation and McCoy cell morphology. Percent killing in tests with CHG gel, the polymyxin B-positive control, and the penicillin G-negative control were calculated by the following formula: [(mean IFU of organism control − mean IFU of test)/mean IFU of organism control]100. Placebo gel alone with no CHG was also tested for its antichlamydial activity by this protocol. A level of 100% killing represents a decrease of at least 10−4 organisms, from the original 106 IFU in the inoculum to 160 IFU, the minimum number of IFU which can be counted in our assay.
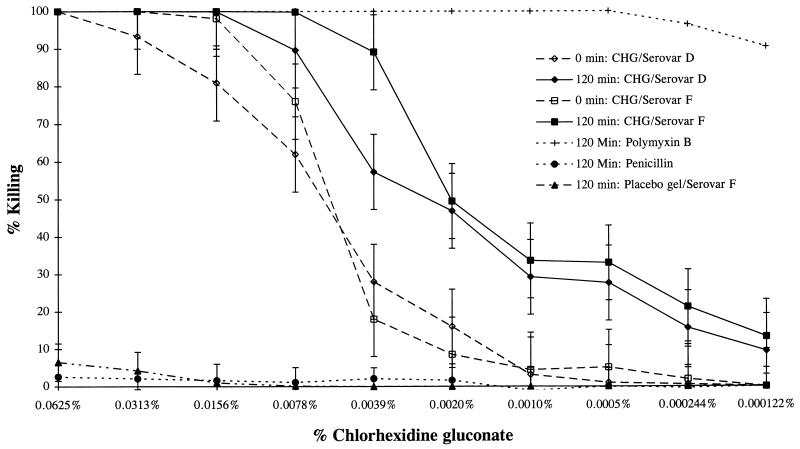
FIG. 1.
Killing of C. trachomatis serovars D and F preexposed to CHG gel for 0 or 120 min. Tests were also performed at time periods of 30, 60, and 90 min, but only the values obtained at 0 and 120 min are indicated. The values from tests with the controls polymyxin B, penicillin G, and placebo gel with no CHG at 120 min are also indicated. The initial test concentrations of polymyxin B and penicillin G were 1,000 μg/ml.
FIG. 3.
Killing of C. trachomatis serovar D after 120 min of exposure to CHG gel adjusted to different pH values. Polymyxin B and penicillin G were tested at pH 7.0 only, but all organism controls were run at each pH and were used to calculate the percent killing at that pH.
Preinoculation MCCs in the presence of human blood.
We also examined the effect of human blood on the MCCs using the same preinoculation method described above, with the exception that 10% human blood at pH 7.0 was added to the CHG gel and the organism control dilutions. Polymyxin B-positive control and penicillin G-negative control dilutions were run in the absence of human blood at pH 7.0. Blood was collected from one of the authors, who was not receiving antibiotics and who has no antichlamydial antibodies. Whole blood was collected in tubes containing sodium citrate, an anticoagulant generally used for routine bacteriologic blood cultures, and was stored at 4°C, and the MCC assays were performed within 1 week of blood collection.
Preinoculation MCCs at different pH values.
In addition, we examined the effect of different pH values on the MCCs using the same preinoculation method described above, except that the SPG pH was adjusted to 4, 5, 6, 7, or 8 with 1 M Na2HPO4 or 1 M KH2PO4 before the C. trachomatis IFU were added. The percent killing by the CHG gel at each different pH value was derived by comparison of the IFU to the organism control IFU at that same pH.
Cell toxicity assay.
The fluorometric and colorimetric growth indicator alamarBlue (Alamar Biosciences, Inc., Sacramento, Calif.) was used to ensure that the CHG gel, diluted 40-fold in the preinoculation MCC protocol, did not cause toxicity to the McCoy cells. A total of 100 μl of each diluted drug mixture was added to three wells containing a McCoy cell monolayer for 1 h, and the drug mixture was then replaced with 200 μl of EMEM with cycloheximide. After 48 h of incubation, 20 μl of alamarBlue was added to each well, the plates were incubated for an additional 6 h, and the absorbance values at 570 and 600 nm were determined. Dilutions of phosphate buffered saline in SPG were used as negative cell controls, and wells with no cells were used as positive controls. The percent inhibition of McCoy cells compared to that of EMEM-negative controls was calculated by the following formula: 100 × {[(mean OD570 − mean OD600 of negative cell control) − (mean OD570 − mean OD600 of test)]/(mean OD570 − mean OD600 of negative cell control)}, where OD570 is the optical density at 570 nm.
RESULTS
Preinoculation MCC.
To closely mimic the topical action of an ideal microbicide, we developed the preinoculation MCC assay described in Materials and Methods in which C. trachomatis was directly exposed to the antimicrobial agent before inoculation of the organisms onto McCoy cells. Using this assay, we found that CHG gel diluted 4- or 8-fold killed both C. trachomatis serovars (Fig. 1) immediately upon exposure and CHG gel diluted to 16- or 32-fold killed both serovars after 120 min of exposure. There was little variation between serovars in the degree of susceptibility to the CHG gel (Fig. 1). Using this method, we saw no visible toxicity to the cultured McCoy cells. The use of gel alone containing no CHG resulted in no killing of Chlamydia (Fig. 1).
Preinoculation MCCs in the presence of human blood.
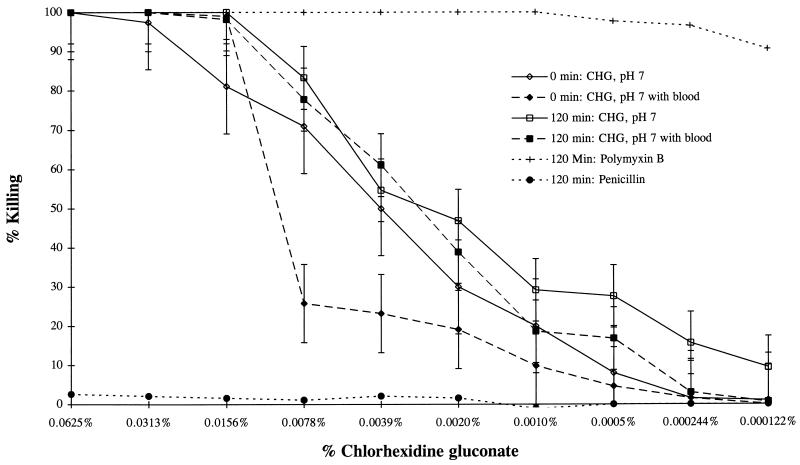
Because we were concerned that blood normally found during the menstrual cycle might alter the activity or duration of a vaginal microbicide, we carried out a preinoculation MCC assay in the presence of 10% human blood at 0 and 120 min after exposure. We found that the presence of blood decreased the CHG gel activity at 0 min but had no effect after 120 min of C. trachomatis exposure (Fig. 2). The anticoagulant sodium citrate was not a factor in this assay because the organism control was also preexposed to 10% whole human blood in the absence of CHG.
FIG. 2.
Killing of C. trachomatis serovar D by CHG gel at pH 7 in the presence and absence of 10% whole human blood. Organism controls were tested at pH 7.0 in the presence and absence of 10% whole human blood and were used to calculate the percent killing under each condition. Polymyxin B and penicillin G were tested at pH 7.0 without blood.
Preinoculation MCCs at different pH values.
Because the pH of the vagina can vary from pH 4 in healthy women to pH 5 to 6 in women with bacterial vaginosis, pH 7 in women who are postmenopausal or bleeding, and pH 8 after semen is deposited, we examined the effect of pH on the preinoculation MCCs. Assays were performed in SPG preadjusted with 1 M Na2HPO4 or 1 M KH2PO4 to pH 4, 5, 6, 7, or 8 before the C. trachomatis inoculum was added. As can be seen in Fig. 3, altering the pH did not significantly affect the MCCs when the organisms were exposed to the pH-adjusted CHG gel for 120 min.
Cell toxicity assay.
To directly measure toxicity not visible microscopically in the preinoculation MCC assay, we determined the percent inhibition of McCoy cells exposed to CHG gel using the oxidation-reduction indicator alamarBlue (Table 1). We found insignificant inhibition of the cells by the first dilution of CHG gel (8.5% inhibition). Once the CHG gel had been further diluted, no cell culture toxicity over that seen with the phosphate-buffered saline control was observed (Table 1).
TABLE 1.
Determination of cell toxicity under preinoculation MCC assay conditions
| CHG concn (%)a | Mean OD570 − mean OD600 | SD | % Inhibition |
|---|---|---|---|
| 0.00625 | 0.379 | 0.064 | 8.5b |
| 0.003125 | 0.398 | 0.045 | 3.9 |
| 0.0015625 | 0.414 | 0.052 | 0.0 |
| 0.0007825 | 0.413 | 0.039 | 0.1 |
| 0.00039 | 0.414 | 0.041 | 0.0 |
| 0.000195 | 0.414 | 0.007 | 0.0 |
| 0.0000975 | 0.414 | 0.045 | −0.1 |
| 0.00005 | 0.414 | 0.038 | 0.0 |
| 0.000025 | 0.414 | 0.032 | 0.0 |
| 0.0000125 | 0.414 | 0.032 | 0.0 |
| 0 | 0.414 | 0.052 | 0.0 |
Concentrations of CHG diluted 40-fold, plated directly onto McCoy cell monolayers, incubated for 1 hour, and replaced with EMEM.
A value of 50% or greater is generally considered toxic.
DISCUSSION
Our goal in these studies was to identify a topical compound that has antimicrobial activity against C. trachomatis, an important STD pathogen. CHG prepared as a gel for topical use has been shown to be broadly active as an antimicrobial agent and has been shown to be nontoxic. In the studies reported here, we showed that CHG gel is active against C. trachomatis when the organisms are exposed before inoculation onto the cell culture system. CHG gel diluted at least four- to eightfold was immediately active against both C. trachomatis serovars tested. In actual use, at least an approximate twofold dilution of CHG gel would be expected since the typical volume of vaginal fluid is 3.5 ml, close to the amount of CHG gel that could be applied vaginally in humans. Upon application, CHG gel would immediately be active and would be minimally diluted, but with time and sexual activity, its concentration would decrease. We found, however, that at times of exposure of up to 120 min, the CHG gel is active at a dilution of 16- or 32-fold. Furthermore, the CHG gel remained active against both C. trachomatis serovars tested, demonstrating that it is most likely broadly antichlamydial. The killing activity of the CHG gel was due to the CHG since the gel alone had no antichlamydial activity.
The antimicrobial activities of topical microbicides applied vaginally may be affected by menstrual blood or the vaginal pH. It has been shown that chlorhexidine activity is decreased or eliminated by serum (9). To examine its antichlamydial activity under various conditions that might be found in the vagina, we tested CHG gel activity against C. trachomatis in the presence of whole blood or at different pH values. Under the conditions of our assay, the killing of C. trachomatis by the CHG gel decreased in the presence of whole human blood at 0 min but was unaffected at 120 min. Similarly, altering the preinoculation pH from 4 to 8 did not affect the antichlamydial activity of the CHG gel. Thus, the antichlamydial activity of CHG gel was present under conditions that might commonly be encountered in the human vagina. Our results, showing that the antichlamydial activity of CHG is not affected by whole blood, differ from the previously reported finding that chlorhexidine activity is decreased by exposure to serum (9). In the previous studies, however, chlorhexidine stock solutions and not CHG gel were examined.
Our results show that the cationic detergent CHG in the CHG gel is able to prevent chlamydial infection in vitro. This finding is not surprising since chlorhexidine has previously been shown to have antichlamydial activity (7). However, our findings may have practical implications. We have shown that CHG gel is active against C. trachomatis. An examination of its activity against other STD pathogens such as N. gonorrhoeae and human immunodeficiency virus is warranted. CHG gel is already formulated as a viscous gel that would stay in the vagina for hours, thus remaining in place long enough to provide protection when it is instilled shortly before sexual contact.
In summary, we have shown that CHG gel is active against C. trachomatis, resulting in 100% killing immediately at a 4- to 8-fold dilution and after 120 min at a 16- to 32-fold dilution. The antichlamydial activity of CHG gel is not affected by the presence of human blood at 120 min or alteration of the pH from 4 to 8. We thus conclude that CHG gel may be an effective topical antimicrobial agent at concentrations that can be used and under conditions that are found in the vagina. Further work to test its activity against other STD pathogens such as N. gonorrhoeae and human immunodeficiency virus are warranted. Furthermore, CHG gel should be examined for its toxicity to vaginal and cervical tissues both in an animal model and in humans. Our results are consistent with the concept that antimicrobial compounds can be developed or identified, which, when self-administered intravaginally, can prevent acquisition of STDs.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-39061 and AI-31448 from the National Institutes of Health.
REFERENCES
- 1.al Tannir M A, Goodman H S. A review of chlorhexidine and its use in special populations. Spec Care Dentist. 1994;14:116–122. doi: 10.1111/j.1754-4505.1994.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 2.Benes S, McCormack W M. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vitro. Antimicrob Agents Chemother. 1985;27:724–726. doi: 10.1128/aac.27.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatch T P, Allan I, Pearce J H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984;157:13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard L, Orenstein N S, King N W. Purification on Renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974;27:102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson E B, Smadel J E. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951;53:326–331. doi: 10.1093/oxfordjournals.aje.a119457. [DOI] [PubMed] [Google Scholar]
- 6.Kelly J P, Reynolds R B, Stagno S, Louv W C, Alexander W J. In vitro activity of the spermicide nonoxynol-9 against Chlamydia trachomatis. Antimicrob Agents Chemother. 1985;27:760–762. doi: 10.1128/aac.27.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyons, J. M., and J. I. J. Ito. 1995. Reducing the risk of Chlamydia trachomatis genital tract infection by evaluating the prophylactic potential of vaginally applied chemicals. Clin. Infect. Dis. 21(Suppl. 2):S174–S177. [DOI] [PubMed]
- 8.Qu X D, Harwig S S, Oren A M, Shafer W M, Lehrer R I. Susceptibility of Neisseria gonorrhoeae to protegrins. Infect Immun. 1996;64:1240–1245. doi: 10.1128/iai.64.4.1240-1245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell A D, Day M J. Antibacterial activity of chlorhexidine. J Hosp Infect. 1993;25:229–238. doi: 10.1016/0195-6701(93)90109-d. [DOI] [PubMed] [Google Scholar]
- 10.Stamm W E, Tam M, Koester M, Cles L. Detection of Chlamydia trachomatis inclusions in McCoy cell cultures with fluorescein-conjugated monoclonal antibodies. J Clin Microbiol. 1983;17:666–668. doi: 10.1128/jcm.17.4.666-668.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suchland R J, Stamm W E. Simplified microtiter cell culture method for rapid immunotyping of Chlamydia trachomatis. J Clin Microbiol. 1991;29:1333–1338. doi: 10.1128/jcm.29.7.1333-1338.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasin B, Harwig S S L, Lehrer R I, Wagar E A. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect Immun. 1996;64:709–713. doi: 10.1128/iai.64.3.709-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]