Abstract
Aims
Commercially available integrated software for echocardiographic measurement of stroke work (SW) is increasingly used for the right ventricle, despite a lack of validation. We sought to assess the validity of this method [echo‐based myocardial work (MW) module] vs. gold‐standard invasive right ventricular (RV) pressure–volume (PV) loops.
Methods and results
From the prospectively recruiting EXERTION study (NCT04663217), we included 42 patients [34 patients with pulmonary arterial hypertension (PAH) or chronic thromboembolic pulmonary hypertension (CTEPH) and 8 patients with absence of cardiopulmonary disease] with RV echocardiography and invasive PV catheterization. Echocardiographic SW was assessed as RV global work index (RVGWI) generated via the integrated pressure–strain MW software. Invasive SW was calculated as the area bounded by the PV loop. An additional parameter derived from the MW module, RV global wasted work (RVGWW), was correlated with PV loop measures. RVGWI significantly correlated with invasive PV loop‐derived RV SW in the overall cohort [rho = 0.546 (P < 0.001)] and the PAH/CTEPH subgroup [rho = 0.568 (P < 0.001)]. Overall, RVGWW correlated with invasive measures of arterial elastance (Ea), the ratio of end‐systolic elastance (Ees)/Ea, and end‐diastolic elastance (Eed) significantly.
Conclusions
Integrated echo measurement of pressure–strain loop‐derived SW correlates with PV loop‐based assessment of RV SW. Wasted work correlates with invasive measures of load‐independent RV function. Given the methodological and anatomical challenges of RV work assessment, evolution of this approach by incorporating more elaborated echo analysis data and an RV reference curve might improve its reliability to mirror invasively assessed RV SW.
Keywords: Right ventricular myocardial work, Echocardiography, Stroke work, Pressure–volume curve, Pulmonary hypertension
Background
Right ventricular (RV) function is significantly associated with prognosis and symptoms in pulmonary hypertension (PH) 1 and in various cardio‐pulmonary diseases. 2 One of the major parameters derived from invasive catheterization is RV stroke work (SW), which represents the active cardiac work in each contraction. 3 Different invasive and non‐invasive surrogates have been previously investigated for the calculation of RV SW, 4 , 5 but without validation by gold‐standard pressure–volume (PV) loops from conductance catheterization. In direct comparison with PV loops, pressure–strain loops may provide an alternative method for the non‐invasive assessment of SW, as suggested by Russell et al. in 2012. 6 A semi‐automated myocardial work (MW) module was recently introduced, which allows bed‐side ready analysis and might pave the way for MW to become a diagnostic tool for clinical routine across various diseases. 7 , 8 The module has already been applied to the right ventricle, 9 , 10 but RV validation data are lacking.
Aims
The aim of our current study is to validate the semi‐automated MW module against RV conductance catheterization, which is the invasive gold standard to assess RV SW and RV–pulmonary arterial (PA) coupling.
Methods
Study design and patients
We prospectively analysed consecutive patients enrolled into the EXERTION study (ClinicalTrials.gov identifier: NCT04663217; ethics committee approval number 117/16). Diagnosis of pulmonary arterial hypertension (PAH) and chronic thromboembolic PH (CTEPH) was made by a multidisciplinary board, according to current guidelines. 11 , 12 Control patients were initially referred for suspicion of PH and dyspnoea on exertion. Post hoc, patients' PH diagnosis was updated to the current guidelines. 12 Patients with left heart disease or chronic thromboembolic disease without PH or atrial fibrillation/flutter at the time of evaluation were excluded. MW analysis was not feasible in 17 patients [e.g. atrial flutter/fibrillation at time of evaluation, missing tricuspid regurgitation (TR) signal, and region of interest (ROI) implausible]. Thirteen patients with left heart disease and three patients with chronic thromboembolic disease without PH were excluded.
Integrated myocardial work module
We applied the MW module (Automated Function Imaging, EchoPAC 204) to the right ventricle (Figure 1 A ), as described previously. 10 Pulmonary valve event timings were determined by pulsed wave Doppler. Tricuspid valve event timings were assessed from direct visualization in the right ventricle‐focused four‐chamber view. To adapt to the right ventricle, we traced the right ventricle‐focused view for all chamber views requested by the software (i.e. the same RV view of the same cycle was traced three times). The ROI was manually aligned to the RV structures by the operators. We used systolic pulmonary artery pressure (PASP) estimated from the TR jet peak velocity [without adding estimated mean right atrial (RA) pressure], as recommended. 13 We entered diastolic pulmonary artery pressure (DPAP) calculated as 1.5 × [mean pulmonary artery pressure (PAMP) − (PASP/3)]. 9 Echocardiographic PAMP was obtained by the following formula: mean RV–RA gradient to mean RA pressure. The mean RV–RA gradient was calculated by tracing the TR velocity–time integral. 9 , 10 The derived MW parameters were RV global work index (RVGWI) and RV global wasted work (RVGWW).
Figure 1.
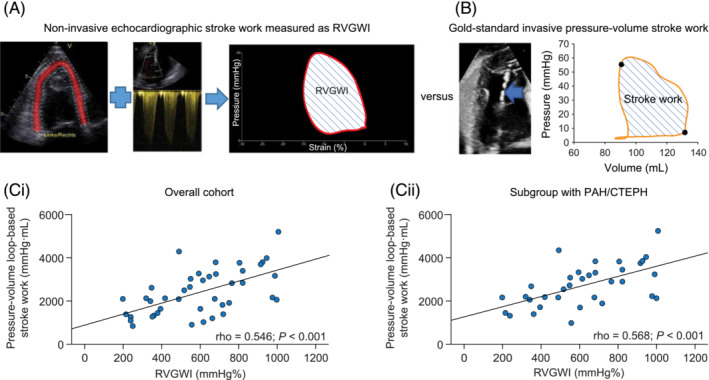
Measurement of right ventricular (RV) stroke work. (A) Echocardiographic RV pressure–strain loop built by the Automated Function Imaging software myocardial work module (Echo PAC Version 204, GE Vingmed Ultrasound). Based on non‐invasive pulmonary artery systolic pressure, longitudinal strain, and the timings of opening and closure of the pulmonary and tricuspid valves, the software generates the RV global work index (RVGWI), representative of stroke work. (B) Invasively measured pressure–volume loop obtained via conductance catheterization (blue arrow). The area bounded by the pressure–volume loop represents RV stroke work. (C) Correlation of echocardiographic RVGWI with invasive pressure–volume loop‐derived RV stroke work (i) in the overall cohort (n = 42) and (ii) in the subgroup with pulmonary arterial hypertension (PAH)/chronic thromboembolic pulmonary hypertension (CTEPH) (n = 34).
Conductance catheterization
Guided by echocardiography, a 4F PV catheter (CA‐Nr 41063, CD Leycom, Zoetermeer, the Netherlands) was positioned in the RV apex for real‐time acquisition of PV loops (Figure 1 B ). End‐systolic elastance (Ees) was calculated as end‐systolic pressure (ESP)/[end‐systolic volume − volume at zero pressure (V0)]. 14 Arterial elastance (Ea) was calculated as ESP/stroke volume. The ratio of Ees/Ea defines RV–PA coupling. 15 SW was calculated as the area bounded by the PV loop. End‐diastolic elastance (Eed) was calculated from a curvilinear adjustment of end‐diastolic pressure/volume ratios, as described previously. 16
Statistical analyses
Adherence to a Gaussian distribution was determined by visual assessment of histograms. Data are expressed as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Differences between groups were analysed with the Pearson χ2 test, the independent‐samples Mann–Whitney U test, or the independent‐samples t‐test, as appropriate. Correlations between variables were determined by Spearman's rho. Intra‐observer and inter‐observer variability was assessed using intra‐class correlation coefficients (ICCs) and 95% confidence intervals (CIs) in 10 random patients. For all analyses, P < 0.05 was considered statistically significant. SPSS Version 28.0 (IBM, Armonk, NY, USA) was used.
Results
Overall, 42 patients were included. Patient characteristics and echocardiographic and invasive PV loop measurements are presented in Table 1 . Eight patients were controls. RVGWI (derived from the MW module) showed significant correlations with invasively measured conductance SW in the overall cohort and in the PAH/CTEPH subgroup (Figure 1 Ci,ii ). As illustrated in Figure 2 , RVGWW and conductance catheter‐derived indices of afterload (Ea), RV–PA coupling, and diastolic stiffness (Eed) were significantly correlated in the overall cohort and within the PAH/CTEPH subgroup. ICC showed acceptable intra‐observer and inter‐observer agreement of RVGWI [inter‐observer, 0.978 (0.910–0.994); intra‐observer, 0.990 (0.961–0.998)] and RVGWW [inter‐observer, 0.964 (0.856–0.991); intra‐observer, 0.916 (0.664–0.979) (95% CIs)].
Table 1.
Patient characteristics, right ventricular myocardial work, and invasive pressure–volume loop measurements
| PAH/CTEPH | Control | P value | |
|---|---|---|---|
| Patients, n | 34 | 8 | |
| Male/female, n/n | 10/24 | 1/8 | 0.328 |
| Age, years | 66 [53–73] | 51 [37–62] | 0.044 |
| BMI, kg/m2 | 29 ± 7 | 28 ± 6 | 0.576 |
| Diagnosis, n (%) | |||
| PAH, Group I | 17 | ||
| CTEPH | 17 | ||
| Right heart catheterization | |||
| Mean pulmonary artery pressure, mmHg | 38 [28–50] | 17 [12–19] | <0.001 |
| Pulmonary vascular resistance, Wood units | 5.7 [3.4–8.1] | 1.1 [0.8–1.4] | <0.001 |
| Cardiac index, L/min/m2 | 2.6 ± 0.6 | 3.4 ± 0.8 | 0.003 |
| Pulmonary artery wedge pressure, mmHg | 9 ± 4 | 9 ± 3 | 0.635 |
| Echocardiography | |||
| RVGWI, mmHg% | 607 [412–820] | 498 [276–705] | 0.289 |
| RVGWW, mmHg% | 60 [30–115] | 24 [15–34] | 0.006 |
| RVGLS, % | −16 ± 5 a | −21 ± 3 | 0.010 |
| PASP, mmHg | 52 ± 19 | 28 ± 12 | 0.002 |
| DPAP, mmHg | 26 [19–31] | 15 [13–21] | 0.002 |
| Pressure–volume loop measurements | |||
| Ea, mmHg/mL | 0.65 [0.45–0.93] | 0.24 [0.16–0.26] | <0.001 |
| Ees, mmHg/mL | 0.73 [0.55–0.98] | 0.38 [0.26–0.62] | 0.003 |
| Ees/Ea ratio | 1.03 [0.90–1.44] | 1.63 [1.26–2.10] | 0.002 |
| Eed, mmHg/mL | 0.18 [0.14–0.27] | 0.14 [0.06–0.20] | 0.075 |
| SW, mmHg/mL | 2630 [2046–3292] | 1240 [1044–1428] | <0.001 |
BMI, body mass index; CTEPH, chronic thromboembolic pulmonary hypertension; DPAP, pulmonary artery diastolic pressure; Ea, arterial elastance; Eed, end‐diastolic elastance; Ees, end‐systolic elastance; PAH, pulmonary arterial hypertension; PASP, pulmonary artery systolic pressure; RVGLS, right ventricular global longitudinal strain; RVGWI, right ventricular global work index; RVGWW, right ventricular global wasted work; SW, stroke work.
Values represent mean ± standard deviation or median [interquartile range], unless otherwise specified.
34.
Figure 2.
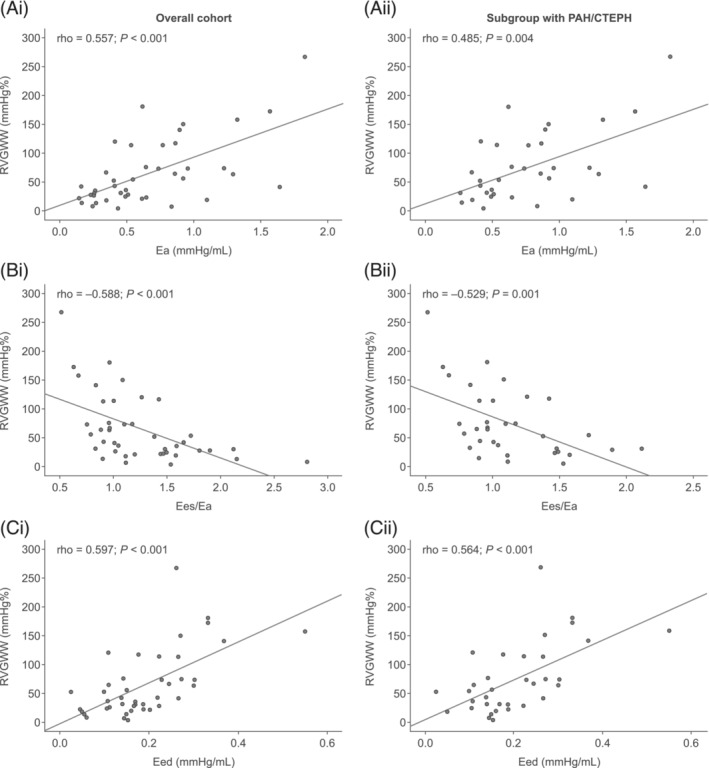
Correlation of right ventricular global wasted work (RVGWW) with invasive conductance catheter parameters (A) arterial elastance (Ea), (B) end‐systolic elastance (Ees)/Ea, and (C) end‐diastolic elastance (Eed) in (i) the overall cohort (n = 42) and (ii) the subgroup with pulmonary arterial hypertension (PAH)/chronic thromboembolic pulmonary hypertension (CTEPH) (n = 34). Correlations were assessed using Spearman's rho coefficient.
Conclusions
Our study aimed to validate echocardiography‐based SW analysis against gold‐standard RV PV loop‐based (invasive) SW and RV–PA coupling (i.e. RV PV loops). Our study shows that RVGWI derived from the left ventricular (LV)‐based semi‐automated pressure–strain software is associated with invasive RV SW in a mixed population of PAH/CTEPH patients and controls, as well as in the solely PAH/CTEPH subgroup. This can be considered a major step forward, because until now, various surrogates and simplifications for the calculation of RV SW have co‐existed and were solely based on LV reference. 17 In general, SW is considered an index of the work performed by the myocardium 18 and is calculated as the product of volume and pressure. 19
As the left ventricle‐based semi‐automatic integrated MW module relies solely on non‐invasive measurements, this method offers for bed‐side assessments. However, several pitfalls remain when this approach is transferred to the right ventricle. First, although we found a significant correlation between RVGWI and invasive RV SW, the correlation is moderate. This is the consequence of the integrated MW module being built on an LV pressure reference curve with different pressure properties. However, as mean pulmonary artery pressure increases from normal values to the level seen in severe PH, the shape of the RV PV loop changes markedly 20 and ESP becomes closer to systolic RV pressure. Thus, the left ventricle‐based software might not be appropriate for patients with mild PH or without PH. Second, the current standard acquisition of the right ventricle is solely focused on the four‐chamber view, the tracing includes repeated measures, and the region of RV septal wall might be influenced by LV function. The moderate correlation between invasive and echocardiographic RV SW in the overall cohort may therefore be partly attributed to the anatomic RV properties and heterogeneous remodelling. Third, diastolic properties are exclusively based on valvular event timing in the integrated MW module (and not adjusting for the diastolic pressure entered), whereas the invasive PV loop area calculation includes an exact determination of diastolic and end‐diastolic pressures. This also limits the degree of correlation between invasive and echocardiographic measurements. Moreover, given the various limitations of the transfer of the application from the LV to the RV. It is mandatory to develop a specific RV MW software, adjusting for the anatomy of the RV, integrating 4D volumes or strain, and taking into account the specific shape of the reference pressure curve.
Interestingly, we found correlations of wasted work (RVGWW) with load‐independent RV parameters including RV–PA coupling. RVGWW as defined by the MW software is equal to the work contributing to myocardial lengthening during systole and shortening during isovolumic relaxation. In general, wasted work is defined as an index of dyssynchronous ventricular contractions. 7 RV dyssynchrony, as assessed by the SD of time to peak strains, was described by Badagliacca and co‐workers as a clinically relevant pathological feature. 21 , 22 In turn, RV dyssynchrony directly relates to wall stress, increased afterload, and diastolic dysfunction and mirrors RV maladaptation in chronic pressure and volume overload. 22 Therefore, the association of higher RVGWW with RV–PA uncoupling mirrors progressive RV dyssynchrony, which is associated with the increase of pulmonary pressures and the impairment of RV global strain 23 in the state of uncoupling. This is of clinical relevance, as an increase in RVGWW implies that work is being done by the ventricle but does not contribute to ejection. Interestingly, there is evidence of improvement of RVGWW after intervention, thus signalling reversibility of this abnormality. 24
The associations found in our study, and further evolution of the echo‐based approach as discussed above, might pave the way for longitudinal non‐invasive assessment of RV contractile function. Of note, Hulshof and co‐workers showed with a non‐automatic approach that RV strain–area loops are of prognostic relevance. 25 Moreover, Butcher and co‐workers were able to demonstrate the prognostic relevance of RVGWI as a measure of SW with association to pulmonary vascular resistance, further emphasizing the clinical value of this approach. 10
This is the first study to validate the integrated echocardiographic measurement of pressure–strain loop‐derived MW for the RV against the invasive gold standard for the evaluation of RV function.
Conflict of interest
The authors have filed a European patent application (EP 20190939.7) for the echocardiography‐derived method for quantification of right ventricular pressure–volume loops. Dr Richter has received support from Bayer; speaker fees from Bayer, Janssen‐Cilag GmbH, and OMT; and consultancy fees from Bayer and Janssen‐Cilag GmbH. Dr Douschan reports personal fees and non‐financial support from Actelion, non‐financial support from Astra Zeneca, non‐financial support from Bayer, non‐financial support from GSK, personal fees and non‐financial support from MSD, non‐financial support from Novartis, non‐financial support from Teva, non‐financial support from Boehringer Ingelheim, non‐financial support from Vifor, and non‐financial support from Menarini outside the submitted work. Dr Ghofrani has received consultancy fees from Bayer, Actelion, Pfizer, Merck, GSK, and Novartis; fees for participation in advisory boards from Bayer, Pfizer, GSK, Actelion, and Takeda; lecture fees from Bayer HealthCare, GSK, Actelion, and Encysive/Pfizer; industry‐sponsored grants from Bayer HealthCare, Aires, Encysive/Pfizer, and Novartis; and sponsored grants from the German Research Foundation, Excellence Cluster Cardiopulmonary Research, and the German Ministry for Education and Research. Dr Seeger has received speaker/consultancy fees from Abivax, Bayer AG, Liquidia Technologies, Pieris Pharmaceuticals, United Therapeutics, and Vectura. Dr Gall has received fees from Actelion, AstraZeneca, Bayer, BMS, GSK, Janssen‐Cilag, Lilly, MSD, Novartis, OMT, Pfizer, and United Therapeutics. Dr Tello has received speaking fees from Actelion and Bayer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding
This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 268555672 – SFB 1213, Project B08. Dr Richter received funding from the JLU‐CAREER programme (German Research Foundation, DFG, 413584448). Dr Douschan was funded by the European Respiratory Society – ERS Clinical Training and Research Fellowship (CTF202004‐00806).
Acknowledgements
For the manuscript, editorial assistance was provided by Claire Mulligan, PhD (Beacon Medical Communications Ltd, Brighton, UK), funded by the University of Giessen.
Open Access funding enabled and organized by Projekt DEAL.
Richter, M. J. , Douschan, P. , Fortuni, F. , Gall, H. , Ghofrani, H. A. , Keranov, S. , Kremer, N. , Kriechbaum, S. D. , Rako, Z. A. , Rieth, A. J. , da Rocha, B. B. , Seeger, W. , Zedler, D. , Yildiz, S. , Yogeswaran, A. , and Tello, K. (2023) Echocardiographic pressure–strain loop‐derived stroke work of the right ventricle: validation against the gold standard. ESC Heart Failure, 10: 3209–3215. 10.1002/ehf2.14453.
Manuel J. Richter and Philipp Douschan contributed equally to this work.
References
- 1. Naeije R, Richter MJ, Rubin LJ. The physiological basis of pulmonary arterial hypertension. Eur Respir J. 2022; 59: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guazzi M, Naeije R. Right heart phenotype in heart failure with preserved ejection fraction. Circ Heart Fail. 2021; 14: e007840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. di Maria MV, Burkett DA, Younoszai AK, Landeck BF II, Mertens L, Ivy DD, Friedberg MK, Hunter KS. Echocardiographic estimation of right ventricular stroke work in children with pulmonary arterial hypertension: comparison with invasive measurements. J Am Soc Echocardiogr. 2015; 28: 1350–1357. [DOI] [PubMed] [Google Scholar]
- 4. Clapham KR, Highland KB, Rao Y, Fares WH. Reduced RVSWI is associated with increased mortality in connective tissue disease associated pulmonary arterial hypertension. Front Cardiovasc Med. 2020; 7: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stevens GR, Garcia‐Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012; 5: 378–387. [DOI] [PubMed] [Google Scholar]
- 6. Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, Haugaa KH, Opdahl A, Fjeld JG, Gjesdal O, Edvardsen T, Smiseth OA. A novel clinical method for quantification of regional left ventricular pressure‐strain loop area: a non‐invasive index of myocardial work. Eur Heart J. 2012; 33: 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roemer S, Jaglan A, Santos D, Umland M, Jain R, Tajik AJ, Khandheria BK. The utility of myocardial work in clinical practice. J Am Soc Echocardiogr. 2021; 34: 807–818. [DOI] [PubMed] [Google Scholar]
- 8. Sade LE, Colak A, Duzgun SA, Hazırolan T, Sezgin A, Donal E, Butcher SC, Özdemir H, Pirat B, Eroglu S, Muderrisoglu H. Approach to optimal assessment of right ventricular remodelling in heart transplant recipients: insights from myocardial work index, T1 mapping, and endomyocardial biopsy. Eur Heart J Cardiovasc Imaging. 2023; 24: 354–363. [DOI] [PubMed] [Google Scholar]
- 9. Butcher SC, Fortuni F, Montero‐Cabezas JM, Abou R, el Mahdiui M, van der Bijl P, van der Velde ET, Ajmone Marsan N, Bax JJ, Delgado V. Right ventricular myocardial work: proof‐of‐concept for non‐invasive assessment of right ventricular function. Eur Heart J Cardiovasc Imaging. 2021; 22: 142–152. [DOI] [PubMed] [Google Scholar]
- 10. Butcher SC, Feloukidis C, Kamperidis V, Yedidya I, Stassen J, Fortuni F, Vrana E, Mouratoglou SA, Boutou A, Giannakoulas G, Playford D, Ajmone Marsan N, Bax JJ, Delgado V. Right ventricular myocardial work characterization in patients with pulmonary hypertension and relation to invasive hemodynamic parameters and outcomes. Am J Cardiol. 2022; 177: 151–161. [DOI] [PubMed] [Google Scholar]
- 11. Delcroix M, Torbicki A, Gopalan D, Sitbon O, Klok FA, Lang I, Jenkins D, Kim NH, Humbert M, Jais X, Vonk Noordegraaf A, Pepke‐Zaba J, Brénot P, Dorfmuller P, Fadel E, Ghofrani HA, Hoeper MM, Jansa P, Madani M, Matsubara H, Ogo T, Grünig E, D'Armini A, Galie N, Meyer B, Corkery P, Meszaros G, Mayer E, Simonneau G. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2020; 57. [DOI] [PubMed] [Google Scholar]
- 12. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, ESC/ERS Scientific Document Group . ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022; 2023: 61. [DOI] [PubMed] [Google Scholar]
- 13. Smiseth OA, Aalen JM. Right ventricular work: a step forward for non‐invasive assessment of right ventricular function. Eur Heart J Cardiovasc Imaging. 2021; 22: 153–154. [DOI] [PubMed] [Google Scholar]
- 14. Richter MJ, Yogeswaran A, Husain‐Syed F, Vadász I, Rako Z, Mohajerani E, Ghofrani HA, Naeije R, Seeger W, Herberg U, Rieth A, Tedford RJ, Grimminger F, Gall H, Tello K. A novel non‐invasive and echocardiography‐derived method for quantification of right ventricular pressure‐volume loops. Eur Heart J Cardiovasc Imaging. 2021; 23: 498–507. [DOI] [PubMed] [Google Scholar]
- 15. Nakaya T, Ohira H, Sato T, Watanabe T, Nishimura M, Oyama‐Manabe N, Kato M, Ito YM, Tsujino I. Right ventriculo‐pulmonary arterial uncoupling and poor outcomes in pulmonary arterial hypertension. Pulm Circ. 2020; 10: 2045894020957223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanderpool RR, Puri R, Osorio A, Wickstrom K, Desai A, Black S, Garcia JGN, Yuan J, Rischard F. EXPRESS: surfing the right ventricular pressure waveform: methods to assess global, systolic and diastolic RV function from a clinical right heart catheterization. Pulm Circ. 2019; 10: 2045894019850993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chemla D, Castelain V, Zhu K, Papelier Y, Creuzé N, Hoette S, Parent F, Simonneau G, Humbert M, Herve P. Estimating right ventricular stroke work and the pulsatile work fraction in pulmonary hypertension. Chest. 2013; 143: 1343–1350. [DOI] [PubMed] [Google Scholar]
- 18. Her C. Right ventricular stroke‐work. An index of distribution of pulmonary perfusion in acute respiratory failure. Chest. 1983; 84: 719–724. [DOI] [PubMed] [Google Scholar]
- 19. Karunanithi MK, Michniewicz J, Copeland SE, Feneley MP. Right ventricular preload recruitable stroke work, end‐systolic pressure‐volume, and dP/dtmax‐end‐diastolic volume relations compared as indexes of right ventricular contractile performance in conscious dogs. Circ Res. 1992; 70: 1169–1179. [DOI] [PubMed] [Google Scholar]
- 20. Richter MJ, Hsu S, Yogeswaran A, Husain‐Syed F, Vadász I, Ghofrani HA, Naeije R, Harth S, Grimminger F, Seeger W, Gall H, Tedford RJ, Tello K. Right ventricular pressure‐volume loop shape and systolic pressure change in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2021; 320: L715–L725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Badagliacca R, Pezzuto B, Papa S, Poscia R, Manzi G, Pascaretta A, Miotti C, Luongo F, Scoccia G, Ciciarello F, Casu G, Sciomer S, Fedele F, Naeije R, Vizza CD. Right ventricular strain curve morphology and outcome in idiopathic pulmonary arterial hypertension. JACC Cardiovasc Imaging. 2021; 14: 162–172. [DOI] [PubMed] [Google Scholar]
- 22. Richter MJ, Badagliacca R, Wan J, Vanderpool R, Dalmer A, Ghofrani HA, Harth S, Seeger W, Gall H, Naeije R, Tello K. Right ventricular dyssynchrony: from load‐independent right ventricular function to wall stress in severe pulmonary arterial hypertension. Pulm Circ. 2020; 10: 2045894020925759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tello K, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Seeger W, Wilhelm J, Gall H, Richter MJ. Cardiac magnetic resonance imaging‐based right ventricular strain analysis for assessment of coupling and diastolic function in pulmonary hypertension. JACC Cardiovasc Imaging. 2019; 12: 2155–2164. [DOI] [PubMed] [Google Scholar]
- 24. Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Gjesdal O, Edvardsen T, Smiseth OA. Assessment of wasted myocardial work: a novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am J Physiol Heart Circ Physiol. 2013; 305: H996–H1003. [DOI] [PubMed] [Google Scholar]
- 25. Hulshof HG, van Dijk AP, Hopman MTE, Heesakkers H, George KP, Oxborough DL, Thijssen DHJ. 5‐year prognostic value of the right ventricular strain‐area loop in patients with pulmonary hypertension. Eur Heart J Cardiovasc Imaging. 2021; 22: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]


