Abstract
Aims
Atrial fibrillation (AF) increases the risk of heart failure (HF); however, little focus has been placed on the prevention of HF in patients with AF. Left ventricular ejection fraction (LVEF) is an established echocardiographic parameter in HF patients. We sought to investigate the association of LVEF with HF events in AF patients without pre‐existing HF.
Methods and results
The Fushimi AF Registry is a community‐based prospective survey of AF patients in Fushimi‐ku, Japan. In this analysis, we excluded patients with pre‐existing HF (defined as having one of the following: prior HF hospitalization, New York Heart Association class ≥ 2 in association with heart disease, or LVEF < 40%). Among 3233 AF patients without pre‐existing HF, we investigated 2459 patients with the data of LVEF at enrolment. We divided the patients into three groups stratified by LVEF [mildly reduced LVEF (40–49%), below normal LVEF (50–59%), and normal LVEF (≥60%)] and compared the backgrounds and incidence of HF hospitalization between the groups. Of 2459 patients [mean age: 72.4 ± 10.5 years, female: 917 (37%), paroxysmal AF: 1405 (57%), and mean CHA2DS2‐VASc score: 3.0 ± 1.6], the mean LVEF was 66 ± 8% [mildly reduced LVEF: 114 patients (5%), below normal LVEF: 300 patients (12%), and normal LVEF: 2045 patients (83%)]. Patients with lower LVEF demonstrated lower prevalence of female and paroxysmal AF (both P < 0.01), but age and CHA2DS2‐VASc score were comparable between the three groups (both P > 0.05). During the median follow‐up period of 6.0 years, 255 patients (10%) were hospitalized for HF (annual incidence: 1.9% per person‐year). Multivariable Cox regression analysis demonstrated that lower LVEF strata were independently associated with the risk of HF [mildly reduced LVEF (40–49%): hazard ratio = 2.98, 95% confidence interval = 1.99–4.45 and below normal LVEF (50–59%): hazard ratio = 2.01, 95% confidence interval = 1.44–2.82, compared with normal LVEF (≥60%)] after adjustment by age, sex, type of AF, and CHA2DS2‐VASc score. LVEF < 60% was significantly associated with the higher risk of HF hospitalization across all major subgroups without significant interaction (P for interaction; all P > 0.05). LVEF had an independent and incremental prognostic value for HF hospitalization in addition to natriuretic peptide levels in AF patients without pre‐existing HF.
Conclusions
Lower LVEF was significantly associated with the higher incidence of HF hospitalization in AF patients without pre‐existing HF, leading to the future risk stratification for and prevention of incident HF in AF patients.
Keywords: Atrial fibrillation, Heart failure, Left ventricular ejection fraction
Introduction
Atrial fibrillation (AF) is associated with the higher risk of mortality and morbidities including thrombo‐embolism and heart failure (HF). 1 In the modern anticoagulation era, HF represents the most common cardiovascular complication in patients with AF, developing at a rate nearly twice that of thrombo‐embolism. 2 , 3 Furthermore, HF accounted for a substantial proportion of deaths among patients with AF, which far exceeds that of death due to thrombo‐embolism. 4 , 5 Although the incidence of thrombo‐embolism has been decreasing, that of HF has not significantly improved over a period of decades. 3 , 6 These findings underscore the importance for prevention of incident HF among AF patients. We previously reported the utility of natriuretic peptide levels for predicting HF events in AF patients without pre‐existing HF 7 ; however, further risk stratification is warranted for the management of AF patients.
Left ventricular ejection fraction (LVEF) measured by echocardiography remains the cornerstone for quantification of the left ventricular systolic performance in clinical practice. In patients with HF, LVEF is known to be a potent predictor of poorer outcomes including all‐cause death or HF rehospitalization. 8 , 9 Thus, LVEF has an essential role in classification and guiding therapy among HF patients. 10 We recently demonstrated that LVEF might also be a useful predictor for incident HF in patients with AF using machine learning technique. 11 Nonetheless, the study focusing on the association between LVEF strata and incident HF has, to the best of our knowledge, never been conducted in a population of AF patients without pre‐existing HF.
Accordingly, the aim of the present study was to investigate the relationship between LVEF and the risk of HF events among AF patients without pre‐existing HF, using the data from a large‐scaled community‐based prospective survey of Japanese AF patients, the Fushimi AF Registry.
Methods
Data source
The Fushimi AF Registry is a community‐based multicentre prospective observational cohort of patients with AF who visited the participating medical institutions in Fushimi‐ku, Kyoto, Japan. The detailed study design, patient enrolment, and the definition of the measurements of the registry were previously described (UMIN Clinical Trials Registry: UMIN000005834). 12 In brief, the inclusion criterion for the registry is the documentation of AF on a 12‐lead electrocardiogram or Holter monitoring at any time. There were no exclusion criteria. A total of 81 institutions participated in the registry, comprising 2 cardiovascular centres (Kyoto Medical Center and Ijinkai Takeda Hospital), 10 small‐ and medium‐sized hospitals, and 69 primary care clinics. We started to enrol patients from March 2011 and enrolment ended at May 2017. We attempted to enrol all consecutive patients with AF under regular outpatient care or under admission. Annual collection of the follow‐up information was mainly conducted through review of the electronic and/or paper medical records, and additional follow‐up information was collected through contact with patients, relatives, and/or referring physicians by mail or telephone at the discretion of the investigators.
Clinical data of the patients were registered on an Internet Database System (https://edmsweb16.eps.co.jp/edmsweb/002001/FAF/top.html) by the doctors in charge at each institution. Data were automatically checked for missing or contradictory entries and values out of the normal range. Additional checks of variables were performed by clinical research co‐ordinators at the general office of the registry. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committees of the Kyoto Medical Center and Ijinkai Takeda Hospital.
Study population and definitions
In the present analysis, we excluded AF patients with pre‐existing HF, which was defined as the presence of one of the following at enrolment: (i) history of hospitalization for HF prior to enrolment, (ii) presence of symptom due to HF (New York Heart Association functional class ≥ 2) in association with heart disease, or (iii) reduced LVEF < 40%. 13 Then, we investigated patients with the data of LVEF among AF patients without pre‐existing HF. Data of transthoracic echocardiography including LVEF, left ventricular diameter, thickness and asynergy, and left atrial diameter were collected at the time of enrolment in the registry. The decision to perform echocardiography was at the discretion of the attending physicians. LVEF was calculated using the biplane Simpson method or the Teichholz method at each participating institutions according to the guidelines. 14 Considering the classification of LVEF in the HF guidelines and previous studies among cardiovascular diseases, 15 , 16 , 17 , 18 we divided the patients into three groups stratified by LVEF [mildly reduced LVEF (40–49%), below normal LVEF (50–59%), and normal LVEF (≥60%)].
B‐type natriuretic peptide (BNP) and N‐terminal pro‐BNP (NT‐proBNP) levels were obtained at the discretion of the attending physicians and measured using the clinical assay of each participating site. For standardization purposes, BNP was converted to NT‐proBNP using the following conversion formula: ‘log10(NT‐proBNP) is equal to 1.1 × log10(BNP) + 0.570’ based on previous reports. 7 , 19 The type of AF was classified into two groups: paroxysmal AF and sustained AF, which was defined as the combination of persistent AF and permanent AF. 12 Antiarrhythmic drugs in this study were defined as class I or class III drugs categorized by the Vaughan Williams classification.
Outcomes
The endpoint in this study was HF hospitalization during the follow‐up period. HF hospitalization was determined based on history, clinical presentation (symptoms and physical examinations), natriuretic peptide levels, imaging findings including chest X‐ray and echocardiography, cardiac catheterization findings, response to HF therapy, and in‐hospital course judged by the attending physicians according to the appropriate guidelines. 20 , 21 We continued follow‐up until death and we defined clinical outcomes as the time to first event.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation when normally distributed and as the median and interquartile range when non‐normally distributed. Distribution was assessed using histogram. Comparisons of differences among groups were performed by the unpaired Student's t‐test, Mann–Whitney U test, one‐way analysis of variance, or Kruskal–Wallis test for continuous variables and χ 2 test for dichotomous variables as appropriate. The relationship between the variables was determined by Spearman's correlation analysis. The Kaplan–Meier method was used to estimate the cumulative incidences of outcomes and log‐rank testing was performed to assess differences among groups. Univariable and multivariable Cox regression analyses were used to investigate the association between LVEF and the incidence of HF hospitalization. The covariates selected to be included in multivariable Model 1 were age, sex, type of AF (paroxysmal or sustained), and the CHA2DS2‐VASc score. Multivariable Model 2 was adjusted for covariates included in Model 1 and the prescription of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, beta‐blockers, and mineralocorticoid receptor antagonists. The unadjusted and adjusted risks of two LVEF groups [mildly reduced LVEF (40–49%) and below normal LVEF (50–59%)] relative to the normal LVEF group (≥60%) for the incidence of HF hospitalization were expressed as hazard ratios (HRs) and their 95% confidence intervals (CIs). We also calculated HR per 5% decrease in LVEF as continuous variable. Subgroup analyses stratified by age, sex, type of AF, prescription of antiarrhythmic drugs, and history of cardiovascular diseases (coronary artery disease, valvular heart disease, and hypertension) were also performed. As the optimal cut‐off value of ‘normal’ LVEF has been under debate, 22 , 23 , 24 we investigated the association of LVEF < 60% relative to LVEF ≥ 60% with the incident HF in this subgroup analysis. The P‐values for interaction were calculated by multivariable Cox regression analysis with adjustment by the covariates mentioned above (age, sex, type of AF, and the CHA2DS2‐VASc score) in order to examine the heterogeneity in the subgroups. Lastly, we specifically investigated the association of LVEF with HF hospitalization among patients with the data of natriuretic peptide levels. We performed multivariable Cox regression analysis adjusted by covariates included in Model 1 and NT‐proBNP levels (multivariable Model 3). The levels of NT‐proBNP were transformed to a log scale in the model. In addition, we stratified the patients into four groups according to LVEF (≥60% or <60%) and NT‐proBNP levels (≥ or <median value) and examined the outcomes between these four groups. All tests were two‐tailed, and a value of P < 0.05 was considered significant. All analyses were performed using JMP Version 14.2.0.
Results
A study flowchart of this analysis is presented in Figure 1 A . As an exploratory analysis, the backgrounds and incidence of HF hospitalization stratified by LVEF among AF patients with pre‐existing HF (N = 1256) are shown in Supporting Information, Table S1 and Figure S1 . In brief, LVEF was significantly associated with the higher risk of incident HF in AF patients with pre‐existing HF.
Figure 1.
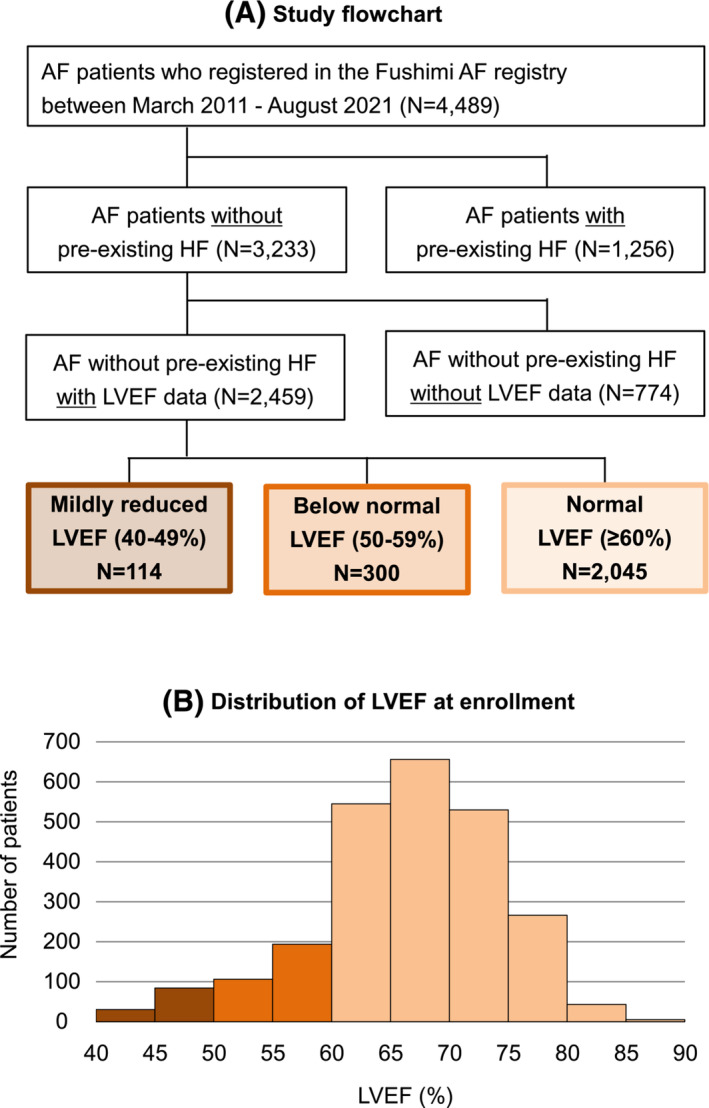
(A) Study flowchart. (B) Distribution of left ventricular ejection fraction (LVEF) at enrolment in atrial fibrillation (AF) patients without pre‐existing heart failure (HF).
Among 3233 AF patients without pre‐existing HF, LVEF data at enrolment were available for 2459 patients (76% of the total). Baseline characteristics and incidence of HF hospitalization were almost comparable between patients with LVEF data and those without it (Supporting Information, Table S2 and Figure S2 ).
Baseline characteristics of atrial fibrillation patients without pre‐existing heart failure
Among 2459 AF patients without pre‐existing HF and with the data of LVEF [mean age: 72.4 ± 10.5 years, women: 917 (37%), paroxysmal AF: 1405 (57%), and mean CHA2DS2‐VASc score: 3.0 ± 1.6], the distribution of LVEF is shown in Figure 1 B . The mean LVEF at enrolment was 66 ± 8% [mildly reduced LVEF (40–49%): 114 patients (5%), below normal LVEF (50–59%): 300 patients (12%), and normal LVEF (≥60%): 2045 patients (83%)]. Median LVEF at enrolment (interquartile range) was 67% (62%, 72%). Baseline characteristics according to the three LVEF strata are shown in Table 1 . Patients with lower LVEF demonstrated lower prevalence of women and paroxysmal AF (both P < 0.001). Age and CHA2DS2‐VASc score were comparable between the three groups. NT‐proBNP levels were higher and left ventricular and left atrial diameters were larger in patients with lower LVEF strata (all P < 0.001).
Table 1.
Baseline characteristics according to left ventricular ejection fraction strata in atrial fibrillation patients without pre‐existing heart failure
| Variables | Mildly reduced LVEF (40–49%) | Below normal LVEF (50–59%) | Normal LVEF (≥60%) | P‐value | No. of patients analysed |
|---|---|---|---|---|---|
| n = 114 | n = 300 | n = 2045 | |||
| Baseline characteristics | |||||
| Age (years) | 73.4 ± 11.0 | 71.7 ± 11.7 | 72.4 ± 10.3 | 0.30 | 2459 |
| Age ≥ 75 years, n (%) | 55 (48%) | 130 (43%) | 937 (46%) | 0.61 | 2459 |
| Women, n (%) | 23 (20%) | 75 (25%) | 819 (40%) | <0.001 | 2459 |
| Body mass index (kg/m2) | 23.0 ± 3.9 | 23.4 ± 4.1 | 23.2 ± 3.7 | 0.72 | 2089 |
| Body weight (kg) | 60.8 ± 13.0 | 61.3 ± 12.7 | 60.2 ± 12.6 | 0.40 | 2180 |
| Systolic blood pressure (mmHg) | 122 ± 19 | 125 ± 20 | 126 ± 19 | 0.031 | 2442 |
| Pulse rate (beats/min) | 78 ± 16 | 79 ± 17 | 77 ± 15 | 0.080 | 2426 |
| Paroxysmal AF, n (%) | 43 (38%) | 147 (49%) | 1215 (59%) | <0.001 | 2459 |
| History of ablation, n (%) | 2 (2%) | 11 (4%) | 184 (9%) | <0.001 | 2459 |
| Co‐morbidities | |||||
| CHADS2 score | 1.9 ± 1.3 | 1.7 ± 1.3 | 1.7 ± 1.2 | 0.30 | 2459 |
| CHA2DS2‐VASc score | 3.1 ± 1.6 | 2.8 ± 1.6 | 3.0 ± 1.5 | 0.13 | 2459 |
| Coronary artery disease, n (%) | 26 (23%) | 50 (17%) | 198 (10%) | <0.001 | 2459 |
| Valvular heart disease, n (%) | 25 (22%) | 43 (14%) | 252 (12%) | 0.009 | 2459 |
| AS/AR/MS/MR/TR, n | 1/7/2/10/6 | 2/12/2/22/15 | 33/54/13/139/65 | 2453 | |
| Cardiomyopathy, n (%) | 4 (4%) | 9 (3%) | 35 (2%) | 0.15 | 2459 |
| Hypertension, n (%) | 80 (70%) | 180 (60%) | 1275 (62%) | 0.16 | 2459 |
| Dyslipidaemia, n (%) | 55 (48%) | 122 (41%) | 927 (45%) | 0.24 | 2459 |
| Diabetes mellitus, n (%) | 34 (30%) | 67 (22%) | 471 (23%) | 0.23 | 2459 |
| History of stroke/SE, n (%) | 24 (21%) | 66 (22%) | 403 (20%) | 0.63 | 2459 |
| Peripheral artery disease, n (%) | 4 (4%) | 9 (3%) | 72 (4%) | 0.90 | 2459 |
| Chronic kidney disease, n (%) | 49 (43%) | 96 (32%) | 601 (29%) | 0.007 | 2459 |
| COPD, n (%) | 10 (9%) | 20 (7%) | 105 (5%) | 0.16 | 2459 |
| Prescription at enrolment | |||||
| Oral anticoagulants, n (%) | 72 (63%) | 148 (50%) | 1094 (54%) | 0.044 | 2446 |
| Warfarin, n (%) | 52 (46%) | 106 (35%) | 776 (38%) | 0.16 | 2446 |
| DOAC, n (%) | 20 (18%) | 42 (14%) | 318 (16%) | 0.65 | 2446 |
| Antiplatelet therapy, n (%) | 43 (38%) | 69 (23%) | 512 (25%) | 0.007 | 2446 |
| ACEi/ARB, n (%) | 59 (52%) | 115 (38%) | 821 (40%) | 0.039 | 2446 |
| Beta‐blocker, n (%) | 36 (32%) | 78 (26%) | 502 (25%) | 0.24 | 2446 |
| MRA, n (%) | 11 (10%) | 12 (4%) | 76 (4%) | 0.008 | 2446 |
| Loop diuretics, n (%) | 25 (22%) | 35 (12%) | 207 (10%) | <0.001 | 2446 |
| Antiarrhythmic drugs, n (%) | 13 (11%) | 30 (10%) | 496 (24%) | <0.001 | 2446 |
| Class I/class III, n | 13/0 | 30/0 | 490/6 | 2446 | |
| Laboratory data | |||||
| BNP (ng/L) | 167 (105, 321) | 94 (44, 197) | 86 (39, 180) | 0.11 | 330 |
| NT‐proBNP (ng/L) | 817 (569, 1596) | 585 (248, 1192) | 438 (151, 959) | <0.001 | 687 |
| Calculated CrCl (mL/min) | 51.5 (31.8, 71.1) | 59.5 (40.3, 79.2) | 58.3 (41.0, 77.8) | 0.060 | 2308 |
| Haemoglobin (g/dL) | 13.2 ± 1.9 | 13.1 ± 2.2 | 13.2 ± 1.9 | 0.81 | 2317 |
| Echocardiography | |||||
| LV end‐diastolic diameter (mm) | 49.7 ± 7.5 | 47.2 ± 5.9 | 45.2 ± 5.1 | <0.001 | 2435 |
| LVEF (%) | 46.0 ± 2.7 | 55.5 ± 2.8 | 68.7 ± 5.4 | <0.001 | 2459 |
| LV asynergy, n (%) | 88 (78%) | 97 (32%) | 119 (6%) | <0.001 | 2451 |
| Left atrial diameter (mm) | 45.7 ± 8.0 | 42.8 ± 7.9 | 42.0 ± 7.5 | <0.001 | 2415 |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AR, aortic regurgitation; ARB, angiotensin receptor blocker; AS, aortic stenosis; BNP, B‐type natriuretic peptide; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; DOAC, direct oral anticoagulants; LV, left ventricular; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; MS, mitral stenosis; NT‐proBNP, N‐terminal pro‐BNP; SE, systemic embolism; TR, tricuspid regurgitation.
Categorical data are presented as numbers (%). Continuous data are presented as the mean ± standard deviation, or median and interquartile range (25%, 75%).
Association of left ventricular ejection fraction with the incidence of heart failure among atrial fibrillation patients without pre‐existing heart failure
During a median follow‐up period of 6.0 years (interquartile range: 3.1–9.0 years), a total of 255 cases of HF hospitalization occurred in AF patients without pre‐existing HF and with the data of LVEF, corresponding to an annual incidence of 1.9% per person‐year. Patients with lower LVEF strata had a higher incidence of HF hospitalization during follow‐up period (Table 2 ). The Kaplan–Meier curves demonstrated that the three LVEF strata could stratify the risk of HF hospitalization during follow‐up period (log‐rank; P < 0.001) (Figure 2 ). Cox regression analyses revealed that LVEF was independently associated with the increased risk of HF hospitalization even after adjustment by the confounders (multivariable Model 1) (mildly reduced LVEF: HR = 2.98, 95% CI = 1.99–4.45 and below normal LVEF: HR = 2.01, 95% CI = 1.44–2.82, compared with normal LVEF). LVEF remained an independent determinant of HF hospitalization after adjustment by the prescription data (multivariable Model 2) and when analysed as continuous variables (Table 2 ).
Table 2.
Incidence and risk of heart failure hospitalization according to left ventricular ejection fraction strata in atrial fibrillation patients without pre‐existing heart failure
| Incidence of events | Univariable Cox regression analysis | Multivariable Cox regression analysis (Model 1) a | Multivariable Cox regression analysis (Model 2) b | Multivariable Cox regression analysis (Model 3) c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidence rate d | Events/no. at risk | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Mildly reduced LVEF (40–49%) | 5.1 | 29/114 | 3.25 (2.20–4.81) | <0.001 | 2.98 (1.99–4.45) | <0.001 | 2.55 (1.70–3.84) | <0.001 | 2.42 (1.34–4.34) | <0.001 |
| Below normal LVEF (50–59%) | 2.9 | 43/300 | 1.89 (1.35–2.63) | 2.01 (1.44–2.82) | 2.05 (1.46–2.87) | 2.14 (1.34–3.41) | ||||
| Normal LVEF (≥60%) | 1.6 | 183/2045 | Reference | Reference | Reference | Reference | ||||
| As continuous variables e | — | — | 1.26 (1.17–1.35) | <0.001 | 1.26 (1.17–1.35) | <0.001 | 1.24 (1.15–1.33) | <0.001 | 1.21 (1.08–1.34) | <0.001 |
CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction.
Multivariable Model 1 was adjusted for age, sex, type of atrial fibrillation, and CHA2DS2‐VASc score.
Multivariable Model 2 was adjusted for covariates included in Model 1 and the prescription of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, beta‐blockers, and mineralocorticoid receptor antagonists.
Multivariable Model 3 was adjusted for covariates included in Model 1 and N‐terminal pro‐B‐type natriuretic peptide levels.
Per person‐year.
HR was calculated per 5% LVEF decrease.
Figure 2.
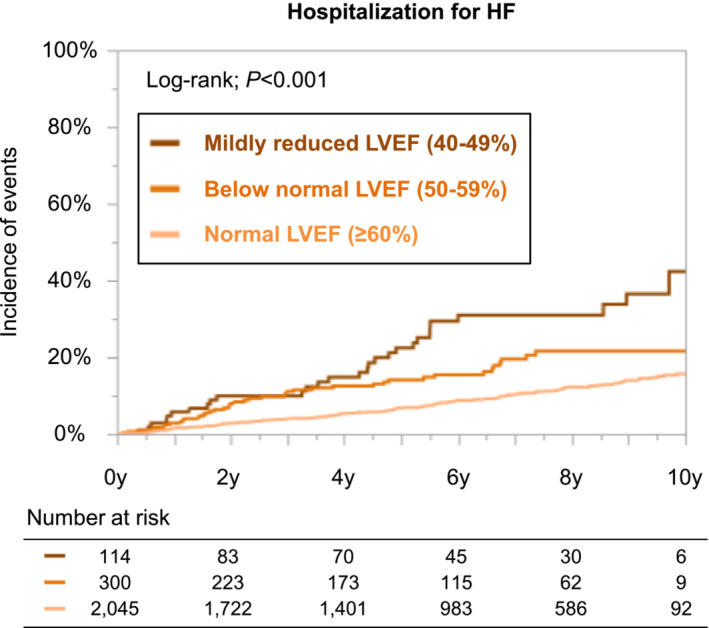
Kaplan–Meier curves for the incidences of heart failure (HF) hospitalization according to the left ventricular ejection fraction (LVEF) strata among atrial fibrillation patients without pre‐existing HF.
The association between LVEF < 60% and incidence of HF hospitalization stratified by major patients' characteristics is shown in Figure 3 . LVEF < 60% was significantly associated with the higher risk of HF hospitalization across all major subgroups without significant interaction (P for interaction; all P > 0.05).
Figure 3.
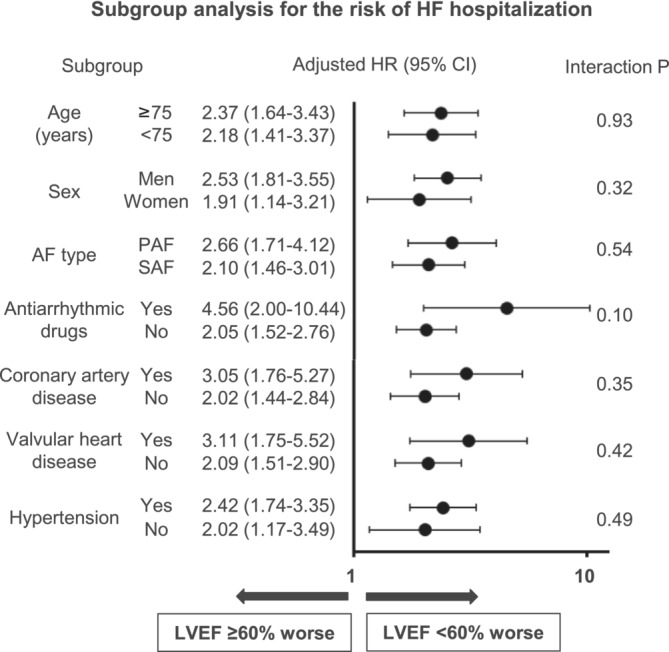
Association of left ventricular ejection fraction (LVEF) < 60% with heart failure (HF) hospitalization among major subgroups in atrial fibrillation (AF) patients without pre‐existing HF. CI, confidence interval; HR, hazard ratio; PAF, paroxysmal AF; SAF, sustained AF.
Patients with natriuretic peptide levels
Among 2459 AF patients without pre‐existing HF and with the data of LVEF, natriuretic peptide levels were available for 1017 patients (330 with BNP and 687 with NT‐proBNP). The LVEF was mildly correlated with NT‐proBNP levels (Spearman's ρ = −0.12, P < 0.001) (Figure 4 A ). The three LVEF strata were significantly associated with the increased risk of HF hospitalization even after adjustment by multivariable analysis including NT‐proBNP levels (multivariable Model 3) (Table 2 ). The Kaplan–Meier curves among the patients divided by LVEF (≥60% or <60%) and NT‐proBNP levels [≥521 or <521 ng/L (median value)] revealed that these four groups could stratify the risk of HF hospitalization during follow‐up period (log‐rank; P < 0.001) (Figure 4 B ).
Figure 4.
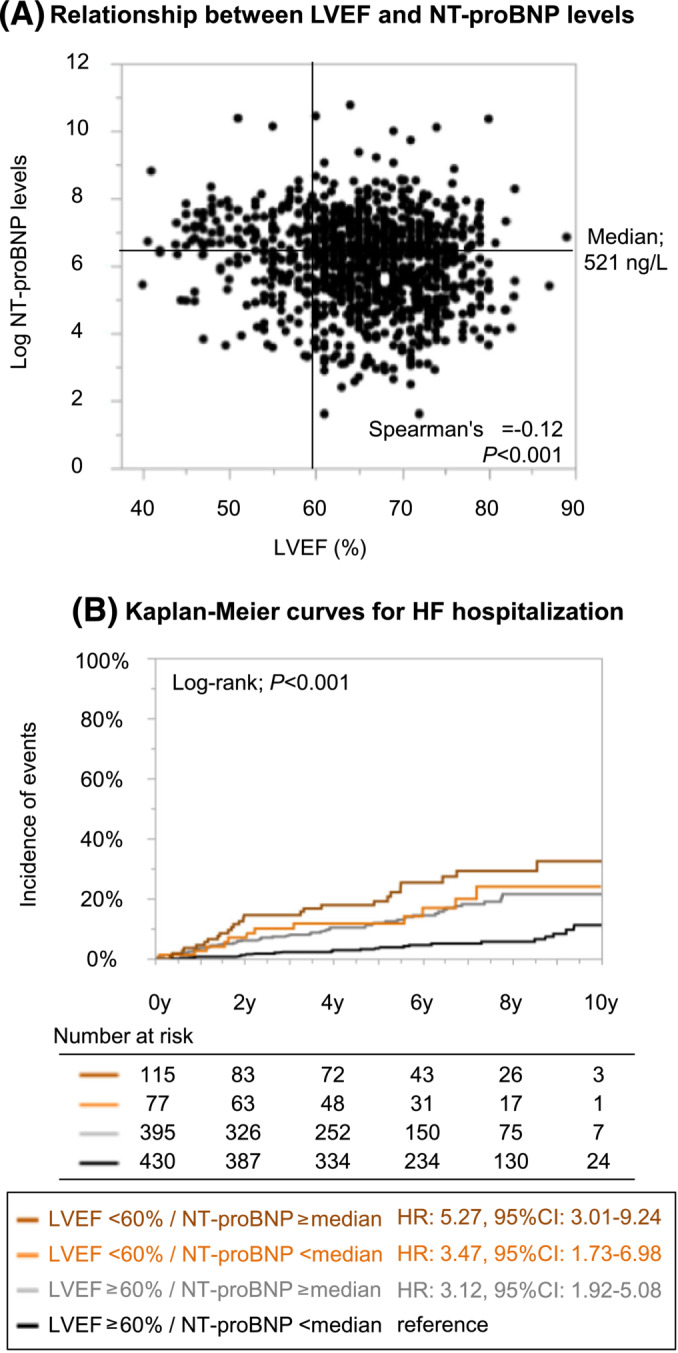
(A) Relationship between left ventricular ejection fraction (LVEF) and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels in atrial fibrillation patients without pre‐existing heart failure (HF). (B) Kaplan–Meier curves for the incidences of HF hospitalization stratified by the LVEF and NT‐proBNP levels. CI, confidence interval; HR, hazard ratio.
Discussion
The major findings of the present study are the following. First, LVEF could stratify the incidence of HF hospitalization in AF patients without pre‐existing HF. Second, even below normal LVEF (<60%) was significantly associated with the higher risk of HF hospitalization across major subgroups. Third, LVEF had independent and incremental prognostic value for HF hospitalization in addition to natriuretic peptide levels in AF patients without pre‐existing HF.
The association of left ventricular ejection fraction with heart failure hospitalization in atrial fibrillation patients without pre‐existing heart failure
Once AF patients develop HF, they have an approximately two‐ to three‐fold higher risk of death than those without. 25 Thus, risk stratification for and prevention of HF events in AF patients without pre‐existing HF is of clinical importance. LVEF is an important descriptor of cardiac function and theoretically can be a significant predictor of future HF events. Indeed, HF readmission rates were higher in HF patients with reduced LVEF and mildly reduced LVEF than in those with HF and preserved LVEF. 26 Even when LVEF was analysed as continuous variables, composite of cardiovascular mortality and HF rehospitalization was more frequent in lower LVEF quartiles among HF patients. 9 However, to date, there is a paucity of literature regarding the association between LVEF and incident HF among AF patients without pre‐existing HF.
One previous study demonstrated that low‐normal LVEF (50–54%) was significantly associated with the incident HF in AF patients without structural heart disease. 27 Besides, we recently suggested that LVEF could be a useful predictor for future HF events using machine learning technique. 11 Using our large‐scaled AF registry with no exclusion criteria over the 5 year follow‐up period, the present results demonstrated that LVEF was an important quantification parameter with respect to the risk stratification for future HF events among AF patients even without pre‐existing HF. Our study suggested the importance of measuring LVEF in all AF patients irrespective of pre‐existing HF. Indeed, European guidelines for AF recommend transthoracic echocardiography as a ‘standard package’ for the evaluation of all patients with AF, 28 and our results support this recommendation.
AF patients with mildly reduced or below normal LVEF are at high risk of developing HF (annual incidences were 5% and 3% in our registry, respectively) and are considered to be in pre‐HF stage. 29 Recently, catheter ablation and/or new HF drugs have been available in daily practice and might be an attractive option in these patients. However, there has been no robust evidence that ablation or HF drugs reduced the risk of all‐cause mortality and/or HF events in patients without HF. In the present study, the number of patients who underwent catheter ablation was so low that we were unable to perform subgroup analysis. At the moment, indications for these treatments should be determined on a case‐by‐case basis.
Optimal threshold of left ventricular ejection fraction for predicting outcomes in atrial fibrillation patients without heart failure
Our analyses suggested that the risk for HF increases even when LVEF is below normal (<60%), which was observed across major subgroups. Several previous studies demonstrated that the risk of mortality extends well beyond currently accepted levels of LVEF (50–55%), with a nadir around an LVEF of 60–65%. 23 , 24 The nadir of the risk for incident HF was also close to 60% in the large‐scaled cohort study. 30 Indeed, revised nomenclature is recently proposed defining HF with ‘reduced’ (<40%), ‘mildly reduced’, and ‘normal’ (>55% in men and >60% in women) LVEF. 22 Taken together with previous studies and ours, we should revisit the optimal cut‐off value of LVEF for risk stratification of HF in patients with AF, in whom LVEF of 50–59% may not be regarded as normal.
Left ventricular ejection fraction and natriuretic peptide levels for predicting heart failure in atrial fibrillation patients
Natriuretic peptide levels such as BNP or NT‐proBNP are important prognostic parameter in HF patients as well as LVEF. We previously reported the prognostic significance of natriuretic peptide levels in AF patients without pre‐existing HF. 7 Of note, natriuretic peptide levels could be a confounder regarding the association between LVEF and incident HF. Nevertheless, LVEF was independently associated with the higher risk of HF hospitalization even taking into account the NT‐proBNP levels in this analysis. Besides, we found that LVEF had the incremental prognostic value for the incidence of HF hospitalization in addition to natriuretic peptide levels, suggesting that the combination of LVEF and NT‐proBNP levels may be used for predicting future HF in AF patients without pre‐existing HF. Measurement of both LVEF and NT‐proBNP might be part of the overall characterization and evaluation of AF patients, given that the contemporary management of AF has moved towards a more holistic or integrated approach considering the improved clinical outcomes with such an approach. 31
Limitations
The present study has several limitations. First, this was an observational study and provides only associative evidence, not causative. We cannot rule out the possibility of unmeasured or residual confounding. In addition, the definition of pre‐existing HF was not based on the recent universal definition of HF, 29 because we started the enrolment of this registry from 2011. Second, the decision to measure LVEF was entirely at the discretion of the attending physicians. Therefore, there was unavoidable selection bias, even though baseline characteristics and incidence of HF were comparable between patients with and without LVEF data. In addition, we did not obtain the data about diastolic dysfunction, speckle tracking, right heart function, or cardiac magnetic resonance imaging in the registry. Third, LVEF data were collected only at enrolment, and we neither obtained the echocardiographic data at the incidence of HF hospitalization nor obtained those during follow‐up period. Thus, we were unable to classify the type of HF hospitalization according to LVEF. Besides, we neither collected the data about aetiology nor collected those about exacerbation factor of HF hospitalization in the registry. The relationship between LVEF and incident HF may vary depending on the cause of HF, and lack of these important data was a major limitation of this study. In addition, both AF and HF are multifactorial entities and HF onset in patients with AF could not only be explained by LVEF. Therefore, our results should be interpreted cautiously. Fourth, the numbers of the patients with mildly reduced or below normal LVEF were small, limiting the robustness of our analysis. Fifth, we had no data about the cardiac rhythm at the time of index echocardiography. Especially in patients with paroxysmal AF, LVEF can fluctuate depending on the cardiac rhythm. Sixth, we did not obtain the calculation method of LVEF and echocardiographic data were site reported. In fact, there could be some intra‐ and inter‐observer variabilities for LVEF measurements. 32 , 33 Thus, we cannot deny the possibility of measurement error and variations in echocardiographic measurements.
Conclusions
LVEF at enrolment could stratify the incidence of HF hospitalization in AF patients without pre‐existing HF, suggesting the importance of measuring LVEF in all AF patients. Even below normal LVEF (<60%) was independently associated with the risk of HF. LVEF had an incremental prognostic value for incident HF in addition to natriuretic peptide levels in AF patients without pre‐existing HF.
Conflict of interest
Dr M. Akao received lecture fees from Pfizer, Bristol‐Myers Squibb, Boehringer Ingelheim, Bayer Healthcare, and Daiichi‐Sankyo. All other authors have reported that they have no relationships relevant to the content of this paper to disclose.
Funding
The Fushimi AF Registry is supported by research funding from Boehringer Ingelheim, Bayer Healthcare, Pfizer, Bristol‐Myers Squibb, Astellas Pharma, AstraZeneca, Daiichi Sankyo Company, Novartis Pharma, MSD, Sanofi‐Aventis, and Takeda Pharmaceutical Company. The sponsors had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. This study was partially supported by the Practical Research Project for Life‐Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus from Japan Agency for Medical Research and Development, AMED (19ek0210082h0003, 18ek0210056h0003) (M. Akao).
Supporting information
Table S1. Baseline characteristics according to LVEF strata in AF patients with pre‐existing HF.
Table S2. Baseline characteristics stratified by the presence of LVEF data among AF patients without pre‐existing HF.
Figure S1. Kaplan–Meier curve for the incidences of HF hospitalization according to LVEF strata among AF patients with pre‐existing HF.
Figure S2. Kaplan–Meier curve for the incidences of HF hospitalization stratified by the presence of LVEF data among AF patients without pre‐existing HF.
Acknowledgements
We sincerely appreciate the efforts of the clinical research co‐ordinators (T. Shinagawa, M. Mitamura, M. Fukahori, M. Kimura, M. Fukuyama, C. Kamata, and N. Nishiyama).
Hamatani, Y. , Iguchi, M. , Minami, K. , Ishigami, K. , Esato, M. , Tsuji, H. , Wada, H. , Hasegawa, K. , Ogawa, H. , Abe, M. , Lip, G. Y. H. , Akao, M. , and the Fushimi AF Registry investigators (2023) Utility of left ventricular ejection fraction in atrial fibrillation patients without pre‐existing heart failure. ESC Heart Failure, 10: 3091–3101. 10.1002/ehf2.14500.
References
- 1. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta‐analysis. BMJ 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 2. Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, et al. Clinical course of atrial fibrillation in older adults: The importance of cardiovascular events beyond stroke. Eur Heart J 2014;35:250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akao M, Ogawa H, Masunaga N, Minami K, Ishigami K, Ikeda S, et al. 10‐year trends of antithrombotic therapy status and outcomes in Japanese atrial fibrillation patients—The Fushimi AF Registry. Circ J 2022;86:726‐736. [DOI] [PubMed] [Google Scholar]
- 4. Gómez‐Outes A, Lagunar‐Ruíz J, Terleira‐Fernández AI, Calvo‐Rojas G, Suárez‐Gea ML, Vargas‐Castrillón E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2016;68:2508‐2521. [DOI] [PubMed] [Google Scholar]
- 5. An Y, Ogawa H, Yamashita Y, Ishii M, Iguchi M, Masunaga N, et al. Causes of death in Japanese patients with atrial fibrillation: The Fushimi Atrial Fibrillation Registry. Eur Heart J Qual Care Clin Outcomes 2019;5:35‐42. [DOI] [PubMed] [Google Scholar]
- 6. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, et al. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: A community‐based study over two decades. Eur Heart J 2006;27:936‐941. [DOI] [PubMed] [Google Scholar]
- 7. Hamatani Y, Iguchi M, Ueno K, Aono Y, Esato M, Tsuji H, et al. Prognostic significance of natriuretic peptide levels in atrial fibrillation without heart failure. Heart 2021;107:705‐712. [DOI] [PubMed] [Google Scholar]
- 8. Meta‐analysis Global Group in Chronic Heart Failure (MAGGIC) . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta‐analysis. Eur Heart J 2012;33:1750‐1757. [DOI] [PubMed] [Google Scholar]
- 9. Janwanishstaporn S, Feng S, Teerlink J, Metra M, Cotter G, Davison BA, et al. Relationship between left ventricular ejection fraction and cardiovascular outcomes following hospitalization for heart failure: Insights from the RELAX‐AHF‐2 trial. Eur J Heart Fail 2020;22:726‐738. [DOI] [PubMed] [Google Scholar]
- 10. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895‐e1032. [DOI] [PubMed] [Google Scholar]
- 11. Hamatani Y, Nishi H, Iguchi M, Esato M, Tsuji H, Wada H, et al. Machine learning risk prediction for incident heart failure in patients with atrial fibrillation. JACC Asia 2022;2:706‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akao M, Chun YH, Wada H, Esato M, Hashimoto T, Abe M, et al. Current status of clinical background of patients with atrial fibrillation in a community‐based survey: The Fushimi AF Registry. J Cardiol 2013;61:260‐266. [DOI] [PubMed] [Google Scholar]
- 13. Iguchi M, Tezuka Y, Ogawa H, Hamatani Y, Takagi D, An Y, et al. Incidence and risk factors of stroke or systemic embolism in patients with atrial fibrillation and heart failure—The Fushimi AF Registry. Circ J 2018;82:1327‐1335. [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440‐1463. [DOI] [PubMed] [Google Scholar]
- 15. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599‐3726. [DOI] [PubMed] [Google Scholar]
- 16. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR‐Preserved trial. Circulation 2021;144:1284‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Prognostic impact of left ventricular ejection fraction in patients with severe aortic stenosis. JACC Cardiovasc Interv 2018;11:145‐157. [DOI] [PubMed] [Google Scholar]
- 18. Stassen J, Ewe SH, Butcher SC, Amanullah MR, Hirasawa K, Singh GK, et al. Moderate aortic stenosis: Importance of symptoms and left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging 2022;23:790‐799. [DOI] [PubMed] [Google Scholar]
- 19. Inohara T, Kim S, Pieper K, Blanco RG, Allen LA, Fonarow GC, et al. B‐type natriuretic peptide, disease progression and clinical outcomes in atrial fibrillation. Heart 2019;105:370‐377. [DOI] [PubMed] [Google Scholar]
- 20. JCS Joint Working Group . Guidelines for treatment of acute heart failure (JCS 2011). Circ J 2013;77:2157‐2201. [DOI] [PubMed] [Google Scholar]
- 21. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure—Digest version. Circ J 2019;83:2084‐2184. [DOI] [PubMed] [Google Scholar]
- 22. Lam CSP, Solomon SD. Classification of heart failure according to ejection fraction: JACC review topic of the week. J Am Coll Cardiol 2021;77:3217‐3225. [DOI] [PubMed] [Google Scholar]
- 23. Wehner GJ, Jing L, Haggerty CM, Suever JD, Leader JB, Hartzel DN, et al. Routinely reported ejection fraction and mortality in clinical practice: Where does the nadir of risk lie? Eur Heart J 2020;41:1249‐1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stewart S, Playford D, Scalia GM, Currie P, Celermajer DS, Prior D, et al. Ejection fraction and mortality: A nationwide register‐based cohort study of 499 153 women and men. Eur J Heart Fail 2021;23:406‐416. [DOI] [PubMed] [Google Scholar]
- 25. Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, et al. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail 2017;5:44‐52. [DOI] [PubMed] [Google Scholar]
- 26. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol 2017;70:2476‐2486. [DOI] [PubMed] [Google Scholar]
- 27. Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Lip GY. Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: The Belgrade Atrial Fibrillation Study. Eur J Heart Fail 2013;15:415‐424. [DOI] [PubMed] [Google Scholar]
- 28. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373‐498. [DOI] [PubMed] [Google Scholar]
- 29. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352‐380. [DOI] [PubMed] [Google Scholar]
- 30. Yeboah J, Rodriguez CJ, Qureshi W, Liu S, Carr JJ, Lima JA, et al. Prognosis of low normal left ventricular ejection fraction in an asymptomatic population‐based adult cohort: The Multiethnic Study of Atherosclerosis. J Card Fail 2016;22:763‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Romiti GF, Pastori D, Rivera‐Caravaca JM, Ding WY, Gue YX, Menichelli D, et al. Adherence to the ‘Atrial fibrillation Better Care’ pathway in patients with atrial fibrillation: Impact on clinical outcomes—A systematic review and meta‐analysis of 285,000 patients. Thromb Haemost 2022;122:406‐414. [DOI] [PubMed] [Google Scholar]
- 32. Thavendiranathan P, Popovic ZB, Flamm SD, Dahiya A, Grimm RA, Marwick TH. Improved interobserver variability and accuracy of echocardiographic visual left ventricular ejection fraction assessment through a self‐directed learning program using cardiac magnetic resonance images. J Am Soc Echocardiogr 2013;26:1267‐1273. [DOI] [PubMed] [Google Scholar]
- 33. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013;61:77‐84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics according to LVEF strata in AF patients with pre‐existing HF.
Table S2. Baseline characteristics stratified by the presence of LVEF data among AF patients without pre‐existing HF.
Figure S1. Kaplan–Meier curve for the incidences of HF hospitalization according to LVEF strata among AF patients with pre‐existing HF.
Figure S2. Kaplan–Meier curve for the incidences of HF hospitalization stratified by the presence of LVEF data among AF patients without pre‐existing HF.


