Abstract
Various suggestions have been made for empirical pharmacodynamic indices of antibiotic effectiveness, such as areas under the drug concentration-time curve in serum (AUC), AUC>MIC, AUC/MIC, area under the inhibitory curve (AUIC), AUC above MIC, and time above MIC (T>MIC). In addition, bacterial growth and killing models, such as the Zhi model, have been developed. The goal of the present study was to compare the empirical behavior of the Zhi model of bacterial growth and killing with the other empirical pharmacodynamic indices described above by using simulated clinical data analyzed with the USC*PACK PC clinical programs for adaptive control of drug therapy, with one model describing a concentration-dependent antibiotic (tobramycin) and another describing a concentration-independent antibiotic (ticarcillin). The computed relative number of CFU was plotted against each pharmacodynamic index, with each axis parameterized over time. We assumed that a good pharmacodynamic index should present a clear and continuous relationship between the time course of its values and the time course of the bacterial killing as seen with the Zhi model. Preliminary work showed that some pharmacodynamic indices were very similar. A good sensitivity to the change in the values of the MIC was shown for AUC/MIC and also for T>MIC. In addition, the time courses of some other pharmacodynamic indices were very similar. Since AUC/MIC is easily calculated and shows more sensitivity, it appeared to be the best of the indices mentioned above for the concentration-dependent drug, because it incorporated and used the MIC the best. T>MIC appeared to be the best index for a concentration-independent drug. We also propose a new composite index, weighted AUC (WAUC), which appears to be useful for both concentration-dependent and concentration-independent drugs.
Success in antibiotic therapy is defined by bacterial killing and by improvement in the patient’s clinical status. It is a retrospective measure. However, before antibiotic therapy is begun, the choice of the drug and the dosage regimen must be planned in advance to achieve maximum predicted efficacy with a tolerable risk of toxicity. Adaptive control of antibiotic dosage regimens, by using pharmacokinetic models, can predict (and therefore control) plasma antibiotic concentrations (2). However, drug levels at the site of the infection itself may be somewhat different from the plasma drug concentrations, except during septicemia when the infection is in the bloodstream. Time delays exist between antibiotic administration and the achievement of antibiotic efficacy. Because of this, plasma antibiotic concentrations at time t cannot be automatically correlated with pharmacodynamic effect at the site of the infection at the same time t, even though general improvement in clinical status can be empirically correlated with such plasma antibiotic concentrations.
Various empirical pharmacodynamic indices for a 24-h period of therapy using plasma or serum antibiotic levels have been proposed to predict antimicrobial effectiveness at steady state and at the site of infection (17–20). At the steady state, their values at the end of a typical 24-h period appear to be related to the success or failure of therapy—some of them for a concentration-dependent drug, some of them for a concentration-independent drug (8, 11, 18, 20). However, the initial exposure to effective serum antibiotic levels is also most important and may be necessary to minimize the emergence of adaptive resistance (4) and as well as the emergence of resistant bacterial subpopulations (5). Indeed, the time course of pharmacodynamic indices during the initial 24 h of therapy appears to be especially important in achieving early success in therapy. With the Zhi model, as the number of CFU is calculated and plotted over time, a dynamic view of the other empirical pharmacodynamic indices (the evolution of each of the other indices over time) has been studied here. The value of each index was computed throughout the initial 24 h of therapy, and the index value was also found at the end of the initial 24-h period, rather than at some subsequent steady state. This was done to emphasize the importance of rapid and effective bacterial killing at the start of therapy, especially for life-threatening infections.
The goal of the present study was to compare the bacterial growth and killing calculated according to the model proposed by Zhi et al. (22) with the various empirical pharmacodynamic index values and their time courses during a typical simulated first day of therapy. In this analysis, we have assumed that a good pharmacodynamic index should show a clear and continuous relationship between its values and the bacterial killing observed with the Zhi model: that is, that during periods of bacterial killing, the value of the pharmacodynamic index under consideration should increase, and during periods of bacterial growth, the index should decrease (or at least increase only very slowly).
MATERIALS AND METHODS
Pharmacokinetic model.
We chose to use a one-compartment pharmacokinetic model with intravenous administration. With maximum a posteriori probability (MAP) Bayesian fitting to individual patient data, plasma antibiotic levels can be predicted in clinical situations with such a linear one-compartment model. Using pharmacokinetic population data available in the USC*PACK PC clinical programs (9, 10), we simulated typical clinical treatment situations for two representative antibiotics by using a representative simulated adult patient (age, 30 years; height, 175 cm [69 in.]; weight, 70 kg; and creatinine clearance, 120 ml/min). We assumed he was being treated for Pseudomonas aeruginosa septicemia. Antibiotic concentrations at the site of the bloodstream infection were assumed to be equal to serum antibiotic concentrations. Two patient data files were created: one for a concentration-dependent drug, tobramycin, and one for a concentration-independent drug, ticarcillin. The dosage regimens used in these simulations were 200 mg of tobramycin infused over 30 min every 12 h (slightly less than 6 mg/kg per day) and 6 g of ticarcillin infused over 30 min every 8 h (slightly more than 260 mg/kg per day). Table 1 shows the pharmacokinetic parameter values for each drug and the characteristics for each dosage regimen.
TABLE 1.
Tobramycin and ticarcillin population pharmacokinetic parameter values and treatment characteristicsa
| Antimicrobial agent | V (liter/kg) | kel (h−1) | D (mg) | Cmax (μg/ml) | Cmin (μg/ml) |
|---|---|---|---|---|---|
| Tobramycin | 0.25 | 0.39 | 400 | 10.4 | 0.12 |
| Ticarcillin | 0.21 | 0.63 | 18,000 | 350.00 | 3.10 |
kel, total elimination rate constant; D, total dose over 24 h; Cmax, peak drug level (at the end of the first intravenous dose); Cmin, trough drug level (just before the second dose).
Zhi pharmacodynamic model.
Bacterial growth and killing were described by computing the relative number of CFU versus time. CFU were computed with the Zhi model (22), which states that
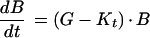 |
1 |
where B is the initial bacterial inoculum (CFU per milliliter), G is the rate constant for exponential bacterial growth of a single bacterial population in the absence of antibiotics (per hour) Kt is the instantaneous rate constant for bacterial kill in the presence of the antibiotic (per hour), which depends on the profile of serum antibiotic levels, Ct (micrograms per milliliter) present at any time t.
Kt follows a sigmoid Emax model:
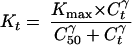 |
2 |
where Kmax is the maximum possible rate constant for bacterial killing (per hour) C50 is the antibiotic concentration which produces a kill rate equal to Kmax/2 (micrograms per milliliter), and γ is the Hill sigmoidicity coefficient.
Killing was computed by using values for G, Kmax, and γ previously obtained (1, 3) for P. aeruginosa in the presence of tobramycin or ticarcillin (Table 2).
TABLE 2.
Pharmacodynamic parameter values of the effect model used in simulation for P. aeruginosa
| Antimicrobial agent | G (h−1) | Kmax (h−1) | γ |
|---|---|---|---|
| Tobramycin | 0.995 | 7.115 | 0.416 |
| Ticarcillin | 0.995 | 1.882 | 0.902 |
The USC*PACK PC clinical programs for adaptive control of tobramycin and ticarcillin therapy compute serum antibiotic concentrations every 6 min. The pharmacodynamic effect of bacterial killing was computed by using these concentration profiles as input to the effect model described in equation 1 above.
When the serum antibiotic level is equal to the pharmacodynamic MIC, the bacterial killing rate equals the bacterial growth rate, the bacterial population remains unchanged, and equation 1 becomes
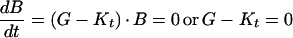 |
3 |
The bacterial apparent growth rate (G − Kt) is then equal to zero. By combining equations 2 and 3, an expression relating C50 to the pharmacodynamic MIC (zMIC) can be obtained (13):
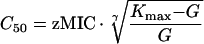 |
4 |
By combining equations 1, 2, and 4, the time course of the bacterial population can be computed by using a model for which the relevant variables (for a single antibiotic and for a single bacterial population with an unchanging growth rate constant and for which the MIC of the antibiotic is unchanging) are the serum antibiotic levels at time t (Ct), B, G, Kmax, the zMIC, and γ (14).
Computation of pharmacodynamic indices.
The pharmacodynamic indices generally proposed to correlate with antimicrobial effectiveness at the steady state have been the area under the drug concentration-time curve in serum (AUC); the AUC when C > MIC (AUC>MIC) (the AUC above the MIC; the AUC divided by the MIC, or AUC/MIC; the area under the inhibitory curve (AUIC), or the AUC when C > MIC divided by the MIC; and the time above the MIC (T>MIC) (Table 3) (18). All indices were computed versus time by employing an approximate integration based on the trapezoidal method (7). The accuracy of this method depends on a factor of 1/n2, for which n is the number of trapezoids which are used (here, 10 per h, with serum drug concentrations computed every 6 min).
TABLE 3.
Review of pharmacodynamic indices for a 24-h period for antimicrobial agents
| Pharmacodynamic index | Mathematical expressiona | Dimension |
|---|---|---|
| AUC | μg · h · ml−1 | |
| AUC>MIC | μg · h · ml−1 | |
| AUC above MIC | μg · h · ml−1 | |
| AUC/MIC | h | |
| AUIC | ||
| h | ||
| T > MIC | T1,i − T0,i, while C > MIC | h |
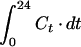 |
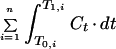 |
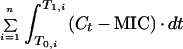 |
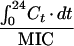 |
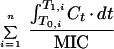 |
A dynamic view of the various empirical pharmacodynamic indices was introduced here in order to relate them to the Zhi model. Each simulation was performed throughout the initial 24 h (first doses) of therapy, and the final index value was obtained at the end of the initial 24-h period, rather than for a typical 24-h period at steady state, as others have done. Continuing this dynamic view, it is noteworthy that the term T>MIC has had several different meanings. Some have used this name to refer instead to the percent of the time during the dose interval in the steady state when the serum drug concentrations are at least the MIC. Another somewhat similar definition has been the percent of the time during a typical day in the steady state that the serum drug concentrations are at least the MIC. However, the name itself specially refers to the total time, during some defined time period, that the concentrations are at least the MIC. In the present paper, we have used the term to mean the total time during the first 24 h of therapy that the serum drug concentrations are at least the MIC. This index will progress from the minimum value of zero toward the maximum value (here 24 h) as the duration of therapy progresses from the very beginning (time zero) to the completion of the first 24 h of therapy.
Because the pharmacodynamic indices are many and varied, preliminary work was first done to compare their dimensional equations, their dynamic evolution over time, and the index value at the end of the initial 24-h period. They were then evaluated to select the most useful indices for comparison with the Zhi model.
Simulation.
Tobramycin and ticarcillin profiles in serum were simulated by using the USC*PACK clinical programs (10). The time courses of the computed CFU were then plotted against the time courses of each pharmacodynamic index (MATLAB software). Each axis was parameterized versus time (as a phase-space plot, like a hysteresis loop), for different MICs of each drug. The MICs of tobramycin studied were 16, 8, 4, 2, 1, 0.5, 0.25, and 0.125 μg/ml, and those of ticarcillin were 128, 64, 32, 16, 8, 4, 2, and 1 μg/ml (21). The initial inoculum was always assumed to be 106 CFU/ml.
RESULTS
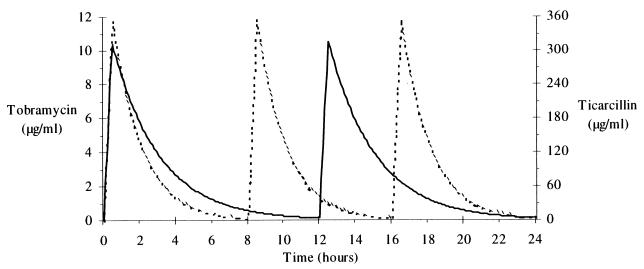
The resulting simulated tobramycin and ticarcillin serum drug level profiles obtained with their respective population models are shown in Fig. 1.
FIG. 1.
Tobramycin (solid line) and ticarcillin (dotted line) plasma drug level profiles.
Study, comparison, and selection of pharmacodynamic indices.
Table 3 shows the different pharmacodynamic indices, their mathematical expressions, and their dimensional equations. Comparison of pharmacodynamic indices was first performed for those indices having the same dimensional units. Several indices were found to be very similar. For example, AUIC and AUC/MIC were indistinguishable. For each MIC and for both antibiotic agents, a linear relationship between AUIC and AUC/MIC was found. This is probably because the area not included in the computation of the AUIC was extremely small, because the serum drug levels were above the MIC the great majority of the time. The method of calculating the index value is simpler for AUC/MIC than for AUIC. In addition, regarding calculation of the AUC>MIC and AUC above the MIC during the first 24-h period (Table 3), Ct − MIC was nearly equal to Ct for low MICs, and for each antibiotic-bacterium pair. The comparison of the mathematical expressions of AUC>MIC and AUC above MIC shows that AUC>MIC is nearly equivalent to AUC above the MIC for low MICs.
Because of this, only four pharmacodynamic indices were finally compared to the computed CFU by using the Zhi model. They were AUC, AUC>MIC, AUC/MIC, and T>MIC.
Comparison between pharmacodynamic indices and the Zhi model of bacterial growth and killing.
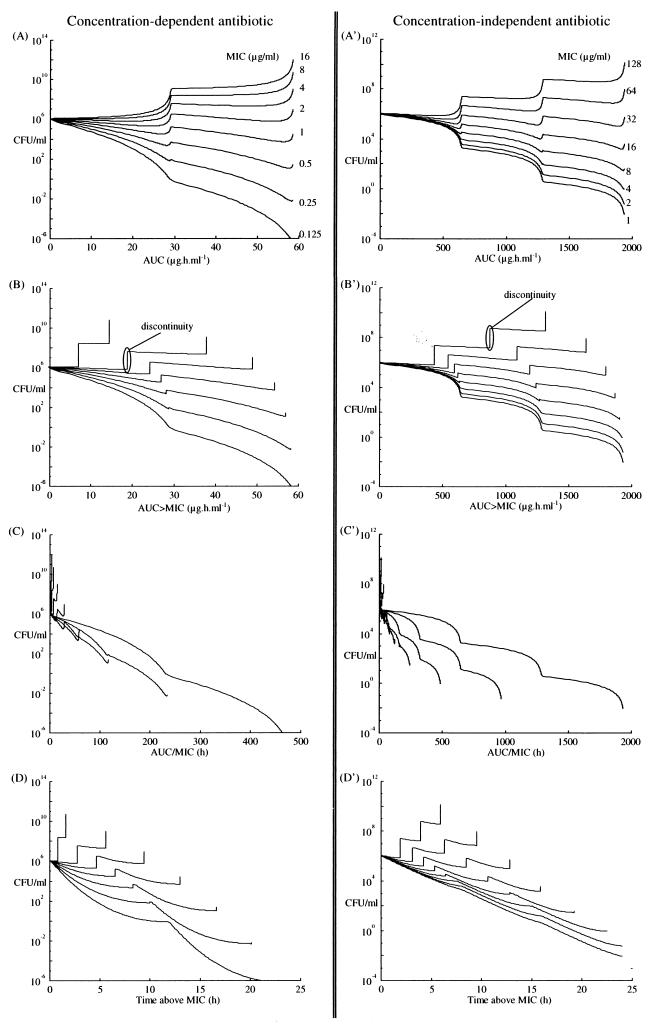
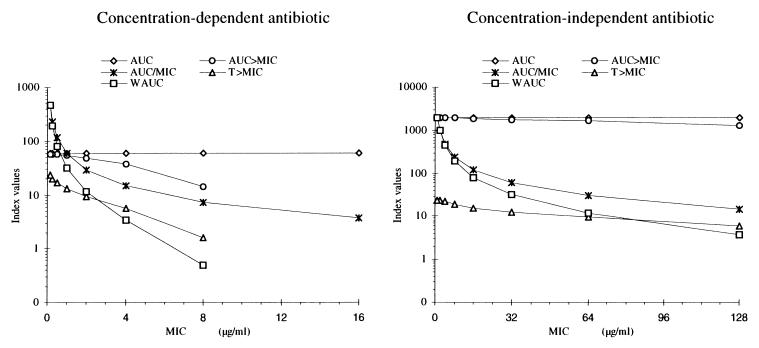
Plots of CFU versus AUC, AUC>MIC, AUC/MIC, and T>MIC, are shown in Fig. 2 for tobramycin (panel A for AUC, B for AUC>MIC, C for AUC/MIC, and D for T>MIC) and for ticarcillin (panel A′ for AUC, B′ for AUC>MIC, C′ for AUC/MIC, and D′ for T>MIC). Figure 3 shows the relationship between final pharmacodynamic index values found at the end of the first 24-h period (on a logarithmic scale) and the MICs.
FIG. 2.
CFU time course versus each pharmacodynamic index for a concentration-dependent drug, tobramycin, and for a concentration-independent drug, ticarcillin, during the first 24-h period of therapy, determined with the previous plasma drug level profiles.
FIG. 3.
Course of pharmacodynamic index values found at the end of the first 24-h period versus the different tested MICs of a concentration-dependent antibiotic and a concentration-independent antibiotic determined with the plasma drug level profiles shown in Fig. 1.
DISCUSSION
Many pharmacodynamic indices have been developed to predict antimicrobial effectiveness. Some of them were not studied here—specifically the peak serum antibiotic level/MIC ratio (6, 15, 16), because no dynamic or cumulative evolution of this index was possible for comparison with the Zhi model.
For the other indices shown in Table 3, two types of index could be distinguished, depending on their dimensional units. The indices of AUC, AUC>MIC, and AUC above MIC are all in micrograms times hours per milliliter. The indices of AUC/MIC, AUIC, and T>MIC are in hours. Contrary to Schentag et al. (20), none of these pharmacodynamic indices was found to be dimensionless.
The indices AUC/MIC, AUC>MIC, AUIC, and AUC above MIC were defined from the AUC by including the MIC in their calculations in different ways, as shown in Table 3. T>MIC is clearly distinguishable from the others. Indeed, it is the only index for which the value is not computed with the AUC. As mentioned above, some indices were very similar and could not be distinguished from each other, by using our dynamic view, especially AUC/MIC versus AUIC and AUC>MIC versus AUC above the MIC. For these reasons, only four pharmacodynamic indices were finally compared with the Zhi model: AUC, AUC/MIC, AUC>MIC (two different ways of including MIC in the calculation), and the T>MIC (independent of the AUC).
Figure 2 shows the CFU versus each of the pharmacodynamic indices (AUC, AUC/MIC, AUC>MIC, and T>MIC) and their evolution during the initial 24-h period. In the MIC ranges tested, and for the first 24 h, the relationships of CFU to each evolving pharmacodynamic index were somewhat similar for both the concentration-dependent antibiotic and the concentration-independent one when the AUC/MIC was less than 250 h. However, for the concentration-independent drug, there was little relationship between AUC/MIC and killing beyond this point. This illustrates the saturation of the effect relationship for the concentration-independent drug. In fact, the relationship of any of the pharmacodynamic indices studied to bacterial killing depended essentially on the AUC and the MIC.
It was evident that AUC alone does not take into account the sensitivity of the bacterium, since MIC is not included in its calculation. For different bacterial sensitivities represented by the different MICs, the values of the AUC were always the same at the end of the initial 24-h period (Fig. 2A and A′). Thus, AUC alone was not a good index.
For the AUC>MIC index, derived from AUC, but which takes into account the relationship of the plasma drug concentration with at least the MIC in the calculation, discontinuities were seen as CFU increased while the index value did not (Fig. 2B and B′). This is because this method does not include concentrations below the MIC, which means that the bacterial killing rate constant at those times is zero. However, this does not seem realistic. Even if the bacterial population increases, the apparent growth rate constant is reduced, and the killing rate is not zero. Because of this assumption, a continuous relationship does not exist between the values of the AUC>MIC and CFU. In addition, there was only a minimal relationship between CFU and AUC>MIC. The relationship between AUC>MIC and CFU was not very different from that found for AUC versus CFU.
In contrast, for the AUC/MIC, the more its values increased, the greater was the killing seen with the Zhi model (Fig. 2C and C′). Conversely, treatment was not effective when AUC/MIC was small. A continuous relationship exists between AUC/MIC values and the resulting CFU. Even if serum antibiotic levels are below the MIC with the apparent growth rate only slightly reduced, AUC/MIC values increased very slowly, showing the small to modest effect of the sub-MIC serum antimicrobial level upon the bacterial growth rate. The difference between concentration-dependent and concentration-independent killing is also shown in Fig. 2C and C′. For those concentrations relatively near the MIC, killing was almost linear with concentration. However, deviations from linearity were seen above this, especially in Fig. 2C′, showing the lack of concentration dependence at the higher AUC/MICs, in the saturable region of the relationship. It is for this reason that when concentrations are reasonably above the MIC, the T>MIC becomes the most significant empirical index for killing. All such relationships were well shown with the Zhi model.
For the index of T>MIC, however, it was also clear, as shown in Fig. 2D and D′, that the dynamic evolution and growth of T>MIC were strongly related to bacterial killing, with a significant negative correlation being found between it and CFU. However, discontinuities were also observed. For all therapy, the maximum possible value of T>MIC is 24 h. Suppose that serum drug levels on one regimen are already above the MIC for a particular dosage regimen of antibiotic and that another regimen of the same antibiotic generates serum drug levels twice as high. The maximum value of T>MIC will still be 24 h for either regimen. Because of this, the effectiveness of antibiotic treatment could not be distinguished if the second regimen was actually somewhat more effective than the first one, the smaller but already effective regimen. On the other hand, the other indices (AUC, AUC>MIC, and AUC/MIC) do not have such maximum values. Therefore, the entire scale of therapeutic effectiveness can be explored with the other indices, whereas that is not the case with T>MIC. Even with T>MIC, which is expressed as linear percent of the dose intervals having serum drug concentrations above the MIC, a similar or the same maximum value (or 100%) is always reached.
The greatest change in the values of the empirical indices found at the end of the initial 24-h period, for two different MICs, was obtained with AUC/MIC. For example, with tobramycin MICs of 0.125 and 1.0 μg/ml, there was no relationship between AUC and MIC (58.52 versus 58.52 μg · h · ml−1), a modest one for AUC>MIC (58.48 versus 57.82 μg · h · ml−1), a greater one for T>MIC (23.7 versus 12.1 h), and the greatest one for AUC/MIC (468.16 versus 58.52 h) (Fig. 3). Somewhat similar results were also seen for ticarcillin, although saturation of the effect relationship was clearly seen at the higher concentrations. AUC/MIC was therefore the pharmacodynamic index which had the greatest sensitivity to differences in the various MICs of either antibiotic, whether the antibiotic was concentration dependent or concentration independent (Fig. 3).
A summary of the characteristics of each index (AUC, AUC>MIC, T>MIC, and AUC/MIC) and of a theoretical ideal index is presented in Table 4. Of the indices presented above, AUC/MIC appeared to be the best because it incorporated and used best the MIC of a concentration-dependent antibiotic and also, interestingly enough, to some degree, that of a concentration-independent antibiotic, although saturation of the effect relationship was clearly seen at higher concentrations. The AUC/MIC for the initial 24-h period has been previously described as the most useful index of fluoroquinolone antimicrobial activity against P. aeruginosa (12). In that study, however, it was shown that AUC/MIC was an index of efficacy only for the concentration-dependent antibiotic (12) and was not sufficient for the concentration-independent antibiotic. Conversely, T>MIC was the only index to be described as an index of efficacy for the concentration-independent antibiotic (18).
TABLE 4.
Summary of the principal characteristics of each index, the ideal index, and our new index, WAUC
| Index characteristic | Result for indexa
|
|||||
|---|---|---|---|---|---|---|
| AUC | AUC>MIC | T>MIC | AUC/MIC | Ideal index | WAUC | |
| Easy determination | + | − | − | + | + | + |
| Incorporation of all pharmacokinetic parameters | + | + | − | + | + | + |
| Incorporation of MICs | − | + | + | + | + | + |
| Sensitivity to change in MICs | − | − | +/− | + | + | + |
| Dynamic view | + | + | + | + | + | + |
| No discontinuities | + | − | − | + | + | + |
| No maximum value | + | + | − | + | + | + |
| Concentration-dependent drug | + | + | − | + | + | + |
| Concentration-independent drug | − | − | + | − | + | + |
| Clear relationship with bacterial killing | − | − | +/− | +/− | + | + |
| Drug combination analysis | − | − | − | − | + | − |
+, presence of the characteristic; −, absence of the characteristic.
In addition, a change in antibiotic administration strategy (for example, more doses at closer intervals) may produce the same AUC/MIC but two different T>MICs. Equally, a change in the dose administered might produce a change in AUC/MIC but not in T>MIC.
Because of this, we also propose a new empirical pharmacodynamic index for which AUC/MIC is weighted by T>MIC, in order to take into account both the concentration-dependent part of the antibiotic efficacy and the concentration-independent part. We therefore propose a new composite pharmacodynamic index, the weighted AUC (WAUC), for the first 24 h, which is the AUC/MIC weighted by the percentage of the total time for which the serum drug level is above the MIC:
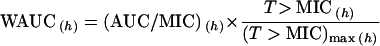 |
5 |
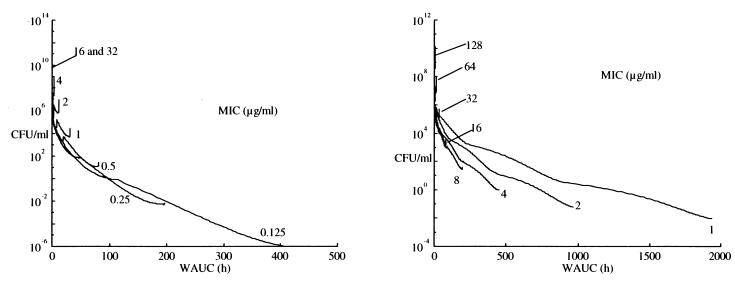
where (T>MIC)max equals 24 h (see above). The units for WAUC are hours. This index considers (i) the total dose administered and the clearance of the drug through the AUC, (ii) the sensitivity of the bacteria to the MIC, and (iii) the percentage of time for which serum drug level is above the MIC through the ratio T>MIC/24 h. This index can be used both for a concentration-dependent drug and for a concentration-independent drug with a high sensitivity to change in MICs (Fig. 3). It shows a more direct relationship between its values and bacterial killing both for the concentration-dependent drug and for the concentration-independent drug (Fig. 4). Obviously, further clinical evaluation of this proposed index is needed before more conclusions can be drawn.
FIG. 4.
CFU course versus our new pharmacodynamic index for a concentration-dependent antibiotic (left panel) and a concentration-independent antibiotic (right panel) during the first 24-h period of therapy, determined with the previous plasma drug level profiles. Some saturation of the relationship is still seen at WAUC values above 400 h.
Conclusion.
The Zhi model describes a saturable Hill model of killing, coupled with assumed logarithmic growth. For concentration-dependent drugs, the concentrations are on the steep slope of the effect relationship, and saturation of the effect is not seen. It is for this reason that the model is concentration dependent when trough concentrations are near or below the MIC; here, AUC/MIC is usually the best empirical index. However, when concentrations are significantly above the MIC, saturation of the effect relationship takes place, and killing becomes relatively independent of concentrations; here, T>MIC is usually the best empirical index.
A good pharmacodynamic index must present a clear relationship between its values and bacterial killing. Actually, none of the empirical indices generally used brings together all of these characteristics, and none approaches the utility of the Zhi model itself to reflect the actual dynamic process of bacterial growth and killing. AUC/MIC appears the best for the concentration-dependent antimicrobial agent, and T>MIC appears best for the concentration-independent antimicrobial agent. Based on this, a new pharmacodynamic index for antimicrobial drugs, WAUC, is also proposed. This new index appears useful for both concentration-dependent and concentration-independent antimicrobial agents. A clear (nearly linear) relationship has been found between its values and bacterial growth and killing reflected by the Zhi model, as shown in Fig. 4, although some saturation of the effect relationship is still seen at higher concentrations. This index is dependent not only on time but also on the concentration and the MIC throughout the duration of the therapy.
None of the pharmacodynamic indices, old or new, could be used in order to estimate the efficacy of a combination of antimicrobial agents. Because of this and because it is now easily available in clinical software, the Zhi model itself (or any similar dynamic model of bacterial growth and killing) probably still represents the current optimal clinical index of therapeutic effectiveness.
The Zhi model actually represents a useful “worst-case model” for the evaluation of the efficacy of a drug dosage regimen. On the one hand, while some organisms may have a reduced or slowed rate of growth as some substrates for their growth become scarce, the Zhi model always makes the worst-case assumption that the organisms are always in their logarithmic phase of most rapid growth. While this may not be entirely realistic, it nevertheless furnishes a useful worst-case assumption for the evaluation of the potential utility of a proposed drug dosage regimen.
The Zhi model contains no provision for describing the emergence of bacterial resistance during therapy. However, if one considers and uses the highest MIC the emerging resistant organism is estimated to attain during therapy, the Zhi model again provides a useful worst-case model for the evaluation of any proposed antibacterial regimen. If a proposed dosage regimen is successful in killing according to the Zhi model and the highest MIC for the organism is estimated to be attained, as described herein, that regimen is quite likely to be effective clinically, because the clinical situation may actually contain a decreasing rate constant for growth rather than the fixed one for the logarithmic phase of growth, and the organisms are not likely to be fully resistant from the very beginning of therapy. Because of this, the Zhi model for a single organism, assumed to be at its most resistant from the very start of therapy, provides a somewhat more stringent and rigorous test of the effectiveness of a dosage regimen than more complex models having several subpopulations, if one analyzes the behavior of the most rapidly growing and most resistant possible strain of organism. The computations are relatively simple and have already been incorporated into clinical software. As more complex models become available, they are likely to be less stringent than the single Zhi model.
REFERENCES
- 1.Bouvier d’Yvoire M J Y, Maire P H. Dosage regimens of antibacterials: implications of a pharmacokinetic/pharmacodynamic model. Clin Drug Invest. 1996;11:229–239. [Google Scholar]
- 2.Charpiat B, Bréant V, Pivot-Dumarest C, Maire P H, Jelliffe R W. Prediction of future serum concentrations with Bayesian fitted pharmacokinetic models: results with data collected by nurses versus trained pharmacy residents. Ther Drug Monit. 1994;16:166–173. doi: 10.1097/00007691-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A., and S. C. Ebert. 1991. Killing and regrowth of bacteria in vitro: a review. Scand. J. Infect. Dis. 74(Suppl.):63–70. [PubMed]
- 4.Daikos G L, Jackson G G, Lolans V T, Livemore D M. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis. 1990;162:414–420. doi: 10.1093/infdis/162.2.414. [DOI] [PubMed] [Google Scholar]
- 5.Dudley M N. Commentary on dual individualization with antibiotics. In: Evans W E, Schentag J J, Jusko W J, editors. Applied pharmacokinetics: principles of therapeutic drug monitoring. 3rd ed. Vancouver, British Columbia, Canada: Applied Therapeutics Co.; 1992. pp. 18.1–18.13. [Google Scholar]
- 6.Ellner P D, Neu H C. The inhibitory quotient. JAMA. 1981;246:1575–1578. doi: 10.1001/jama.246.14.1575. [DOI] [PubMed] [Google Scholar]
- 7.Gibaldi M, Perrier D. Drug and the pharmaceutical sciences: pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 8.Ingerman M J, Pitsakis P G, Rosenberg A F, Levison M E. The importance of pharmacodynamics in determining the dosing interval in therapy for experimental Pseudomonas endocarditis in the rats. J Infect Dis. 1986;153:707–714. doi: 10.1093/infdis/153.4.707. [DOI] [PubMed] [Google Scholar]
- 9.Jelliffe R W, Schumitzky A, Van Guilder M, Liu M, Hu L, Maire P, Gomis P, Barbaut X, Tahani B. Individualizing drug dosage regimens: roles of population pharmacokinetic and dynamic models, Bayesian fitting, and adaptive control. Ther Drug Monit. 1993;15:380–393. [PubMed] [Google Scholar]
- 10.Laboratory of Applied Pharmacokinetics. ADCAPT. USC*PACK PC on line user’s manual. 1997. web.avo.fr/slecoq. http// web.avo.fr/slecoq. [Google Scholar]
- 11.Legget J E, Ebert S, Fantin B, Craig W A. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis model. Scand J Infect Dis. 1991;74:179–184. [PubMed] [Google Scholar]
- 12.Madaras-Kelly K J, Ostergaard B E, Baeker Hovde L, Rotschafer J C. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1996;40:627–632. doi: 10.1128/aac.40.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maire P, Barbaut X, Thalabard J C, Mentré F, Jelliffe R W. Pharmacocinétique clinique appliquée aux antibiotiques. In: Freney J, Renaud F, Hansen W, Bollet C, editors. Manuel de bactériologie clinique. 2nd ed. 1. Collection OptionBio. Paris, France: -Elsevier; 1994. pp. 479–518. [Google Scholar]
- 14.Maire P H, Barbaut X, Thalabard J C, Vergnaud J M, Roux D, Roy M, Jelliffe R W. Adaptive control of therapeutic drug regimens: relations between clinical situations—outcomes and simulations using nonlinear dynamic models. In: Greenes R, Peterson H, Protti D, editors. MEDINFO’95. Proceedings of the 8th World Congress on Medical Informatics. Edmonton, Alberta, Canada: International Medical Informatics Association; 1995. pp. 1111–1115. [PubMed] [Google Scholar]
- 15.Moore R D, Craig R S, Lietman P S. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984;77:657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- 16.Moore R D, Lietman P S, Craig R S. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 17.Rotschafer J C, Zabinski R A, Walker K J. Pharmacodynamic factors of antibiotic efficacy. Pharmacotherapy. 1992;12:64S–70S. [PubMed] [Google Scholar]
- 18.Rotschafer J C, Walker K J, Madras-Kelly K J, Sullivan C J. Antibiotic pharmacodynamics. In: Culter N R, Sramek J J, Narang P K, editors. Pharmacodynamics and drug development: perspectives in clinical pharmacology. Chichester, United Kingdom: John Wiley & Sons, Inc.; 1994. pp. 315–343. [Google Scholar]
- 19.Schentag J J, Nix D E, Adelman M H. Mathematical examination of dual individualization principles: relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin and tobramycin. DICP Ann Pharmacother. 1991;25:1050–1057. doi: 10.1177/106002809102501003. [DOI] [PubMed] [Google Scholar]
- 20.Schentag J J, Ballow C H, Paladino J A, Nix D E. Dual individualization with antibiotics: integrated antibiotic. Management strategies for use in hospitals. In: Evans W E, Schentag J J, Jusko W J, editors. Applied pharmacokinetics: principles of therapeutic drug monitoring. 3rd ed. Vancouver, British Columbia, Canada: Applied Therapeutics Inc.; 1992. pp. 17.1–17.20. [Google Scholar]
- 21.Wiedemann B, Atkinson B A. Susceptibility to antibiotics: species incidence and trends. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 962–1208. [Google Scholar]
- 22.Zhi J, Nightingale C H, Quintiliani R. Microbial pharmacodynamics of piperacillin in neutropenic mice with systemic infection due to Pseudomonas aeruginosa. J Pharmacokinet Biopharm. 1988;16:355–375. doi: 10.1007/BF01062551. [DOI] [PubMed] [Google Scholar]