Abstract
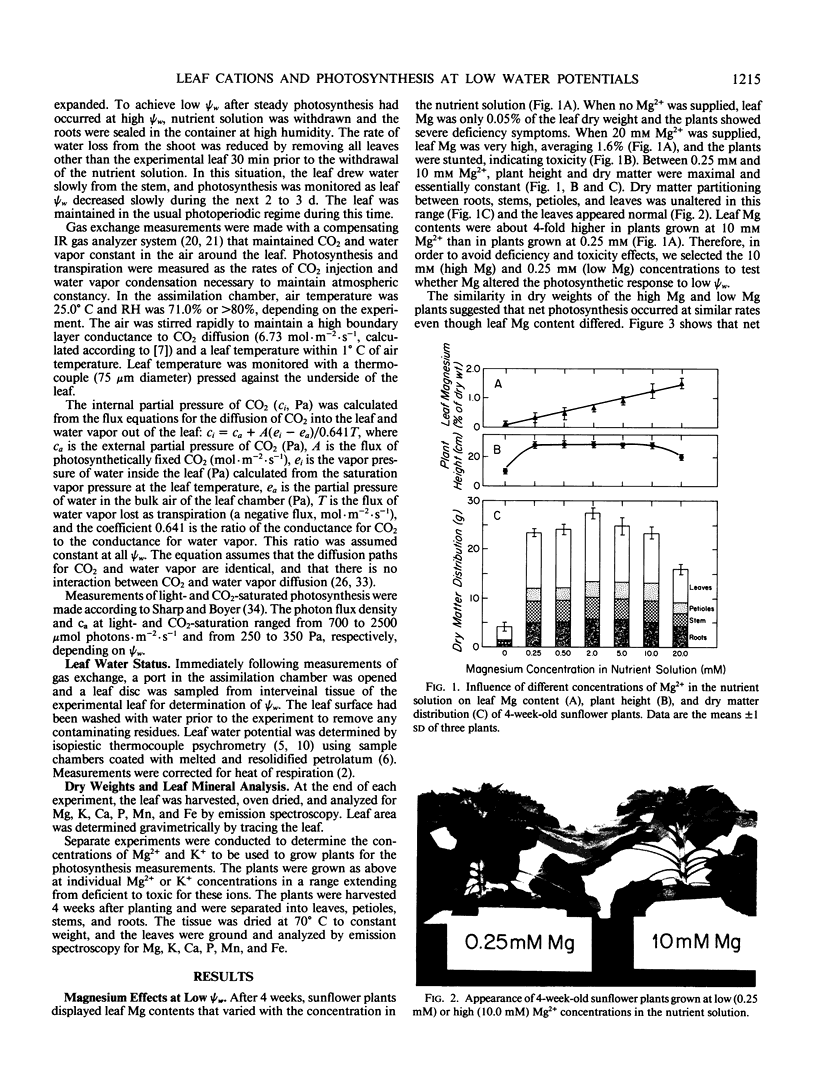
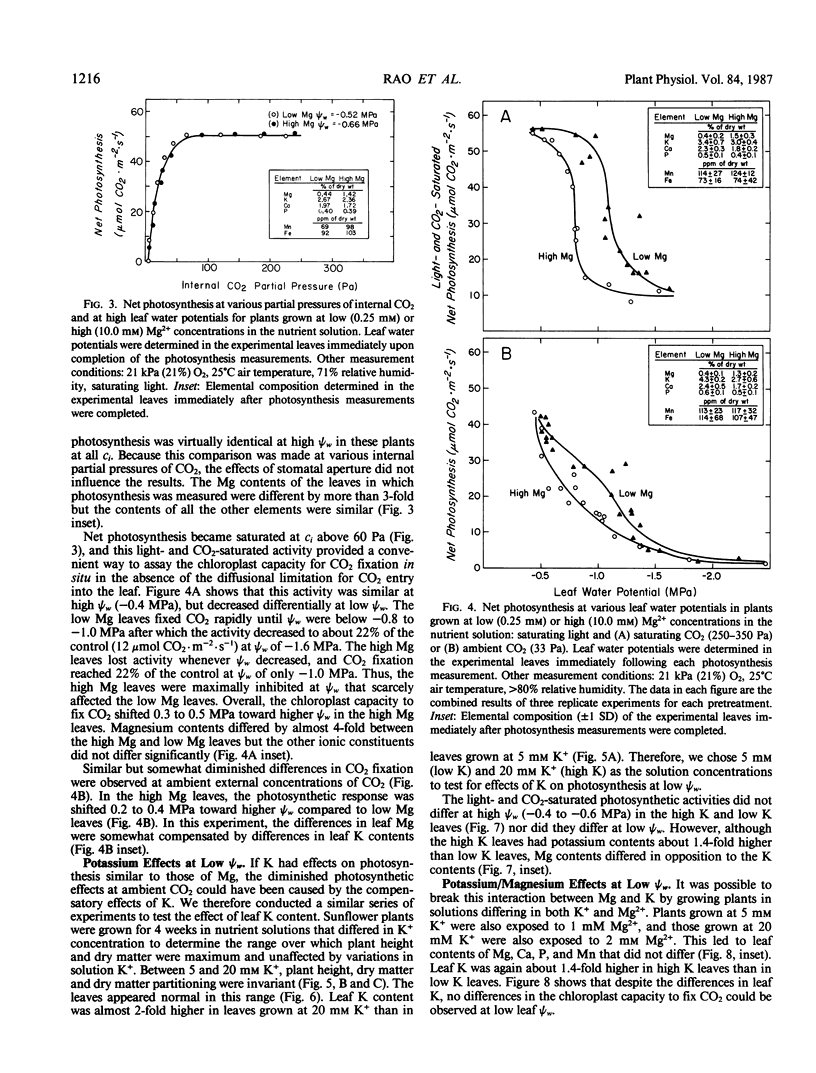
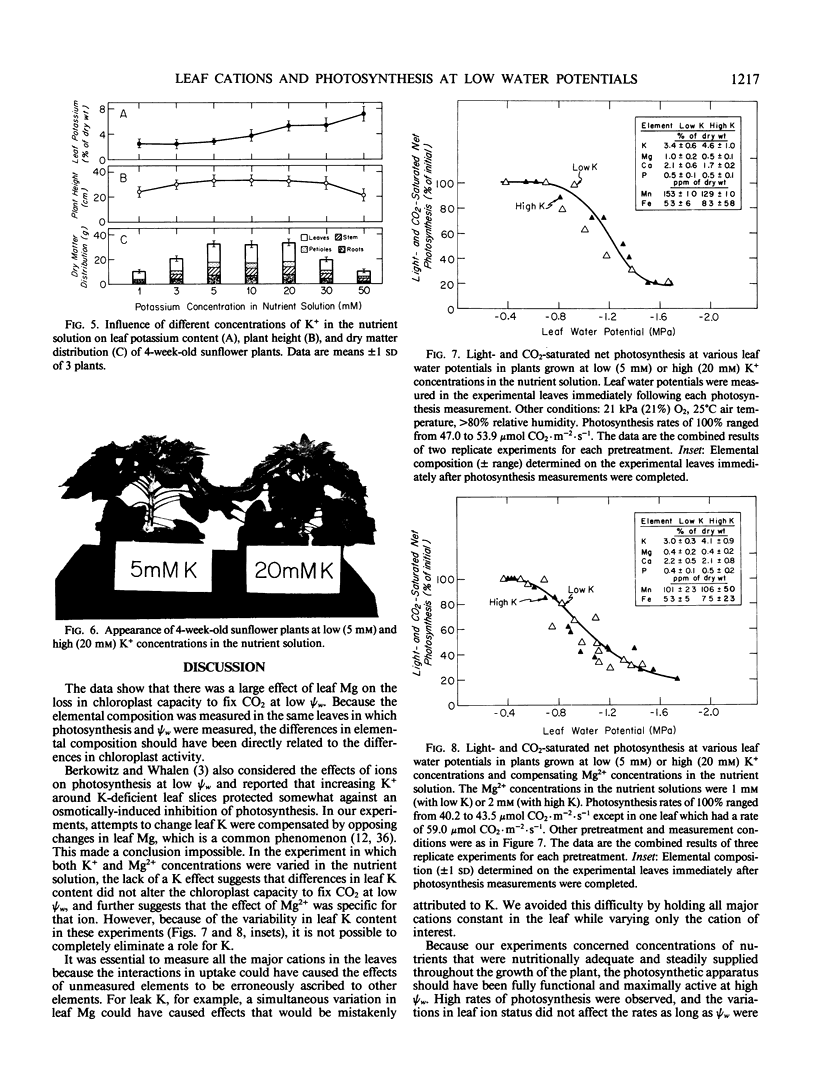
We grew sunflower (Helianthus annuus L.) plants in nutrient solutions having nutritionally adequate but low or high Mg2+ concentrations and determined whether photosynthesis was effected as leaf water potentials (ψw) decreased. Leaf Mg contents were 3- to 4-fold higher in the plants grown in high Mg2+ concentrations (10 millimolar) than in those grown in low concentrations (0.25 millimolar). These contents were sufficient to support maximum growth, plant dry weight, and photosynthesis, and the plants appeared normal. As low ψw developed, photosynthesis was inhibited but moreso in high Mg leaves than in low Mg leaves. The effect was particularly apparent under conditions of light- and CO2-saturation, indicating that the chloroplast capacity to fix CO2 was altered. The differential inhibition observed in leaves of differing Mg contents was not observed in leaves having differing K contents, suggesting that the effect may have been specific for Mg. Because Mg2+ inhibits photophosphorylation and coupling factor activities at concentrations likely to occur as leaves dehydrate, Mg may play a role in the inhibition of chloroplast reactions at low ψw, especially in leaves such as sunflower that markedly decrease in water content as ψw decreases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz G. A., Whalen C. Leaf k interaction with water stress inhibition of nonstomatal-controlled photosynthesis. Plant Physiol. 1985 Sep;79(1):189–193. doi: 10.1104/pp.79.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Isopiestic technique: measurement of accurate leaf water potentials. Science. 1966 Dec 16;154(3755):1459–1460. doi: 10.1126/science.154.3755.1459. [DOI] [PubMed] [Google Scholar]
- Boyer J. S. Leaf water potentials measured with a pressure chamber. Plant Physiol. 1967 Jan;42(1):133–137. doi: 10.1104/pp.42.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Nonstomatal inhibition of photosynthesis in sunflower at low leaf water potentials and high light intensities. Plant Physiol. 1971 Nov;48(5):532–536. doi: 10.1104/pp.48.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Potter J. R. Chloroplast response to low leaf water potentials: I. Role of turgor. Plant Physiol. 1973 Jun;51(6):989–992. doi: 10.1104/pp.51.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck R. W., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: III. Differing Inhibition of Electron Transport and Photophosphorylation. Plant Physiol. 1974 Mar;53(3):474–479. doi: 10.1104/pp.53.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B., Ort D. R., Boyer J. S. Impairment of photosynthesis by chilling-temperatures in tomato. Plant Physiol. 1981 Aug;68(2):329–334. doi: 10.1104/pp.68.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. A., Boyer J. S. Acclimation of photosynthesis to low leaf water potentials. Plant Physiol. 1984 Jan;74(1):161–166. doi: 10.1104/pp.74.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelena V. A., Boyer J. S. Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol. 1982 May;69(5):1145–1149. doi: 10.1104/pp.69.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty P., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: IV. Quantum Yield Is Reduced. Plant Physiol. 1976 May;57(5):704–709. doi: 10.1104/pp.57.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Active/inactive state transitions of mitochondrial ATPase molecules influenced by Mg2+, anions and aurovertin. FEBS Lett. 1975 Aug 1;56(1):55–61. doi: 10.1016/0014-5793(75)80110-4. [DOI] [PubMed] [Google Scholar]
- Portis A. R. Evidence of a Low Stromal Mg Concentration in Intact Chloroplasts in the Dark: I. STUDIES WITH THE IONOPHORE A23187. Plant Physiol. 1981 May;67(5):985–989. doi: 10.1104/pp.67.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. R., Boyer J. S. Chloroplast Response to Low Leaf Water Potentials: II. Role of Osmotic Potential. Plant Physiol. 1973 Jun;51(6):993–997. doi: 10.1104/pp.51.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D., Imai K., Farquhar G. D., Cowan I. R. A Direct Confirmation of the Standard Method of Estimating Intercellular Partial Pressure of CO(2). Plant Physiol. 1982 Mar;69(3):657–659. doi: 10.1104/pp.69.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R. E., Boyer J. S. Photosynthesis at low water potentials in sunflower: lack of photoinhibitory effects. Plant Physiol. 1986 Sep;82(1):90–95. doi: 10.1104/pp.82.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Younis H. M., Boyer J. S., Govindjee Conformation and activity of chloroplast coupling factor exposed to low chemical potential of water in cells. Biochim Biophys Acta. 1979 Nov 8;548(2):328–340. doi: 10.1016/0005-2728(79)90139-7. [DOI] [PubMed] [Google Scholar]




