Abstract
Aims
The aim of this study was to determine microvascular function in the acute phase of Takotsubo syndrome (TTS) and to identify inflammatory mediators that could reflect TTS‐induced pathology.
Methods and results
The study included 20 females [median age 65 years; interquarile range (IQR) = 58–70 years] with TTS according to the Mayo diagnostic criteria. During heart catheterization, we determined the index of microvascular resistance (IMR) and drew blood samples almost simultaneously from the aorta and coronary sinus. Cardiac magnetic resonance imaging (MRI) was done in the acute phase. We present descriptive coronary physiology and cardiac MRI data and compare inflammatory biomarkers between samples from the aorta, coronary sinus, and venous samples after 3 months using the Wilcoxon signed‐rank test. For comparison, we also analysed the actual biomarkers in venous blood from 15 healthy female controls. A supplementary analysis explored Spearman's rank correlation between the inflammatory biomarkers, IMR, MRI data, and cardiac biomarkers. The median IMR was 16.5 mmHg·s (IQR = 10.5–28.2 mmHg·s), which was only slightly higher than that in the reference populations. Seven (35%) of the study subjects had IMR > 25 mmHg·s, suggesting a microvascular dysfunction. IMR was not affected by time from symptom onset. According to MRI, the apical region of the left ventricle was affected in 65% of the subjects. The median ejection fraction was 41% (IQR = 31–48%). Biomarker analyses revealed elevation of markers for extracellular matrix remodelling and fibrosis, inflammation, immune activation, and upstream inflammation as compared with healthy controls. Only the levels of interleukin (IL)‐1 receptor antagonist and soluble T‐cell immunoglobulin mucin domain‐3 (sTIM‐3) were higher in the coronary sinus than in the aorta. No variable was significantly correlated with IMR. The IL‐6 level in the aorta was inversely correlated with the left ventricular ejection fraction. Growth differentiation factor‐15, osteoprotegerin, and von Willebrand factor levels in both aorta and coronary sinus were positively correlated with N‐terminal‐pro‐brain‐natriuretic peptide, while the correlations of IL‐6 and sTIM‐3 with N‐terminal‐pro‐brain‐natriuretic peptide were restricted to the aorta and coronary sinus, respectively. While most of the markers were within normal limits after 3 months, matrix metalloproteinase‐9 increased during follow‐up to reach levels higher than those in the healthy controls.
Conclusion
The median IMR was only slightly elevated in this study, but about one‐third of the patients had values indicating microvascular dysfunction. The present study supports the involvement of several inflammatory pathways in TTS, including monocyte/macrophage activation, with sTIM‐3 as a potential novel marker.
Keywords: Index of microvascular resistance (IMR), Inflammatory markers, Takotsubo cardiomyopathy
Background
Acute stress‐induced cardiomyopathy, called Takotsubo syndrome (TTS), is an acute heart failure condition that mimics acute coronary syndrome (ACS). Recent publications suggest that its prognosis is less benign than initially believed, with long‐term outcomes comparable with those of ACS. 1 , 2 TTS is often related to physical and/or emotional triggers 3 , 4 and is characterized by regional hypokinesia/akinesia of the left ventricle (LV) that cannot be attributed to the myocardial territory supplied by a coronary artery. 5 Diagnostic criteria have developed from the Mayo criteria of 2004 6 to the recent interTAK criteria. 5
Excessive sympathetic stimulation is considered central in TTS development, which contributes to microvascular dysfunction. 7 , 8 , 9 , 10 TTS also involves activation of inflammatory pathways with interleukin (IL)‐6 as a central mediator. 11 However, these issues remain unclear, and data on other inflammatory mediators are scarce.
Aims
We hypothesized that TTS is characterized by microcirculatory impairment, in which inflammation may contribute. The main goals of the study were to (i) calculate the index of microvascular resistance (IMR) in patients with TTS and (ii) examine the degree and composition of systemic and myocardial inflammation, as assessed by the gradient of inflammatory biomarkers across the heart, and their relation to IMR and myocardial function.
Methods
Additional methodologic information is presented in the supporting information
Study design
This study enrolled 20 patients during 2012–2016 and was approved by the South‐East Department of the Norwegian Regional Committee for Medical and Health Research Ethics (approval no. 2011/1361) and the review board of the hospital.
Study participants
Patients aged 20–80 years with suspected ACS who were referred for coronary angiography at a tertiary referral centre in Norway for interventional cardiology (Oslo University Hospital, Rikshospitalet) were eligible for inclusion in this study. If the coronary angiogram revealed no obstructive coronary artery disease and/or a fractional flow reserve of >0.80, TTS was diagnosed according to the Mayo criteria 6 : (i) regional hypokinesis/akinesis/dyskinesis of the LV with or without apical involvement extending beyond a single coronary artery, (ii) electrocardiogram abnormalities (ST elevation and/or T‐wave inversion), and (iii) absence of pheochromocytoma and myocarditis.
Informed consent was obtained after the diagnostic heart catheterization. Relevant exclusion criteria were uncontrolled endocrinologic disturbances, significant mental disorders including dementia, and the inability to follow the protocol. All participants had normal levels of meta‐ and normetanephrine. Patients who fulfilled the diagnostic criteria had a second heart catheterization including coronary physiologic measurements within 24–48 h after admission and cardiac magnetic resonance imaging (MRI), and blood sampling.
Magnetic resonance imaging
All subjects underwent MRI with a 1.5‐T clinical scanner (Avanto or Sonata, Siemens Healthineers, Erlangen, Germany) and were administered with 0.2 mmol/kg gadolinium‐based contrast agents (gadoterate meglumine, Dotarem, Guerbet, Paris, France). Cardiac volumes and functions were manually measured using short axis summation with Simpson's rule.
Heart catheterization and coronary physiologic assessments
Left heart catheterization was performed mostly using a transradial approach and a 6‐French (Fr) catheter. Coronary physiologic measurements were performed using a pressure‐ and thermistor‐equipped guide wire (PressureWire Certus, St. Jude Medical, St. Paul, MN), which was preceded by intracoronary administration of 200 μg of glyceryl trinitrate. Hyperaemia was obtained via intravenous adenosine infusion at 140 μg/kg/min in the femoral vein. Coronary physiologic assessments were performed as previously described 12 and as outlined in the supporting information. The coronary sinus was catheterized using a 6‐Fr catheter through a 6‐Fr sheath inserted in the femoral vein.
Biomarkers
On top of routine biochemical work, EDTA plasma was obtained almost simultaneously from the aorta and coronary sinus during the second heart catheterization in order to analyse various inflammatory markers (for details, see supporting information). In addition to IL‐6 that has been associated with TTS, 11 we examined levels of growth‐differentiation factor 15 (GDF‐15), 13 osteoprotegerin (OPG), 14 , 15 galectin‐3 (Gal‐3), 16 and matrix metalloproteinase‐9 (MMP‐9) 17 that all have been related to myocardial and extracellular matrix (ECM) remodelling in heart failure patients. We also included other biomarkers (Table 2 ), reflecting markers of activation of T‐cells, monocytes/macrophages and endothelial cells, and IL‐1 receptor antagonist (IL‐1Ra) as a marker of the IL‐1 system, which all could be involved in TTS pathogenesis.
Table 2.
Comparisons of biomarkers sampled at different locations and time points, and with a control group of 15 age‐ and sex‐matched healthy females. Data are median (interquartile range) values
| Aorta | Coronary sinus | Venous at 3 months | Controls | Aorta vs. coronary sinus (P) | Aorta vs. venous at 3 months (P) | Aorta vs. controls (P) | Venous at 3 months vs. controls (P) | |
|---|---|---|---|---|---|---|---|---|
| n | 15 | 15 | 9 | 15 | 15 pairsa | 7 pairsa | 15 vs. 15b | 9 vs. 15b |
| ECM/fibrosis | ||||||||
| GAL‐3 (ng/mL) | 5.8 (4.2–7) | 5 (4.2–7.4) | 5.8 (5.3–6.6) | 5.8 (4.3–6.9) | 0.69 | 0.043 | 0.63 | 0.33 |
| GDF‐15 (ng/mL) | 2.6 (1.3–4.2) | 2.7 (1–5.7) | 1.7 (1.3–3.2) | 1.1 (0.83–1.9) | 0.23 | 1 | 0.0095 | 0.053 |
| MMP‐9 (ng/mL) | 32 (15–83) | 30 (19–65) | 46 (39–47) | 30 (19–37) | 0.19 | 0.043 | 0.49 | 0.025 |
| Endothelial cell activation | ||||||||
| OPG (ng/mL) | 17 (11–25) | 18 (12–28) | 9.6 (8.8–13) | 9 (8–10) | 0.14 | 0.063 | 0.0002 | 0.30 |
| VCAM‐1 (ng/mL) | 0.55 (0.49–0.62) | 0.59 (0.51–0.66) | 0.72 (0.57–0.77) | 0.66 (0.56–0.8) | 0.78 | 0.028 | 0.11 | 0.53 |
| vWf (AU/mL) | 23 (15–36) | 24 (15–27) | 16 (16–20) | 21 (14–28) | 0.57 | 0.61 | 0.40 | 0.33 |
| Immune activation/leukocytes | ||||||||
| sCD163 (ng/mL) | 416 (328–482) | 469 (336–539) | 582 (411–731) | 547 (483–759) | 0.26 | 0.018 | 0.044 | 0.98 |
| sTIM‐3 (ng/mL) | 4.6 (2.9–8.4) | 5.7 (2.7–11) | 2.3 (1.9–3) | 1.7 (1.5–2.3) | 0.017 | 0.043 | 0.0002 | 0.089 |
| sCD25 (ng/mL) | 0.79 (0.56–1.3) | 0.81 (0.57–1.4) | 0.91 (0.72–1.6) | 0.67 (0.61–0.98) | 0.95 | 0.61 | 0.55 | 0.13 |
| Inflammation | ||||||||
| IL‐6 (pg/mL) | 1.6 (0.72–15) | 2.8 (0.77–16) | 0.65 (0.55–0.85) | 0.51 (0.46–0.60) | 0.36 | 0.091 | 0.0001 | 0.14 |
| IL‐1Ra (pg/mL) | 30 (19–40) | 35 (19–52) | 16 (15–18) | 18 (13–19) | 0.031 | 0.18 | 0.0045 | 0.61 |
Significant P values in bold.
ECM, extracellular matrix; Gal‐3, galectin‐3; GDF‐15, growth differentiation factor‐15; MMP‐9, matrix metalloproteinase‐9; OPG, osteoprotegerin; VCAM‐1, vascular cellular adhesion molecule‐1; vWf, von Willebrand factor; sCD163, soluble CD163; sTIM‐3, soluble T‐cell immunoglobulin mucin domain‐3; sCD25, soluble CD25; IL‐6, interleukin‐6; IL‐1Ra, interleukin‐1 receptor antagonist, AU, arbitrary units.
Wilcoxon signed‐rank test.
Mann–Whitney U test.
Statistical analyses
Descriptive statistics are presented as median and interquartile‐range (IQR) or number (percentage) values. Pairwise comparisons were performed on the aorta samples with coronary sinus and venous samples after 3 months using the Wilcoxon signed‐rank test. Independent samples were compared with those of a control population (n = 15) using the Mann–Whitney U test.
In an exploratory analysis, we determined Spearman's rank correlations between IMR, selected biomarkers and MRI variables. We chose 5% as the significance level in two‐sided tests. Stata software (version 17, StataCorp, College Station, TX) was used for the analyses.
Results
The median age of the subjects was 65 years (IQR = 58–70 years), and all were female. An emotional stress trigger was suspected in eight subjects, a physical in three and no apparent trigger in the remaining participants. TTS was not secondary to a physical illness in any of the included subjects. Peak cardiac troponin T and N‐terminal‐pro‐brain natriuretic peptide (NT‐proBNP) were 404 ng/L and 235 pmol/L, respectively. Table 1 lists the basic characteristics and the findings for coronary physiology, MRI evaluations, and biochemistry.
Table 1.
Characteristics of the study cohort (n = 20). Data are median (interquartile range) or n (%) values
| Age, years | 64.5 (58–70) |
| BMI, kg/m2 | 24 (22–27) |
| Never smoked | 10 (50) |
| Current or former smoker | 10 (50) |
| Diabetes mellitus | 4 (20) |
| Treated arterial hypertension | 6 (30) |
| Relevant cardiac medication | |
| ACEi/ARB | 6 (30) |
| Beta‐blocker | 7 (35) |
| Statin | 6 (30) |
| Aspirin | 6 (30) |
| Biochemistry | |
| Haemoglobin, g/dL | 13.5 (12.8–14.0) |
| Creatinine, μmol/L, | 58 (50–66) |
| HbA1c, % (n = 19) | 5.6 (5.2–5.7) |
| NT‐pro‐BNP, pmol/L (n = 19) | 235 (150–793) |
| Troponin T, ng/L (peak value) | 404 (163–682) |
| C‐reactive protein, mg/L (peak value) | 4.0 (1.3–21.5) |
| CK‐MB, μg/L (peak value) | 12 (5–22) |
| Coronary physiology | |
| Index of myocardial resistance | 16.5 (10.5–28.2) |
| Fractional flow reserve | 0.93 (0.88–0.96) |
| Coronary flow reserve | 2.4 (1.8–4.5) |
| Time from symptom onset to invasive coronary physiology examination, h | 40 (23–56) |
| Cardiac magnetic resonance imaging | |
| End diastolic volume, mL/m2 | 82 (74–91) |
| Stroke volume, mL/m2 | 32 (28–39) |
| Myocardial mass, g/m2 | 64 (56–70) |
| Ejection fraction (%) | 41 (31–48) |
| Affected region | |
| Apical | 13 (65) |
| Midventricular | 3 (15) |
| Multiple regions | 3 (15) |
| Regional | 1 (5) |
BMI, body mass index; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HbA1c, glycated haemoglobin; NT‐pro‐BNP, N‐terminal‐pro‐brain‐natriuretic peptide; CK‐MB, creatine kinase‐myocardial band.
Coronary physiology
The coronary physiology assessment was performed in the left anterior descending artery in 17 of the subjects. Only two had a LV end‐diastolic pressure >16 mmHg. The median IMR was 16.5 mmHg·s (IQR = 10.5–28.2 mmHg·s) with IMR 50–60 mmHg·s in two subjects and 25–40 in five (Table 1 , Figure 1 A ). The median IMR was only slightly higher than that in a reference population with atrioventricular nodal reentrant tachycardia12 and a female population with chest pain and no obstructive coronary artery disease 18 (Figure 1 A ). There was no apparent decrease in IMR with time after symptom onset (Figure 1 B ). Table 1 lists the values for fractional flow reserve, coronary flow reserve, and time from symptom onset to physiologic assessment.
Figure 1.
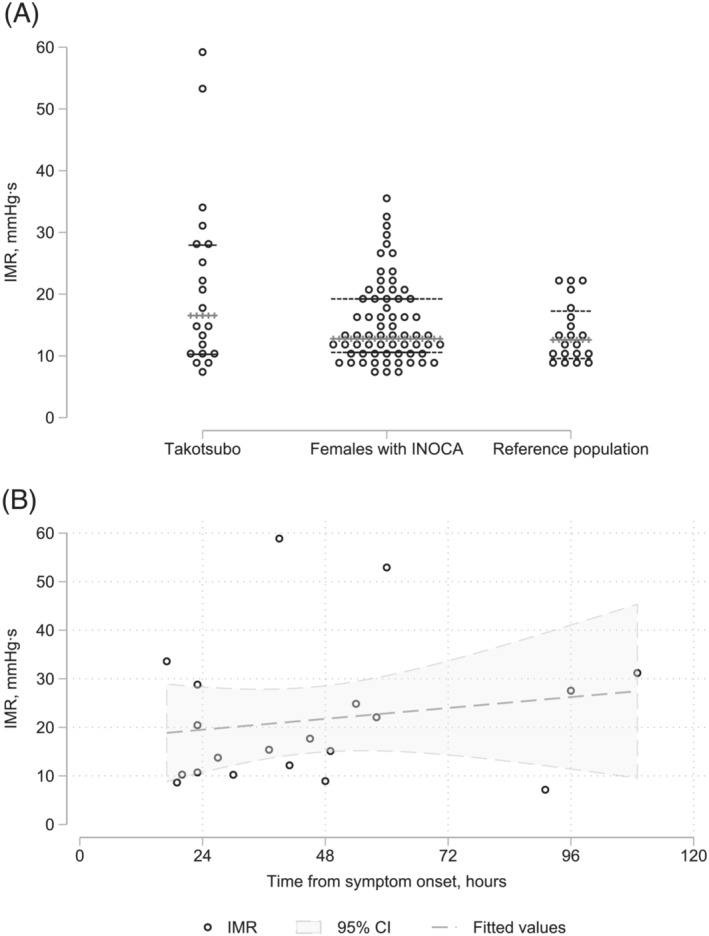
(A) Index of myocardial resistance (IMR) (median and interquartile‐range values) for 20 patients with Takotsubo cardiomyopathy compared with a population of females with ischaemia and no obstructive coronary artery disease (INOCA) 13 and a reference population with supraventricular tachycardia (10 males and 10 females). 12 (B) IMR according to time from symptom onset. Data are fitted values with 95% confidence intervals (CIs) shaded (n = 20).
Magnetic resonance imaging
MRI analyses are shown in Table 1 . The apical region was affected in 65% of the subjects. The median ejection fraction was 41% (IQR = 31–48%).
Biomarkers
Biomarkers from the aorta and coronary sinus and from venous blood (only available from 3 months follow‐up) were compared with venous blood samples from 15 female healthy controls with median age 66 years (IQR 64–68 years) (Table 2 ). Several significant findings were revealed. First, GDF‐15 and OPG reflecting ECM remodelling and fibrogenesis, and soluble T‐cell immunoglobulin mucin domain‐3 (sTIM‐3), IL‐6 and IL‐1‐Ra that reflect inflammation and immune activation, were significantly increased whereas soluble CD163 (sCD163), thought to reflect a pro‐resolving macrophage phenotype, was significantly decreased in aorta from TTS patients as compared with venous blood in healthy controls. Of these, sTIM‐3 and IL‐1Ra were significantly increased in the coronary sinus compared with in the aorta. Second, while none of the variables were significantly correlated with IMR, IL‐6 levels in the aorta were inversely correlated with LV ejection fraction (Table S1 ). GDF‐15, OPG, and von Willebrand factor levels in both the aorta and coronary sinus were positively correlated with NT‐proBNP, whereas correlations of IL‐6 and sTIM‐3 with NT‐proBNP were restricted to the aorta and coronary sinus, respectively. Finally, while most of the markers where within their normal limits after 3 months, MMP‐9 increased during follow‐up to reach levels higher than those in the healthy controls.
Discussion
Whereas median IMR in this study was below the normal threshold (25 mmHg·s); IMR of 35% of the subjects were consistent with microvascular dysfunction. This contrasted with no IMR values exceeding 25 mmHg·s in a healthy reference population (only with atrioventricular nodal reentrant tachycardia). 12 Several previous case studies found extremely high IMR values in TTS, 19 , 20 including values >100 mmHg·s. This apparent discrepancy may be due to coronary physiologic assessments in the present study protocol requiring patients to lay supine for 30–60 min during the cardiac MRI, thereby possibly excluding patients with most severe heart failure. Rivero et al. found that IMR declined over time. 21 That study, 21 however, performed no serial individual assessments, as in the present study, and most investigations were performed <25 h after symptom onset, as compared with a median time of 40 h in the present study.
The present study confirms previous reports of increased IL‐6 levels associated with impaired cardiac function in TTS. 11 However, we also report that in addition to IL‐1‐Ra, sTIM‐3 had higher levels in the coronary sinus than in the aorta, suggesting intracardiac release. sTIM‐3 is thought to reflect T‐cell exhaustion, but also monocyte/macrophage activation. 22 The lack of increased levels of sCD25, a reliable marker of T‐cell activation, indicated that the increased sTIM‐3 levels in these TTS‐patients may reflect enhanced monocyte/activation. Indeed, sTIM‐3 promotes an inflammatory phenotype in monocytes/macrophages that includes the induction of IL‐6. 23 Infiltration of this cell type into the myocardium has recently been found in TTS. 24 Our finding of decreased sCD163, a potential marker of a pro‐resolving monocyte/macrophage phenotype, in TTS patients further supports an inflammatory monocyte/macrophage phenotype in TTS. There have been few reports of elevated GDF‐15 levels in TTS 25 and we found that this was accompanied by another marker of ECM remodelling, OPG. Both markers are associated with myocardial remodelling 14 and provide prognostic information for patients with myocardial infarction. 13 , 15 The present study suggests that these markers could also be relevant for TTS. 26
Limitations
The relatively small number of subjects included and the single centre design constitute major limitations to the generalization of the findings. The study protocol required cardiac MRI of all participants, which may have introduced an inclusion bias in which the most symptomatic partients were excluded. This may explain that the IMR value was abnormal in only 35% of the subjects. The 40‐h delay from symptom debut until invasive assessment makes the time‐dependant development of IMR hard to evaluate.
The selection of biomarkers was based on previous studies in relation to myocardial remodelling, but several other biomarkers could have been chosen, like for instance IL‐9. 27 For technical reasons, we have not performed MRI mapping of the left ventricle.
Conclusions
The IMR value in the current study was above the usual threshold of 25 mmHg·s in 35% of the patients. The IMR showed little association with the analysed biomarkers in the acute phase of TTS. Notwithstanding the present study has yielded further data to support the involvement of several inflammatory pathways in TTS, including monocyte/macrophage activation, with sTIM‐3 as a potential novel marker. However, these data will have to be confirmed in larger study populations including several centres.
Conflict of interest
None declared.
Supporting information
Data S1. Supporting Information.
Table S1. Associations of biomarkers with index of myocardial resistance, cardiac biomarkers and magnetic resonance imaging variables. Values are Spearman's rank correlation coefficients.
Solberg, O. G. , Aaberge, L. , Bosse, G. , Ueland, T. , Gullestad, L. , Aukrust, P. , and Stavem, K. (2023) Microvascular function and inflammatory activation in Takotsubo cardiomyopathy. ESC Heart Failure, 10: 3216–3222. 10.1002/ehf2.14461.
References
- 1. Uribarri A, Núñez‐ Gil IJ, Conty DA, Vedia O, Almendro‐ Delia M, Durán Cambra A, Martin‐ Garcia AC, Barrionuevo‐ Sánchez M, Martínez‐ Sellés M, Raposeiras‐Roubín S. Short‐ and long‐ term prognosis of patients with Takotsubo syndrome based on different triggers: importance of the physical nature. JAMA. 2019; 8: e013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D, Candreva A, Ding KJ, Micek J, Szawan KA. Long‐term prognosis of patients with Takotsubo syndrome. J Am Coll Cardiol. 2018; 72: 874–882. [DOI] [PubMed] [Google Scholar]
- 3. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015; 373: 929–938. [DOI] [PubMed] [Google Scholar]
- 4. Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ. Natural history and expansive clinical profile of stress (Takotsubo) cardiomyopathy. J Am Coll Cardiol. 2010; 55: 333–341. [DOI] [PubMed] [Google Scholar]
- 5. Ghadri J‐R, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W. International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018; 39: 2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST‐segment elevation myocardial infarction. Ann Intern Med. 2004; 141: 858–865. [DOI] [PubMed] [Google Scholar]
- 7. Rigo F, Sicari R, Citro R, Ossena G, Buja P, Picano E. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: a Doppler transthoracic echo study. Ann Med. 2009; 41: 462–470. [DOI] [PubMed] [Google Scholar]
- 8. Yalta K, Yalta T. Physically triggered Takotsubo cardiomyopathy has a worse prognosis: potential roles of systemic inflammation and coronary slow flow phenomenon. Int J Cardiol. 2017; 242: 31–32. [DOI] [PubMed] [Google Scholar]
- 9. Cuisset T, Quilici J, Pankert M, Poyet R, Lambert M, Bonnet J‐L. Usefulness of index of microcirculatory resistance to detect microvascular dysfunction as a potential mechanism of stress‐induced cardiomyopathy (Tako‐tsubo syndrome). Int J Cardiol. 2011; 153: e51–e53. [DOI] [PubMed] [Google Scholar]
- 10. Kume T, Akasaka T, Kawamoto T, Yoshitani H. Assessment of coronary microcirculation in patients with Takotsubo‐like left ventricular dysfunction. Circ J. 2005; 69: 934–939. [DOI] [PubMed] [Google Scholar]
- 11. Scally C, Abbas H, Ahearn T, Srinivasan J. Myocardial and systemic inflammation in acute stress‐induced (Takotsubo) cardiomyopathy. Circulation. 2019; 139: 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solberg OG, Ragnarsson A, Kvarsnes A, Endresen K, Kongsgård E, Aakhus S, Gullestad L, Stavem K, Aaberge L. Reference interval for the index of coronary microvascular resistance. EuroIntervention. 2014; 9: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 13. Hagström E, James SK, Bertilsson M, Becker RC, Himmelmann A, Husted S, Katus HA, Steg PG, Storey RF, Siegbahn A, Wallentin L, PLATO Investigators . Growth differentiation factor‐15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. Eur Heart J. 2016; 37: 1325–1333. [DOI] [PubMed] [Google Scholar]
- 14. Erkol A, Oduncu V, Pala S, Kızılırmak F, Kılıcgedik A, Yılmaz F, Güler A, Karabay CY, Kırma C. Plasma osteoprotegerin level on admission is associated with no‐reflow phenomenon after primary angioplasty and subsequent left ventricular remodeling in patients with acute ST‐segment elevation myocardial infarction. Atherosclerosis. 2012; 221: 254–259. [DOI] [PubMed] [Google Scholar]
- 15. Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, Squire IB, Gullestad L, Bollerslev J, Dickstein K, Aukrust P. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004; 44: 1970–1976. [DOI] [PubMed] [Google Scholar]
- 16. Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, Adourian A, Bohm M, van Veldhuisen DJ, Komajda M, Cleland JGF, Wikstrand J, McMurray JJV, Aukrust P, on behalf of the CORONA Study Group . Galectin‐3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur Heart J. 2012; 33: 2290–2296. [DOI] [PubMed] [Google Scholar]
- 17. Cohen L, Sagi I, Bigelman E, Solomonov I, Aloshin A, Ben‐Shoshan J, Rozenbaum Z, Keren G, Entin‐Meer M. Cardiac remodeling secondary to chronic volume overload is attenuated by a novel MMP9/2 blocking antibody. PLoS ONE. 2020; 15: e0231202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solberg OG, Stavem K, Ragnarsson A, Beitnes JO, Skårdal R, Seljeflot I, Ueland T, Aukrust P, Gullestad L, Aaberge L. Index of microvascular resistance to assess the effect of rosuvastatin on microvascular function in women with chest pain and no obstructive coronary artery disease: a double‐blind randomized study. Catheter Cardiovasc Interv. 2019; 94: 660–668. [DOI] [PubMed] [Google Scholar]
- 19. Daniels DV, Fearon WF. The index of microcirculatory resistance (IMR) in takotsubo cardiomyopathy. Catheter Cardiovasc Interv. 2011; 77: 128–131. [DOI] [PubMed] [Google Scholar]
- 20. Morrow AJ, Nordin S, O'Boyle P, Berry C. ‘Acute micro‐coronary syndrome’: detailed coronary physiology in a patient with Takotsubo cardiomyopathy. BMJ Case Rep. 2019; 12: e229618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rivero F, Cuesta J, García‐Guimaraes M, Bastante T, Alvarado T, Antuña P, Alfonso F. Time‐related microcirculatory dysfunction in patients with Takotsubo cardiomyopathy. JAMA Cardiol. 2017; 2: 699–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ocaña‐Guzman R, Torre‐Bouscoulet L, Sada‐Ovalle I. TIM‐3 regulates distinct functions in macrophages. Front Immunol. 2016; 7: 22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang J, Li C, Fu J, Wang X, Feng X, Pan X. Tim‐3 regulates inflammatory cytokine expression and Th17 cell response induced by monocytes from patients with chronic hepatitis B. Scand J Immunol. 2019; 89: e12755. [DOI] [PubMed] [Google Scholar]
- 24. Wilson HM, Cheyne L, Brown PAJ, Kerr K, Hannah A, Srinivasan J, Duniak N, Horgan G, Dawson DK. Characterization of the myocardial inflammatory response in acute stress‐induced (Takotsubo) cardiomyopathy. JACC Basic Transl Sci. 2018; 3: 766–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stiermaier T, Adams V, Just M, Blazek S, Desch S, Schuler G, Thiele H, Eitel I. Growth differentiation factor‐15 in Takotsubo cardiomyopathy: diagnostic and prognostic value. Int J Cardiol. 2014; 173: 424–429. [DOI] [PubMed] [Google Scholar]
- 26. Warisawa T, Naganuma T, Nakamura S. Reversible microvascular dysfunction in Takotsubo syndrome shown using index of microcirculatory resistance. Circ J. 2016; 80: 750–752. [DOI] [PubMed] [Google Scholar]
- 27. Marra AM, Arcopinto M, Salzano A, Bobbio E, Milano S, Misiano G, Ferrara F, Vriz O, Napoli R, Triggiani V, Perrone‐Filardi P, Saccà F, Giallauria F, Isidori AM, Vigorito C, Bossone E, Cittadini A. Detectable interleukin‐9 plasma levels are associated with impaired cardiopulmonary functional capacity and all‐cause mortality in patients with chronic heart failure. Int J Cardiol. 2016; 15: 114–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Table S1. Associations of biomarkers with index of myocardial resistance, cardiac biomarkers and magnetic resonance imaging variables. Values are Spearman's rank correlation coefficients.


