Abstract
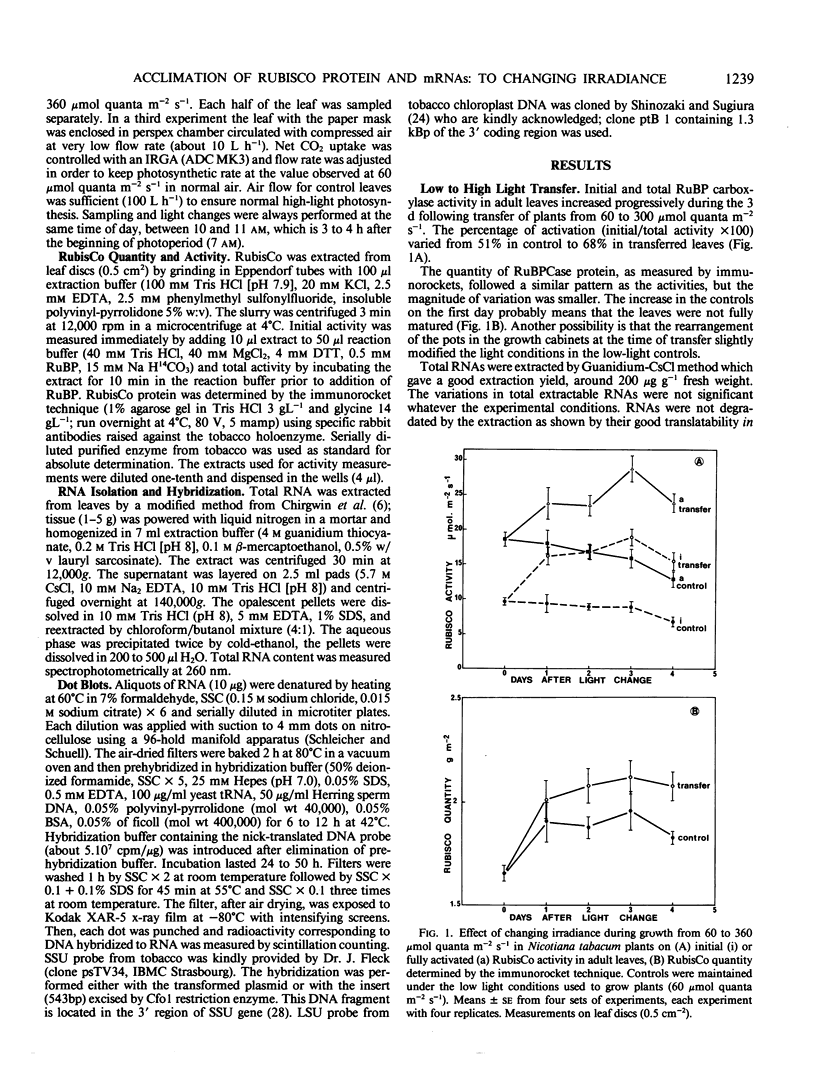
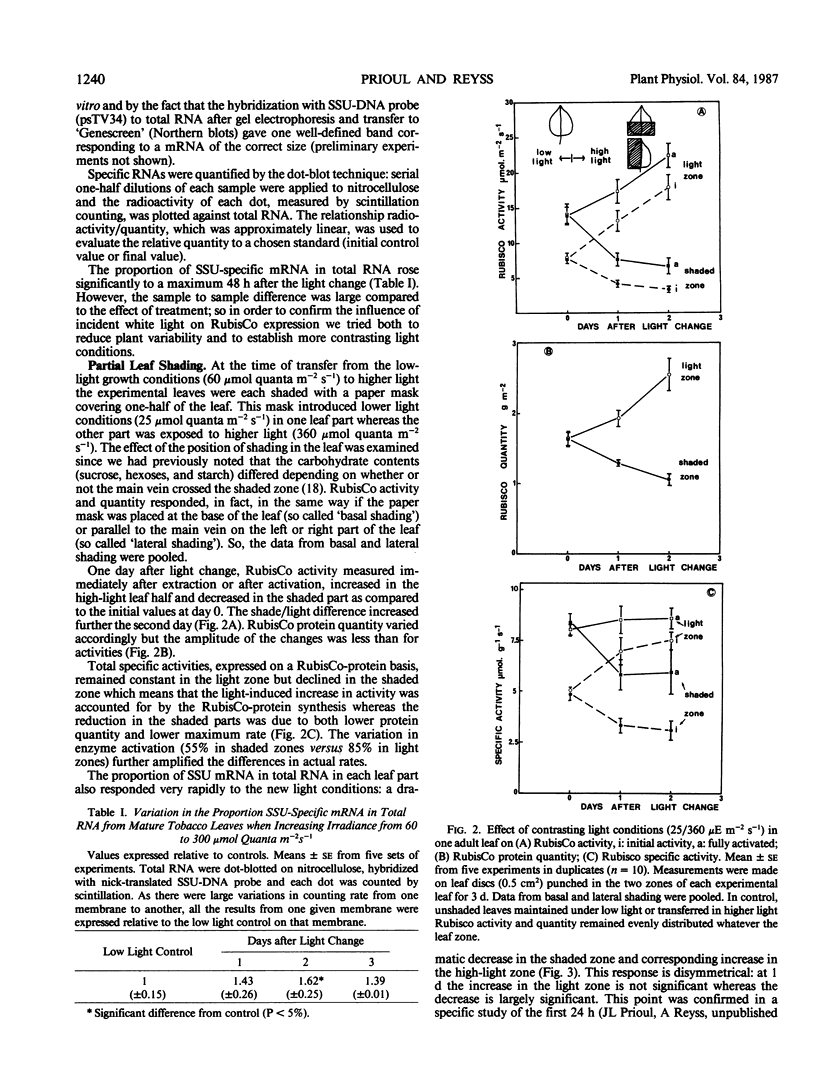
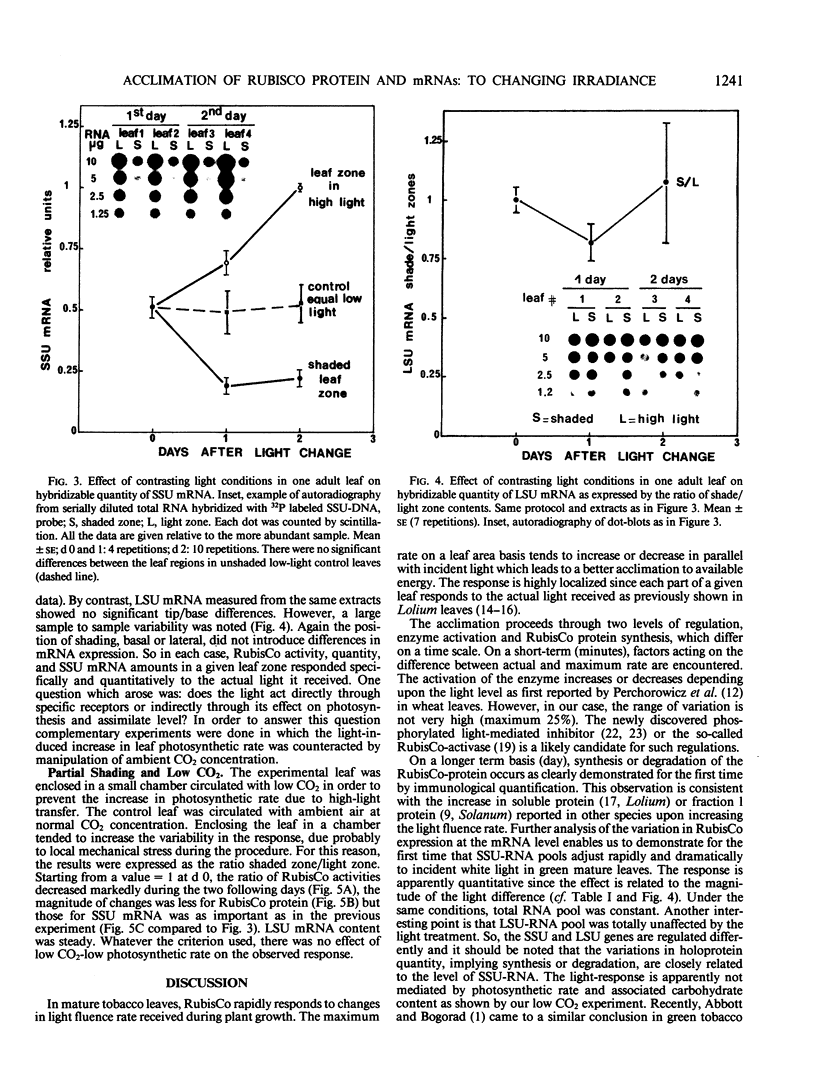
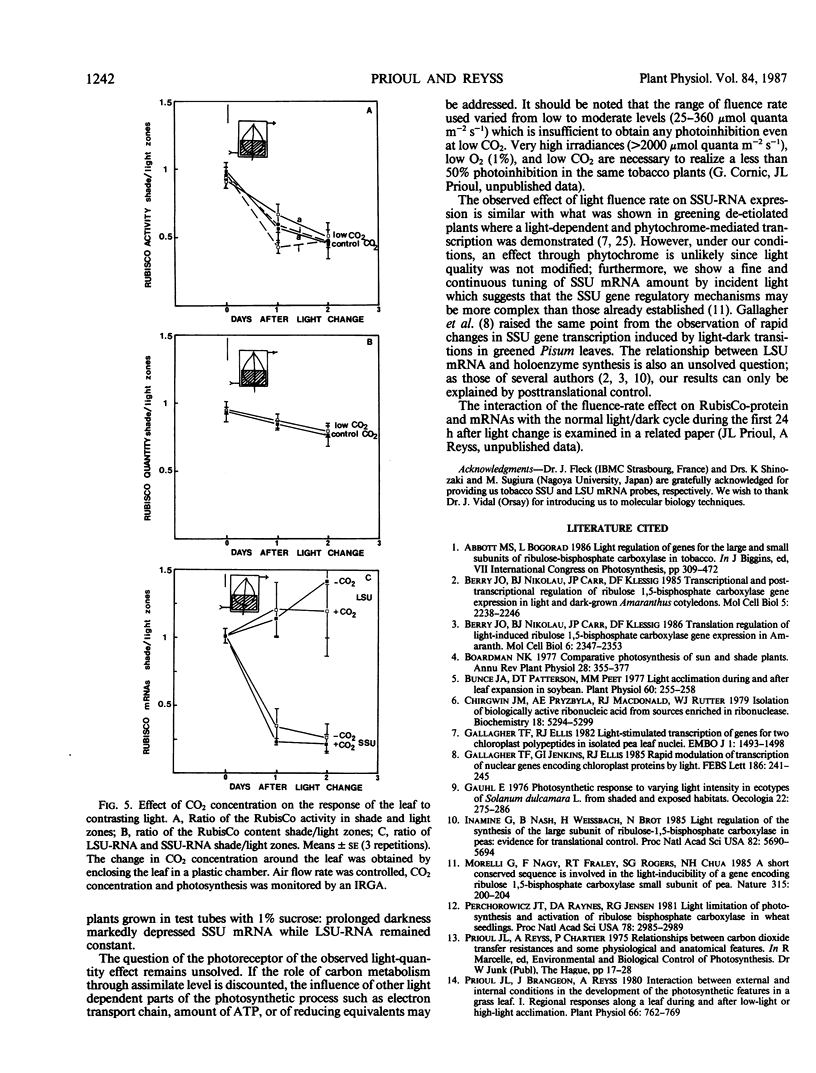
The transfer of Nicotiana tabacum plants grown in low light (60 micromoles quanta per square meter per second) to higher light (360 micromoles quanta per square meter per second) was previously shown to induce adaptive stimulation of photosynthetic capacities. The variations of ribulose bisphosphate carboxylase/oxygenase (RubisCo) expression in mature leaves was examined as a result of this acclimation. Maximum or initial activities increased markedly after low- to high-light transfer with a maximum effect after 2 to 3 days. The higher activity is mainly explained by RubisCo protein synthesis as shown by immunorocket technique. Small subunits of RubisCo (SSU) mRNA relative content determined by hybridization of total RNA with DNA probe by Dot-blot method, followed the same pattern as RubisCo quantity. The magnitude of this response was amplified when more contrasting light conditions (25 versus 360 micromoles per square meter per second) were established on the same leaf: RubisCo activity, RubisCo protein, and SSU mRNA contents decreased in the shaded zone and increased in the high-light zone within 1 day. After 2 days the shade/light ratio was 1 to 3 for RubisCo protein and 1 to 4 for SSU-RNA, whereas the ratios remained equal to one in controls. Hybridization of the same RNA extracts with large subunits of RubisCo (LSU) probe showed no variation in LSU-RNA content. So in green adult leaves, the expression of SSU and LSU genes is regulated differently. The observed white light quantitative effect on RubisCo expression was not dependent on the photosynthetic rate or assimilate content since low CO2 concentration around the leaf after the light shift did not modify the response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Transcriptional and post-transcriptional regulation of ribulose 1,5-bisphosphate carboxylase gene expression in light- and dark-grown amaranth cotyledons. Mol Cell Biol. 1985 Sep;5(9):2238–2246. doi: 10.1128/mcb.5.9.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Translational regulation of light-induced ribulose 1,5-bisphosphate carboxylase gene expression in amaranth. Mol Cell Biol. 1986 Jul;6(7):2347–2353. doi: 10.1128/mcb.6.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce J. A., Patterson D. T., Peet M. M. Light acclimation during and after leaf expansion in soybean. Plant Physiol. 1977 Aug;60(2):255–258. doi: 10.1104/pp.60.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine G., Nash B., Weissbach H., Brot N. Light regulation of the synthesis of the large subunit of ribulose-1,5-bisphosphate carboxylase in peas: Evidence for translational control. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5690–5694. doi: 10.1073/pnas.82.17.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci U S A. 1981 May;78(5):2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioul J. L., Brangeon J., Reyss A. Interaction between External and Internal Conditions in the Development of Photosynthetic Features in a Grass Leaf: I. REGIONAL RESPONSES ALONG A LEAF DURING AND AFTER LOW-LIGHT OR HIGH-LIGHT ACCLIMATION. Plant Physiol. 1980 Oct;66(4):762–769. doi: 10.1104/pp.66.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioul J. L., Brangeon J., Reyss A. Interaction between External and Internal Conditions in the Development of Photosynthetic Features in a Grass Leaf: II. REVERSIBILITY OF LIGHT-INDUCED RESPONSES AS A FUNCTION OF DEVELOPMENTAL STAGES. Plant Physiol. 1980 Oct;66(4):770–774. doi: 10.1104/pp.66.4.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Berry J. A., Freas S. M., Krump M. A. Regulation of ribulose bisphosphate carboxylase activity in vivo by a light-modulated inhibitor of catalysis. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8024–8028. doi: 10.1073/pnas.82.23.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Binding of a Phosphorylated Inhibitor to Ribulose Bisphosphate Carboxylase/Oxygenase during the Night. Plant Physiol. 1985 Aug;78(4):839–843. doi: 10.1104/pp.78.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene. 1982 Nov;20(1):91–102. doi: 10.1016/0378-1119(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Silverthorne J., Tobin E. M. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet T., Fleck J., Durr A., Fritsch C., Pinck M., Hirth L. Expression of the gene coding for the small subunit of ribulosebisphosphate carboxylase during differentiation of tobacco plant protoplasts. Eur J Biochem. 1982 Sep 1;126(3):489–494. doi: 10.1111/j.1432-1033.1982.tb06806.x. [DOI] [PubMed] [Google Scholar]