Abstract
Aims
Previous cost‐effectiveness analysis suggests that CardioMEMS is cost‐effective compared with usual care for patients with persistent New York Heart Association class III symptoms and at least one heart failure (HF) hospitalization within 12 months. The aim of the paper is to perform an update of the cost‐effectiveness analysis of CardioMEMS using the most recent data from the published literature.
Methods and results
A Microsoft Excel Markov model from a previous UK cost‐effectiveness study of CardioMEMS was updated using the clinical effectiveness of pulmonary artery pressure (PAP)‐guided treatment derived from the pivotal trials. The model included the device costs (and the implantation procedure and related complications), costs of remote monitoring, costs of HF‐related hospitalizations, and costs of usual care. Quality‐adjusted life years (QALYs) were estimated based on utilities from pivotal trials and published literature. Cost‐effectiveness results were estimated as incremental cost per QALY gained of CardioMEMS compared with usual care. Scenario analyses were also performed using data from real‐world studies that showed a significant decrease in HF‐related hospitalizations. In the base case analysis over a time horizon of 10 years, PAP‐guided HF therapy increased cost compared with usual care by £6337 (i.e. from £22 770 in usual care to £29 107 in PAP‐guided HF therapy) and the QALYs per patient for usual care and PAP‐guided patients were 2.62 and 2.94, respectively, reflecting an increase of 0.32 QALYs with PAP‐guided treatment. The resultant incremental cost‐effectiveness ratio (ICER), the ratio between incremental costs and the QALYs, is estimated at £19 761/QALY. Scenario analyses suggest that the ICER for CardioMEMS can range from being dominant to £27 910/QALY. Probabilistic sensitivity analyses suggested that PAP‐guided HF therapy has 81.9% probability of being cost‐effective at a threshold of £30 000/QALY.
Conclusions
Our model suggests that CardioMEMS is likely to be cost‐effective in the United Kingdom, at the currently considered thresholds of £20 000–30 000/QALY.
Keywords: Heart failure, Cost‐effectiveness, Pulmonary artery pressure monitoring
Introduction
A total of 1–2% of adults in Europe live with a diagnosis of heart failure (HF), with the true prevalence of HF likely to be higher. 1 Many HF patients develop increased pulmonary artery pressure (PAP), which is associated with lower quality of life (QoL), increased risk of hospitalization, and higher mortality. Several trials evaluating the CardioMEMS™ HF System to monitor PAP and facilitate personalized therapy have shown meaningful reductions in HF hospitalizations (HFHs) and improved QoL, including the pivotal randomized CHAMPION trial in the United States. 2
Previous cost‐effectiveness analyses of CardioMEMS were based on the data from the CHAMPION trial. There have been further studies evaluating CardioMEMS, as outlined in a recent systematic review, including a new randomized clinical trial (GUIDE‐HF 3 ) as well as real‐world studies in the United Kingdom (COAST 4 ) and the European Union (MEMS‐HF 5 in Germany, the Netherlands, and Ireland). All these studies demonstrate reduced HFHs with the use of CardioMEMS; however, the impact of these recently published data on estimates of the cost‐effectiveness of this strategy has not been evaluated.
The aim of the paper is to perform an update of the cost‐effectiveness analysis of CardioMEMS compared with usual care from a UK National Health Service (NHS) perspective published in 2017, 6 using the most recent data from the published literature. The analysis was conducted for patients with persistent New York Heart Association (NYHA) class III symptoms and at least one HFH within 12 months, in line with the market authorization for CardioMEMS. Costs and quality‐adjusted life years (QALYs) were estimated for both usual care and patients with CardioMEMS, and incremental cost‐effectiveness ratios (ICERs) were calculated and compared with the typical thresholds used for decision making by the local health technology assessment body [the National Institute for Health and Care Excellence (NICE)] of £20 000–30 000/QALY. 7
Methods
Model overview
To estimate the cost‐effectiveness of PAP‐guided treatment of HF using the CardioMEMS implantable pressure sensor compared with usual care strategies, a Microsoft Excel Markov model from a previous UK cost‐effectiveness study of CardioMEMS 6 was updated using the most appropriate data from the published literature. Patients were assumed to be NYHA class III with at least one HFH in past 12 months and aged 70 (typical age of HF patients who are remotely monitored in the United Kingdom and Europe).
The clinical effectiveness of PAP‐guided treatment was derived from the pivotal trials, 2 , 3 and scenario analyses were also performed using data from real‐world studies that showed a significant decrease in hospitalizations related to HF. 4 , 5 Costs estimated in the model included costs of the device (and the implantation procedure and related complications), costs of remote monitoring, costs of HF‐related hospitalizations, and costs of usual care. Cost‐effectiveness results were estimated as incremental cost per QALY gained, using mean values of 1000 probabilistic sensitivity analysis (PSA) runs, with each PSA run using different estimates for the risks, hazard ratios (HRs), costs, and utilities to capture the uncertainty in these input parameters.
Model structure
The model structure was based on a previous UK cost‐effectiveness study 6 of CardioMEMS and included two health states: ‘heart failure’ and ‘dead’. In each monthly model cycle, patients alive were at risk of HFH or dying, and the probabilities of these were based on whether they had the CardioMEMS intervention or not. HFH was not modelled as a health state but rather an event that patients might experience, with associated costs and loss of QoL, consistent with the previous cost‐effectiveness models. Patients who experienced HFH would then transition back to the ‘heart failure’ health state at the end of that monthly cycle. QALYs and healthcare costs were accrued according to their hospitalization and treatment status. The model incorporated half‐cycle correction and used a 10 year time horizon; the costs and QALYs were estimated using an NHS perspective and discounted at an annual discount rate of 3.5%, as recommended by NICE. 7
Baseline mortality and hospitalization risk
Baseline mortality was estimated from the monthly probability of death used in the previous version of the model based on a study by Griffiths et al. 8 who estimated mortality rates based on the CARE‐HF trial, a randomized controlled trial conducted on NYHA class III and IV HF patients with a prior hospitalization event. The mortality rates used in the model are adjusted using UK interim life tables and increases with age in 5‐yearly steps (see Appendix A for more details). These mortality rates are similar to the recent data presented in the UK national HF audit 2019 summary report. 9
A recent systematic review of studies on CardioMEMS suggested that the baseline hospitalization rate can range from 0.7 per year (CHAMPION randomized trial 2 ) to 1.5 (COAST 4 and MEMS‐HF 5 real‐world data). This results in a monthly probability of hospitalization of 5.6% or 11.8%, respectively. In the base case analysis, an average of these probabilities is used in the model, that is, 8.7% per month.
Treatment effect
Unpublished data shared by the company suggested that the HR for HFHs was similar in CHAMPION 2 and GUIDE‐HF. 3 As such, the HR for CardioMEMS was taken as 0.67 based on the long‐term follow‐up of the CHAMPION trial, 10 in line with the previous cost‐effectiveness analysis. This HR is applied to the baseline monthly risk of HFH to estimate the monthly HFH in CardioMEMS arm. Scenario analyses were performed using the HRs from COAST 4 and MEMS‐HF 5 studies, which show much larger effects, and also assuming an HR of 0.8.
Due to the uncertainty regarding the mortality effect at this time, it was decided not to model the mortality effect; that is, the mortality risk is assumed to be the same for both cohorts (those with and those without CardioMEMS™) within the model. Scenario analyses were performed using the mortality HR of 0.8 from the CHAMPION trial 2 and an HR of 1.81 from the subgroup analysis of the GUIDE‐HF study. 3
Prior to entering the Markov model, a small proportion of patients in the CardioMEMS cohort were assumed to experience an implant‐related complication, based on data from the CHAMPION trial. 2
Health‐related quality of life
Health‐related QoL (or utilities) for the patients were the same as the values used in the previous version of the model. Utilities were based on the European Quality of Life (EuroQoL) Five Dimensions, three‐level questionnaire (EQ‐5D‐3L) data collected from the patients within the CHAMPION trial at 1, 3, 6, and 12 months as presented in Appendix A. Trial utilities were modelled out to 5 years, and beyond this, utility of 0.57 was used for both arms. Also, utility values were assumed to decrease at a rate of 0.008 per year, based on a longitudinal study in a population of Swedish HF patients. 8 , 11
After 5 years, a disutility for each HFH of −0.1 is applied to reflect the impact of hospitalizations on QoL, based on data reported by Klersy et al., 12 and is assumed to last for the whole cycle duration (i.e. 1 month). This disutility is similar to the value used in other cost‐effectiveness studies. 13
Costs
The current cost of the CardioMEMS device in the United Kingdom is £9500 (~€11 100). The implant procedure was assumed to be similar to that of a standard cardiac catheterization day case procedure and the costs of implant procedure were estimated as the average of the standard cardiac catheterization day case procedures (EY43A to EY43F) from the NHS reference costs at £1215 14 (~€1420).
The cost of an implant complication was estimated as £1175 (~€1375) by inflating the value used in the previous cost‐effectiveness study to 2020 costs. In the previous study, the cost of complications was taken as £1090 (~€1275), which was estimated using a weighted average of the eight complications in the CHAMPION trial mapped to NHS reference costs. 14
The cost of standard HF care including medication costs and outpatient visits (i.e. excluding hospitalizations) for patients with NYHA class III symptoms is £39 (~€45) per month based on inflating the previous estimates to current costs and is applied to stable HF patients in both cohorts.
The cost of HFH is estimated from NHS reference costs as £4093 (~€4785), calculated as the weighted average of costs of HRG codes EB03A:EB03E (non‐elective long stay) in line with the approach used in NICE Technology Appraisal 679. 15
Monitoring costs were included in the base case analyses and sourced from literature related to CardioMEMS. 16 , 17 The cost of remote monitoring was estimated to be £37.60 (~€44) per month based on 40 min of nurse (band 5) and 5 min of physician time, with hourly costs of £41 and £123, respectively (Table 1 ).
Table 1.
Summary of model parameters
| Parameter | Mean value | Distribution used in the PSA | Source |
|---|---|---|---|
| Baseline monthly probabilities | |||
| Baseline monthly mortality risk | 0.016 | See Appendix A | National HF Audit 2019/2020, 9 Griffiths et al. 8 |
| Baseline monthly HF hospitalization risk | 0.087 | Beta (4379, 45 949) | CHAMPION, 2 COAST, 4 MEMS‐HF 5 |
| CardioMEMS effectiveness parameters | |||
| HR for mortality | 1 | Normal (1, 0.11) | Assumption |
| HR for hospitalization | 0.67 | LogNormal (0.67, 0.064) | CHAMPION, 2 , 10 GUIDE‐HF 3 |
| Risk of implant complication | 0.0272 | Fixed | CHAMPION trial 2 |
| Utility values | |||
| Utilities for usual care patients from the CHAMPION trial 2 | |||
| Trial utility at 1 month | 0.645 | Normal (0.645, 0.016) | CHAMPION trial 2 |
| Trial utility at 3 months | 0.569 | Normal (0.569, 0.019) | CHAMPION trial 2 |
| Trial utility at 6 months | 0.566 | Normal (0.566, 0.020) | CHAMPION trial 2 |
| Trial utility at 12 months | 0.547 | Normal (0.547, 0.020) | CHAMPION trial 2 |
| Utilities for CardioMEMS patients from the CHAMPION trial 2 | |||
| Trial utility at 1 month | 0.688 | Normal (0.688, 0.014) | CHAMPION trial 2 |
| Trial utility at 3 months | 0.646 | Normal (0.646, 0.016) | CHAMPION trial 2 |
| Trial utility at 6 months | 0.617 | Normal (0.617, 0.019) | CHAMPION trial 2 |
| Trial utility at 12 months | 0.653 | Normal (0.653, 0.017) | CHAMPION trial 2 |
| Utilities after 5 years | |||
| Utility values | 0.57 | Fixed | CHAMPION trial 2 |
| Utility decrement per hospitalization | 0.10 | Normal (0.10, 0.01) | Klersy et al. 12 |
| Costs | |||
| Monthly costs of usual care for HF | £39 | Fixed | Cowie et al. 6 |
| Costs of the CardioMEMS device | £9500 | Fixed | Manufacturer |
| Monthly monitoring costs for CardioMEMS | £38 | Fixed | Estimate |
| Costs of implantation procedure | £1215 | Normal (£1215, £50) | NHS reference costs 14 |
| Costs of complications | £1175 | Normal (£1175, £50) | Cowie et al. 6 |
| Costs of HF hospitalization | £4093 | Fixed | NICE TA 679, 15 NHS reference costs 14 |
HF, heart failure; HR, hazard ratio; NHS, National Health Service; NICE TA, National Institute for Health and Care Excellence Technology Appraisal; PSA, probabilistic sensitivity analysis.
Scenario and sensitivity analyses
Analyses were performed by amending the following parameters: HR for mortality reduction from the CHAMPION 2 trial (0.8), HR for mortality from the GUIDE‐HF 3 trial (1.81); HR for HFH from the COAST 4 study (0.178), HR for HFH from the MEMS‐HF study (0.38) and assuming HR of 0.8 for HFH; and baseline HFH rate (5.6%) from the CHAMPION 2 trial, baseline HFH rate (11.8%) from the COAST 4 study, cost of HFH (£2518–5188), from the lowest to highest costs of HFH in the NHS reference costs. 14
Results
Base case results
In the base case analysis over a time horizon of 10 years, PAP‐guided HF therapy increased cost compared with usual care by £6337 (i.e. from £22 770 in usual care to £29 107 in PAP‐guided HF therapy). The model estimated a mean survival of 4.79 years for patients being treated with usual care practices and for patients with treatment guided by PAP monitoring (as no mortality effect associated with CardioMEMS was modelled). The QALYs per patient for usual care and PAP‐guided patients were 2.62 and 2.94, respectively, reflecting an increase of 0.32 QALYs with PAP‐guided treatment. The resultant ICER, the ratio between incremental costs and the QALYs, is estimated at £19 671/QALY.
Probabilistic sensitivity analysis
The results of the PSA displayed on a scatterplot (ellipse indicates 95% confidence interval) show a tight cluster of results in the north‐east quadrant. Lines indicating willingness to pay (WTP) thresholds of an ICER of £20 000/QALY and £30 000/QALY have been drawn for reference (Figure 1 ); these lines represent the WTP thresholds below which NICE typically recommends a new treatment be made available to NHS patients. The majority of points on the scatterplot are underneath these thresholds, as the probability of PAP being cost‐effective at a WTP threshold of £30 000/QALY is 81.9% (Figure 2 ).
Figure 1.
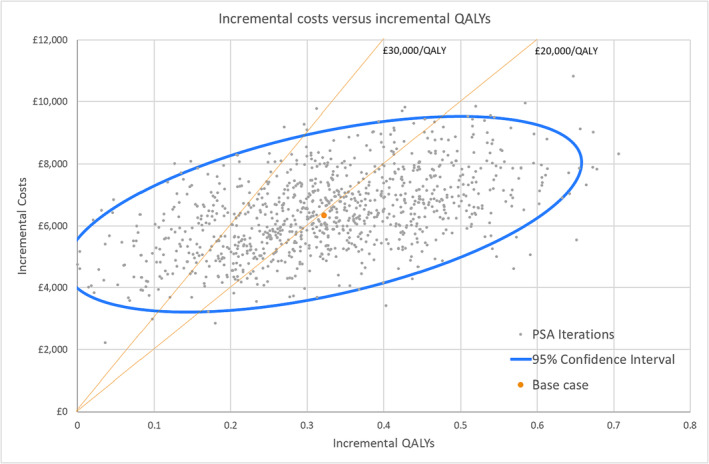
Cost‐effectiveness scatter plot. Each of the dots on the scatter plot is one of 1000 mean incremental cost and incremental quality‐adjusted life year (QALY) results of 1000 model runs each with different input values sampled from the input distributions. PSA, probabilistic sensitivity analysis.
Figure 2.
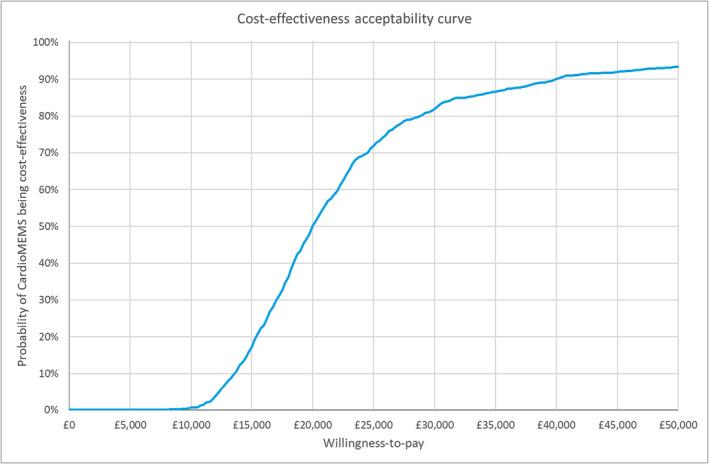
Cost‐effectiveness acceptability curve.
Discussion
The results of our cost‐utility analysis suggest that the CardioMEMS™ HF System could provide cost‐effective benefits to HF patients in the United Kingdom: The base case ICER of £19 761/QALY is lower than the threshold range of £20 000–30 000/QALY typically used by NICE. The ICER estimated in our analysis is similar to that estimated in the previous cost‐utility analysis of CardioMEMS™ HF System, which suggested a base case ICER of £19 274/QALY. This is because the conservative choices we made when assuming no mortality benefit and inclusion of monitoring costs (which were different to the assumptions made in the previous cost‐effectiveness analyses of CardioMEMS, which included mortality benefit but excluded monitoring costs) were offset by the higher baseline hospitalization rates and higher HFH costs used in the current analyses.
Incorporating the 20% beneficial effect on mortality observed in the CHAMPION trial in a one‐way sensitivity analysis shows that the ICER could be as low as £14 234/QALY, whilst using the mortality ratio from GUIDE‐HF resulted in CardioMEMS being dominated by usual care (Table 2 ). Whilst the mortality benefit in CHAMPION did not reach statistical significance, this trend can be included in economic modelling, which recommends the use of point estimates even when effect differences lack conventional statistical significance. However, in the scenario where we modelled an HR for mortality above 1, we would be testing the assumption that the device increases mortality and the model would produce a negative ICER, indicating that it costs money to reduce outcomes. There is of course no suggestion to date that the CardioMEMS system would increase mortality. However, this is still an area of uncertainty and we recommend that long‐term mortality be studied as part of any future registry or clinical trial in the European healthcare setting.
Table 2.
Results of the scenario and sensitivity analyses
| ID | Scenario | Usual care | CardioMEMS | ICER | ||||
|---|---|---|---|---|---|---|---|---|
| LYs | QALYs | Costs | LYs | QALYs | Costs | |||
| 1 | Base case analyses (inputs from Table 1 ) | 4.79 | 2.62 | £22 770 | 4.79 | 2.94 | £29 107 | £19 671/QALY |
| 2 | £2518 for cost of HF hospitalization (assumption) | 4.79 | 2.62 | £14 870 | 4.79 | 2.94 | £23 735 | £27 519/QALY |
| 3 | £5188 for cost of HF hospitalization (assumption) | 4.79 | 2.62 | £28 262 | 4.79 | 2.94 | £32 842 | £14 217/QALY |
| 4 | Baseline HF hospitalization rate of 5.6% (using data from the CHAMPION trial) | 4.79 | 2.62 | £15 455 | 4.79 | 2.94 | £24 085 | £26 900/QALY |
| 5 | Baseline HF hospitalization rate of 11.8% (using data from COAST and MEMS‐HF) | 4.79 | 2.62 | £30 085 | 4.79 | 2.94 | £34 186 | £12 679/QALY |
| 6 | HR for HF hospitalization of 0.8 (assumption) | 4.79 | 2.62 | £22 770 | 4.79 | 2.94 | £31 719 | £27 910/QALY |
| 7 | HR for HF hospitalization of 0.178 (using data from the COAST study) | 4.79 | 2.62 | £22 770 | 4.79 | 2.95 | £18 940 | Dominant |
| 8 | HR for HF hospitalization of 0.38 (using data from the MEMS‐HF study) | 4.79 | 2.62 | £22 770 | 4.79 | 2.94 | £23 169 | £1226/QALY |
| 9 | HR for mortality of 0.8 (using data from the CHAMPION trial) | 4.79 | 2.62 | £22 770 | 5.17 | 3.17 | £30 544 | £14 234/QALY |
| 10 | HR for mortality of 1.81 (using data from subgroup analyses of the GUIDE‐HF trial) | 4.79 | 2.62 | £22 770 | 3.59 | 2.23 | £24 558 | Dominated |
HF, heart failure; HR, hazard ratio; ICER, incremental cost‐effectiveness ratio; LYs, life years; QALYs, quality‐adjusted life years.
The time spent monitoring the patient also influences our estimates of cost‐effectiveness. Recent experience over 18 months of using CardioMEMS in real‐world clinical practice in the United Kingdom indicated that HF nurses spend on average 40 min per patient per month. This time includes the time spent by the nurse reviewing the tracings, calling the patient with medication changes, ordering and checking the blood tests required, and discussing data and actions with the physician. The physician spends on average of 5 min per patient per month in reviewing the data with the nurses and setting up the treatment plan (M. Cowie, 4 personal communication). As such, we have incorporated these monitoring costs in the cost‐effectiveness analysis. If the actual resource use estimates are substantially different to those assumed in the model, the ICER is likely to be different than that estimated in our analysis. Also, the costs of usual care in our analyses are £39 per month based on a general clinical practice in the United Kingdom, which appears quite low when compared with the level of management in the control groups in the CHAMPION and GUIDE‐HF randomized trials. The control groups in CHAMPION and GUIDE‐HF included a very resource‐intensive follow‐up protocol that is not possible or usual to replicate in normal clinical practice; however, there is no methodological guidance yet to account for the costs of the control group management strategies in cost‐effectiveness modelling. As these are important aspects of the model, we recommend that resource utilization be studied as part of any future registry or clinical trial in the European healthcare setting.
The baseline mortality rate for HF in the usual care cohort was taken from previous work by Griffiths et al. in their model to estimate the cost‐effectiveness of ivabradine as part of a submission to NICE. They used the CARE‐HF trial 18 to estimate age‐related mortality and we assumed that there were enough similarities between that population and the target population for CardioMEMS™ to use the figures as an appropriate baseline risk of death. We have also triangulated this value with the mortality rate estimated from the UK HF audit 2019 summary report, 9 which suggests a monthly mortality probability of 0.016 for those hospitalized within the preceding 12 months. Baseline risk of hospitalization for the usual care cohort was estimated as an average of the probabilities reported in the CHAMPION 2 trial and COAST. 4 We believe that this decision represents a conservative approach and is likely to lead to an overestimate of the cost per QALY. Indeed, including a higher baseline risk of HFH from ‘real‐world’ data from COAST (United Kingdom) and MEMS‐HF (Germany, the Netherlands, and Ireland) results in a lower ICER of £12 679/QALY (Table 2 ).
The HR for HFH for CardioMEMS™ used in the model was 0.67, which is based on the long‐term data reported from CHAMPION. 2 , 10 We believe that our analysis represents a conservative estimate leading to an overestimation of the cost per QALY. The magnitude of treatment effect could be affected by the resource‐intensive follow‐up protocols of control groups in CHAMPION and GUIDE‐HF as highlighted earlier. We believe that the UK COAST and MEMS‐HF are more representative of ‘usual care’ even with the inherent issues with historical control studies. Indeed, real‐world studies such as COAST 4 and MEMS‐HF 5 showed much larger effects, and in the scenario analyses performed using the data from COAST 4 (HR = 0.178), a CardioMEMS™‐based strategy was in fact ‘dominant’; that is, patients managed using CardioMEMS™ had lower total costs and higher QALYs compared with usual care. In the scenario analyses performed using data from MEMS‐HF (HR = 0.38), CardioMEMS™‐based strategy had an ICER of £1226/QALY compared with usual care, which is close to being ‘dominant’ (Table 2 ).
All models and modelling analyses make assumptions and simplify reality in some way, which leads to limitations. The model did not consider other events such as pressure sensor failure, given the rarity of these events. Some of the costs in the model were fixed during PSA rather than modelled as distributions, but these are not expected to impact the cost‐effectiveness results or conclusions. We have made conservative choices when defining the base case and we believe that the ICER is likely to be an overestimate. For example, CardioMEMS could also reduce outpatient medical care (e.g. urgent care visits, outpatient visits, telehealth visits, and outpatient infusion of diuretics), and incorporating these cost savings would further reduce the ICER. Similarly, incorporating a societal perspective would reduce the ICER as patients on CardioMEMS are expected to fewer transportation costs, less caregiver burden (i.e. less caregiver time and costs), and lower levels of absenteeism from work (for both patients and caregivers). Our analysis is from a UK NHS perspective, which may not be generalizable to other European countries. There are studies currently underway in Germany (PASSPORT‐HF 19 ) and the Netherlands (MONITOR‐HF 20 ), which may provide more information to update the cost‐effectiveness on CardioMEMS in these countries. Future cost‐effectiveness studies may explore the scenario where all reusable parts of the CardioMEMS monitoring system could be leased instead of being purchased (and discarded) after a patient's death. This would further improve the cost‐effectiveness as preserving parts of the system for re‐use is likely to lower costs (and also come with the benefits of protection of the environment and better use of resources).
Conclusions
Our model suggests that CardioMEMS is likely to be cost‐effective in the United Kingdom, at the currently considered threshold of value for money. Our findings are supportive of the recent decisions by NICE in England to change its interventional procedure guidance recommendation 21 (from a registry‐ or trial‐only‐based setting to ‘normal’ clinical practice) on the basis of adequate evidence of safety and efficacy for percutaneous implantation of PAP sensors for monitoring treatment of chronic HF and the European Society of Cardiology 2021 guidelines recommendation 22 (that monitoring of PAP using wireless haemodynamic monitoring system may be considered in symptomatic patients with HF to improve outcomes—recommendation class IIb).
Conflict of interest
MRC is employed by Astrazeneca, and ZI & PB are employed by Abbott Laboratories.
Appendix A. Detailed information about the model inputs
A.1. Mortality
A.1.1. Baseline mortality probability estimated from heart failure audit
The difference in 1 year mortality and 30 day mortality for New York Heart Association (NYHA) III/IV group was converted into a monthly mortality probability (using time duration of 11 months) on the assumption that most patients would receive CardioMEMS beyond 1 month after heart failure (HF) hospitalization. 9 The 1 year mortality probability was estimated as 0.35 by applying the hazard ratio (HR) for the NYHA III/IV group (1.12) to the baseline mortality of 0.32. The 30 day mortality probability was estimated as 0.19 by applying the HR for the NYHA III/IV group (1.19) to the baseline mortality probability of 0.16. The difference in 1 year mortality and 30 day mortality is estimated as 0.16, which is converted into a monthly probability of 0.016 using the formula 1 − (1 − 0.16)^(1/11).
A.1.2. Baseline mortality probability estimated from Griffiths et al.
Baseline mortality was estimated from the monthly probability of death used in the previous version of the model based on the study by Griffiths et al. 8 who estimated mortality rates based on the CARE‐HF trial, a randomized controlled trial conducted on NYHA class III and IV HF patients with a prior hospitalization event. The mortality rates used in the model are adjusted using UK interim life tables and increases with age in 5‐yearly steps (see Table A1 for more details). These mortality rates are similar to the recent data presented in the national HF audit 2019 summary report, which suggests an average monthly mortality probability of 0.016.
Table A1.
Monthly risk of mortality by age group
| Age group | Monthly risk of mortality | Source |
|---|---|---|
| Mortality risk age 45–50 | 0.00125 | Griffiths et al. 8 |
| Mortality risk age 50–55 | 0.00197 | |
| Mortality risk age 55–60 | 0.00296 | |
| Mortality risk age 60–65 | 0.00460 | |
| Mortality risk age 65–70 | 0.00698 | |
| Mortality risk age 70–75 | 0.01044 | |
| Mortality risk age 75–80 | 0.01566 | |
| Mortality risk age 80–85 | 0.02136 | |
| Mortality risk age 85–90 | 0.02301 | |
| Mortality risk age 90+ | 0.01864 |
As we have triangulated this value with the mortality rate estimated from the national HF audit 2019 summary report, the mortality rate from Griffiths et al. 8 was used in the model.
A.2. Utilities
Health‐related quality of life (or utilities) for the patients were the same as the values used in the previous version of the model. Utilities were based on the European Quality of Life (EuroQoL) Five Dimensions, three‐level questionnaire (EQ‐5D‐3L) data collected from the patients within the CHAMPION trial at 1, 3, 6, and 12 months as presented in Table A2 .
Table A2.
Utility values estimated from the CHAMPION 2 trial
| Usual care patients | CardioMEMS patients | |
|---|---|---|
| Trial utility at 1 month | 0.645 | 0.688 |
| Trial utility at 3 months | 0.569 | 0.646 |
| Trial utility at 6 months | 0.566 | 0.617 |
| Trial utility at 12 months | 0.547 | 0.653 |
Cowie, M. R. , Thokala, P. , Ihara, Z. , Adamson, P. B. , and Angermann, C. (2023) Real‐time pulmonary artery pressure monitoring in heart failure patients: an updated cost‐effectiveness analysis. ESC Heart Failure, 10: 3046–3054. 10.1002/ehf2.14496.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al.; CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3 [DOI] [PubMed] [Google Scholar]
- 3. Lindenfeld J, Abraham WT, Maisel A, Zile M, Smart F, Costanzo MR, et al. Hemodynamic‐GUIDEd management of heart failure (GUIDE‐HF). Am Heart J 2019;214:18–27. doi: 10.1016/j.ahj.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 4. Cowie MR, Flett A, Cowburn P, Foley P, Chandrasekaran B, Loke I, et al. Real‐world evidence in a national health service: Results of the UK CardioMEMS HF System Post‐Market Study. ESC Hear Fail 2022;9:48–56. doi: 10.1002/ehf2.13748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angermann CE, Assmus B, Anker SD, Asselbergs FW, Brachmann J, Brett ME, et al.; for the MEMS‐HF Investigators . Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF). Eur J Heart Fail 2020;22:1891–1901. doi: 10.1002/ejhf.1943 [DOI] [PubMed] [Google Scholar]
- 6. Cowie MR, Simon M, Klein L, Thokala P. The cost‐effectiveness of real‐time pulmonary artery pressure monitoring in heart failure patients: A European perspective. Eur J Heart Fail 2017;19:661–669. doi: 10.1002/ejhf.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Institute for Health and Care Excellence . Guide to the methods of technology appraisal 2013 Process and methods. 2013. www.nice.org.uk/process/pmg9. Accessed 8 August 2023 [PubMed]
- 8. Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the UK National Health Service perspective. Heart 2014;100:1031–1036. doi: 10.1136/heartjnl-2013-304598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Institute for Cardiovascular Outcomes Research . National heart failure audit 2019/20 summary report. 2020. https://www.nicor.org.uk/wp‐content/uploads/2019/09/Heart‐Failure‐2019‐Report‐final.pdf. Accessed 8 August 2023
- 10. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB; CHAMPION Trial Study Group . Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow‐up results from the CHAMPION randomised trial. Lancet (London, England) 2016;387:453–461. doi: 10.1016/S0140-6736(15)00723-0 [DOI] [PubMed] [Google Scholar]
- 11. Berg J, Lindgren P, Mejhert M, Edner M, Dahlström U, Kahan T. Determinants of utility based on the EuroQol five‐dimensional questionnaire in patients with chronic heart failure and their change over time: Results from the Swedish Heart Failure Registry. Value Health 2015;18:439–448. doi: 10.1016/j.jval.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 12. Klersy C, de Silvestri A, Gabutti G, Raisaro A, Curti M, Regoli F, et al. Economic impact of remote patient monitoring: An integrated economic model derived from a meta‐analysis of randomized controlled trials in heart failure. Eur J Heart Fail 2011;13:450–459. doi: 10.1093/eurjhf/hfq232 [DOI] [PubMed] [Google Scholar]
- 13. Thokala P, Baalbaki H, Brennan A, Pandor A, Stevens JW, Gomersall T, et al. Telemonitoring after discharge from hospital with heart failure: Cost‐effectiveness modelling of alternative service designs. BMJ Open 2013;3:e003250. doi: 10.1136/bmjopen-2013-003250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Cost Collection for the NHS . National schedule of NHS costs 2020/21. 2021. https://www.england.nhs.uk/costing‐in‐the‐nhs/national‐cost‐collection/. Accessed 8 August 2023
- 15. National Institute for Health and Care Excellence . Dapagliflozin for treating chronic heart failure with reduced ejection fraction: Technology appraisal guidance [TA679]. 2021. https://www.nice.org.uk/guidance/ta679. Accessed 8 August 2023
- 16. Lucas R, Sharma R, Mason M, Singleton M, Lane R, Cowie M. “UK experience of using an implantable pulmonary artery pressure monitoring system (CardioMEMS) for heart failure monitoring” European Society of Cardiology 2017. poster.
- 17. Dauw J, Sokolski M, Middleton JT, Nijst P, Dupont M, Forouzan O, et al. Ambulatory haemodynamic‐guided management reduces heart failure hospitalizations in a multicentre European heart failure cohort. ESC Heart Fail 2022;9:3858–3867. doi: 10.1002/ehf2.14056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cleland JGF, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. doi: 10.1056/NEJMoa050496 [DOI] [PubMed] [Google Scholar]
- 19. Störk S, Bernhardt A, Böhm M, Brachmann J, Dagres N, Frantz S, et al. Pulmonary artery sensor system pressure monitoring to improve heart failure outcomes (PASSPORT‐HF): Rationale and design of the PASSPORT‐HF multicenter randomized clinical trial. Clin Res Cardiol 2022;111:1245–1255. doi: 10.1007/s00392-022-01987-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brugts JJ, Veenis JF, Radhoe SP, Linssen GCM, van Gent M, Borleffs CJW, et al. A randomised comparison of the effect of haemodynamic monitoring with CardioMEMS in addition to standard care on quality of life and hospitalisations in patients with chronic heart failure: Design and rationale of the MONITOR HF multicentre randomised clinical trial. Neth Heart J 2020;28:16–26. doi: 10.1007/s12471-019-01341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institute for Health and Care Excellence . Interventional Procedures Guidance (IPG 711). Percutaneous implantation of pulmonary artery pressure sensors for monitoring treatment of chronic heart failure. 2021. https://www.nice.org.uk/guidance/ipg711. Accessed 8 August 2023
- 22. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]


