Abstract
Aims
In recent years, survival in patients with breast cancer has increased. Despite the improvement in outcomes of those patients, the risk of treatment‐related cardiotoxicity remains high, and its presence has been associated with a higher risk of treatment termination and thus lower therapeutic efficacy. Prior trials demonstrated that a preventive initiation of heart failure drugs, including the renin–angiotensin–aldosterone inhibitors, might reduce the risk of treatment‐related cardiotoxicity. However, to date, no study investigated the efficacy of sacubitril/valsartan, a novel antineurohormonal drug shown to be superior to the previous therapies, in the prevention of cardiotoxicity in patients with early‐stage breast cancer, which is the aim of the trial.
Methods and results
MAINSTREAM is a randomized, placebo‐controlled, double‐blind, multicentre, clinical trial. After the run‐in period, a total of 480 patients with early breast cancer undergoing treatment with anthracyclines and/or anti‐human epidermal growth factor receptor 2 drugs will be randomized to the highest tolerated dose of sacubitril/valsartan, being preferably 97/103 mg twice daily or placebo in 1:1 ratio. The patients will be monitored, including routine transthoracic echocardiography (TTE) and laboratory biomarker monitoring, for 24 months. The primary endpoint of the trial will be the occurrence of a decrease in left ventricular ejection fraction by ≥5% in TTE within 24 months. The key secondary endpoints will be the composite endpoint of death from any cause or hospitalization for heart failure, as well as other imaging, laboratory, and clinical outcomes, including the occurrence of the cancer therapy‐related cardiac dysfunction resulting in the necessity to initiate treatment. The first patients are expected to be recruited in the coming months, and the estimated completion of the study and publication of the results are expected in December 2027, pending recruitment.
Conclusions
The MAINSTREAM trial will determine the efficacy and safety of treatment with sacubitril/valsartan as a prevention of cardiotoxicity in patients with early breast cancer (ClinicalTrials.gov number: NCT05465031).
Keywords: Breast cancer, Cardio‐oncology, Cardiotoxicity, Heart failure, Randomized trial, Sacubitril/valsartan
Introduction
Breast cancer is the most prevalent malignancy in Western countries, and its worldwide prevalence is expected to significantly increase in the future. 1 Taking into consideration a notably lower age at diagnosis in patients with breast cancer than in other malignancies, its social impact defined by premature mortality and living with a disability is also most prominent. At present, the majority of patients are treated with surgical cancer excision and radiotherapy with advanced (neo)adjuvant chemotherapy. Although associated with improved prognosis, chemotherapy might lead to the development of cardiotoxicity or, more specifically, cancer therapy‐related cardiac dysfunction (CTRCD), as it has been introduced in the most recent European Society of Cardiology (ESC) guidelines on cardio‐oncology. 2 CTRCD can be defined as either clinically relevant heart failure (HF), or asymptomatic decrease in left ventricular ejection fraction (LVEF), or development of other imaging or biomarker‐based indications of cardiac dysfunction. 2 , 3 , 4 Its development is associated with increased morbidity and mortality and, according to previous analyses, in 2.0–17.0%, results in the temporary or definite cessation of life‐saving chemotherapy, thus increasing the risk of malignancy recurrence and worse prognosis. 5 , 6 Moreover, patients who develop cardiotoxicity are at an elevated risk of death and symptomatic HF in the future. 7
In recent years, attempts have been made to identify drugs that could serve as cardioprotective pharmacotherapy preventing the development of cardiotoxicity in patients with cancer. Although the results of the previously published trials demonstrated a slight improvement in the echocardiographic measures of cardiac dysfunction, or a reduction in cardiac troponin levels, which are associated with myocardial injury, in no trial, a durable improvement in LVEF has been identified. 8 , 9 , 10
The introduction of sacubitril/valsartan into treatment schemes for patients with HF led to a significant improvement in the prognosis of patients with reduced (HFrEF) or mildly reduced ejection fraction (HFmrEF), and the drug is currently recommended in all eligible patients with HFrEF. 11 , 12 Due to its most potent influence on the renin–angiotensin–aldosterone (RAA) axis, which has been demonstrated to play an important role in the development of chemotherapy‐induced cardiotoxicity, its early introduction might bring benefits in patients with breast cancer requiring chemotherapy. Moreover, preclinical studies and retrospective analyses have indicated the benefit of sacubitril/valsartan in the mitigation of the risk of cardiotoxicity caused by systemic chemotherapy. 13 , 14 Thus, the Sacubitril/Valsartan in PriMAry preventIoN of the cardiotoxicity of systematic breaST canceR trEAtMent (MAINSTREAM) trial has been designed to evaluate the efficacy and safety of sacubitril/valsartan in the prevention of cardiotoxicity in early breast cancer patients undergoing treatment with anthracyclines with or without treatment directed against human epidermal growth factor receptor 2 (HER‐2).
Study design of the MAINSTREAM trial
MAINSTREAM is a prospective, multicentre, randomized, placebo‐controlled, double‐blind, parallel‐group, investigator‐initiated clinical trial. The study is powered to demonstrate the difference between the groups in the primary endpoint, which has been defined as the occurrence of a decrease in LVEF by ≥5% assessed by transthoracic echocardiography (TTE) within 24 months. Approximately 600 patients will be recruited in three tertiary supraregional Polish oncology centres. Of those patients, after an estimated dropout, during the single‐blinded phase of drug uptitration to the target dose, 480 will be randomized in a 1:1 ratio into sacubitril/valsartan or matching placebo. The trial design complies with the Declaration of Helsinki and has received the approval of the Bioethics Committee of the National Research Institute of Oncology, Gliwice, Poland, under Number KB/430‐131/22. The authors exclusively contributed to the drafting, editing, and revision of the manuscript and are solely responsible for the design and conduction of this study and for drafting and editing of the paper and its final contents. The trial has been registered in ClinicalTrials.gov as NCT05465031.
Study population
The complete inclusion and exclusion criteria are summarized in Table 1 . In brief, patients with histologically confirmed and phenotypically assessed breast cancer at an early stage, defined as stages I–III and oligometastatic stage IV, with a radical treatment plan, which includes surgery and the post‐operative and/or pre‐operative systemic treatment, will be included in the study. The patients must be classified in the 0–2 classes of the Eastern Cooperative Oncology Group. In the baseline echocardiography analysis, the LVEF must be ≥50% and the patients must be in the sinus rhythm. The patients who underwent prior therapy with anthracyclines and/or left‐sided radiotherapy suffered from myocardial infarction within the preceding 3 months prior to the study, or have symptomatic, clinically relevant HF, will be excluded from the study. Similarly, patients with contraindications, or prone to the adverse effects of the studied drug, which includes patients with symptomatic hypotension, hyperkalaemia defined as K+ higher than 5.5 mmol/L and estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 on the screening visit, will also be considered ineligible to participate in this trial. Patients must not have been on treatment with angiotensin‐converting enzyme inhibitor (ACE‐I)/angiotensin II receptor blocker (ARB)/angiotensin receptor–neprilysin inhibitor (ARNI) at least in the 36 h prior to study enrolment. According to the ESC guidelines on cardio‐oncology, the inclusion and exclusion criteria for the trial define the vast majority of the studied population in the low or moderate risk of CTRCD; however, the inclusion of patients at high risk of either anthracycline‐associated or anti‐HER‐2‐associated CTRCD, such as patients aged ≥80 years, is not impossible. No very‐high‐risk patients wl be considered eligible for enrolment.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
‐ Written informed consent ‐ Female gender, aged 18 years and over ‐ Patients with histologically confirmed breast cancer and complete assessment of tumour phenotype (ER, PR, HER‐2, and Ki‐67) ‐ Ability to take oral medication and willingness to adhere to the planned regimen ‐ Tumour stage IA–IIIC or oligometastatic stage IV ‐ Radical treatment plan including surgery ‐ Plan of use of systemic treatment (pre‐operative, post‐operative, or combined) with anthracyclines and/or anti‐HER‐2 drugs ‐ ECOG 0–2 general status ‐ LVEF ≥ 50% as assessed by echocardiography ‐ Sinus rhythm |
‐ Prior anthracycline‐based chemotherapy and/or thoracic radiotherapy (prior to diagnosis of the cancer being the present cause of therapy) ‐ Clinically relevant HF (NYHA II–IV) ‐ MI within the last <3 months ‐ Symptomatic hypotension or SBP < 90 mmHg ‐ Significant valvular disease, symptomatic coronary artery disease (CCS > 2), significant AV block, and symptomatic sinus node dysfunction ‐ Expected survival <12 months ‐ eGFR < 30 mL/min/1.73 m2 (screening visit) ‐ K+ > 5.5 mmol/L (screening visit) ‐ Contraindications to ACE‐I/ARB or sacubitril/valsartan if not listed among criteria ‐ Active untreated liver disease ‐ Pregnancy ‐ Conditions/circumstances that may lead to non‐compliance with medical staff recommendations (e.g. active drug/alcohol dependence and poorly controlled mental illness) |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AV, atrioventricular; CCS, Canadian Cardiovascular Society; ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate; ER, oestrogen receptor; HER‐2, human epidermal growth factor receptor 2; HF, heart failure; Ki‐67, marker of proliferation Ki‐67; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PR, progesterone receptor; SBP, systolic blood pressure.
Treatment protocol
The details of the study procedures and the treatment protocol planned for each visit are summarized in Figure 1 and Table 2 . After assessment of the inclusion and exclusion criteria, and all examinations, including the echocardiography assessment, the patients will begin the single‐blinded phase of treatment with sacubitril/valsartan during the screening visit. The magnetic resonance imaging (MRI) study may be performed according to the availability of the method in each participating facility. The initial dosing of the drug will be 49/51 mg twice daily, which should be uptitrated to the target dose of 97/103 mg twice daily at the run‐in visit scheduled at 6–8 days from the initial evaluation and drug introduction, tolerance permitting. After further 6–8 days of single‐blinded treatment with target dose of sacubitril/valsartan, providing satisfactory tolerance of the drug, the patients will undergo randomization at the randomization visit.
Figure 1.
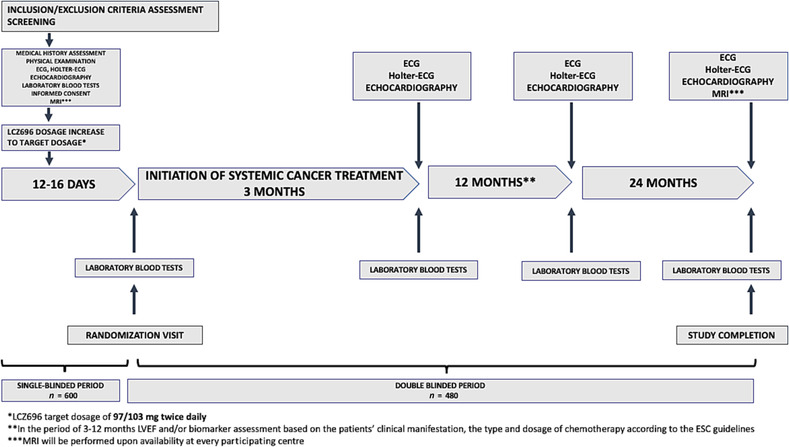
The study design. ECG, electrocardiography; ESC, European Society of Cardiology; LVEF, left ventricular ejection fraction; MRI, magnetic resonanse imaging.
Table 2.
The planned study scheme, including the timing of study procedures for each visit
| Procedure | Study visit | |||||
|---|---|---|---|---|---|---|
| Screening | Sacubitril/valsartan dose increase during the run‐in visit | Randomization | Visit after 3 months | Visit after 12 months | Visit after 24 months | |
| Informed written consent | X | |||||
| Analysis of the inclusion/exclusion criteria | X | X | ||||
| Medical history assessment and physical examination (including NYHA assessment) | X | X | X | X | X | X |
| 12‐lead Holter ECG | X | X | X | X | ||
| 12‐lead ECG | X | X | X | X | ||
| Laboratory blood test | X | X | X | X | ||
| Sacubitril/valsartan tolerability assessment | X | X | X | X | X | |
| Cardiac MRI | X a | X a | ||||
| Echocardiography | X | X | X | X | ||
| Pregnancy blood test | X | X | X | X | ||
| Blood sample for genetic testing | X | |||||
| QoL SF‐36 assessment | X | X | ||||
| Adverse event monitoring | X | X | X | X | X | X |
| Endpoint assessment | X | X | X | |||
ECG, electrocardiography; MRI, magnetic resonance imaging; NYHA, New York Heart Association; QoL, quality of life; SF‐36, 36‐Item Short Form Survey.
‘X’ indicates that the procedure should be performed according to the protocol during the marked visit.
MRI will be performed upon availability at each participating centre.
Randomization
Providing the patient had signed informed consent, is fully eligible for randomization, and underwent the single‐blinded study treatment regimen with satisfactory tolerance, the patient will be randomized with the use of an electronic, centralized randomization system, blinded to the patient's characteristics, to either interventional group, which will be administered with sacubitril/valsartan, or the placebo group. The system will dynamically randomly allocate patients to the two groups in a 1:1 ratio.
Double‐blinded period
After randomization, the patients will follow treatment with either sacubitril/valsartan or a matching placebo, for a maximum of 24 months. During this period, three study visits are planned, at 3, 12, and 24 months from randomization. At each visit, the patients will be assessed for the medical presentation, will undergo laboratory and imaging studies, and will be assessed for the presence of adverse events. In patients who will be unable to tolerate the target dose of the study drug of 97/103 mg twice daily, the dose can be down‐titrated to 49/51 mg twice daily at the investigator's discretion (after having considered whether there is any other concomitant medication that could act as a parallel contributor to the lower tolerance of the drug). If the dose of 49/51 mg twice daily cannot be tolerated, the dose could be temporarily lowered by half for a period of 2 weeks. However, if after that period the uptitration of the dose is impossible, the patients will have to be excluded from further participation in the trial. The physicians will be strongly encouraged to foster the continuation of the target dose of the study drug, based on their assessment of the patient's condition.
The outcomes
All outcomes to be monitored in the study are listed in Table 3 . The primary endpoint of the study has been defined as the occurrence of a decrease in LVEF by ≥5% assessed by TTE within 24 months. Among the secondary endpoints of the study, the clinical composite endpoint of death from any cause or hospitalization for HF and its components will be analysed, as well as the wide spectrum of echocardiographic, electrocardiographic, laboratory, and clinical outcomes, including the CTRCD resulting in the need to implement treatment consistent with the ESC guidelines on cardio‐oncology. 2 To maintain utmost objectivity, all examinations performed in relation to the study, including the echocardiographic, electrocardiographic, and MRI analyses, will be sent using 3D Option IMAGE‐COM (TOMTEC Imaging Systems GmbH, Germany)—a multi‐modality, vendor‐neutral 3D/4D viewer—to the core laboratory where their results will be analysed by two independent investigators blinded to the patient's randomization status.
Table 3.
Study outcomes
|
Primary outcome: ‐ Decrease in left ventricular ejection fraction ≥5% assessed on echocardiography within 24 months of randomization |
|
Secondary outcomes: [time frame: from randomization till the end of blinded therapy (final assessment—at 24 months)] ‐ A composite endpoint: death from any cause or hospitalization for heart failure ‐ Death from any cause ‐ Death from cardiovascular causes ‐ Hospitalization for other cardiovascular causes ‐ Cancer therapy‐related cardiac dysfunction resulting in the necessity to initiate treatment according to the European Society of Cardiology on cardio‐oncology ‐ Decrease in left ventricular ejection fraction ≥5% (MRI) within 24 months of randomization ‐ Development of other imaging or arrhythmic pathologies (e.g. diastolic dysfunction, new pericardial effusion, cardiac tamponade, pericarditis, myocarditis, supraventricular/ventricular arrhythmias, conduction disturbances, and changes in corrected QT interval) ‐ Changes in BNP, NT‐proBNP, and cTn levels according to the cut‐off values described in the European Society of Cardiology guidelines |
BNP, brain natriuretic peptide; cTn, cardiac troponin; MRI, magnetic resonance imaging; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Breast cancer treatment
The patients should be treated according to the standard protocols of the participating centres, with the intention of radical treatment based on the surgical excision of cancer, with addition of either pre‐surgical or post‐surgical chemotherapy and/or radiotherapy. The choice of the scheme of oncological treatment will be at the discretion of the treating oncologist and will be performed after analysis of the risk of recurrence, the potential response to a particular type of treatment, with consideration of possible drug‐related adverse effects, and patient's comorbidities and preferences. All decisions, including the scheme of therapy, will be performed in accordance with the recommendations of the Polish Society of Clinical Oncology. 15
Pre‐operative and post‐operative chemotherapy with alkylating drugs, which include anthracyclines, usually in multi‐drug regimens, will begin most commonly, with the initiation of anthracyclines (4 or 3 cycles of EC ‐ epirubicin + cyclophosphamide). In routine perioperative treatment, trastuzumab will be administered̨ for 12 months, but shorter regimens (6 months) may be considered in individual situations; however, due to the cardiotoxic effects of trastuzumab, it will not be routinely given concomitantly with anthracyclines.
Genetic testing
As there are currently no data regarding the potential differences in the pharmacodynamics and pharmacokinetics, and consequently efficacy and safety of sacubitril/valsartan prophylactic treatment in patients with breast cancer and systemic therapy, there may be genetic biomarkers defining the disease susceptibility and prognosis, as well as the response to treatment. For each patient who gives informed consent to participate in the study, an additional blood sample will be taken at the first visit. After completion of the study, the subanalysis of the trial will be attempted based on the genetic profile aiming at defining the genetic pattern characterizing the response to sacubitril/valsartan for the prevention of cardiotoxicity caused by anthracyclines and/or anti‐HER‐2‐targeted drugs.
Statistical calculations
All sample size calculations were performed based on the estimated differences between the groups for the occurrence of the primary endpoint. Based on the available evidence, we estimated that the incidence of left ventricular systolic dysfunction in the group of patients not receiving cardiotoxicity prevention would be ~20%. We have estimated a 50% relative and 10% absolute reduction of the risk of this event. Under these assumptions, and taking into account the 20% dropout due to drug intolerance and other factors, the inclusion of 240 patients in the study and control group respectively resulted in the 80% power of the study, for the aggregate of 480 randomized patients, and the occurrence of primary endpoint in 48 patients from the placebo group and in 24 patients from the study group during 24 months of follow‐up. Analysis of the necessary minimum sample size was performed with the power procedure using Pearson's χ 2 test for two independent proportions. The level of statistical significance was taken as α = 0.05. Calculations were performed using the SAS statistical package Version 9.4 (SAS Institute Inc., Cary, NC, USA).
The categorical variables will be presented using frequency tables for both absolute numbers and percentages. The continuous variables will be presented with the use of the mean with standard deviation for data following normal distribution or a median with quartiles 1 and 3 for data demonstrating a non‐normal distribution. For baseline characteristics comparison and follow‐up data, t‐tests and Mann–Whitney U tests will be used for respectively normally and non‐normally distributed continuous data. For categorical data, Pearson's χ 2 or Fisher's exact test will be used, as appropriate.
The analysis of the primary endpoint will be performed according to the intention‐to‐treat concept (analysis of groups as randomized and including all patients with outcome data available), with a secondary analysis pre‐specified as a per‐protocol (PP) analysis, using the Cox proportional hazards regression model. The secondary endpoints will be analysed with the use of either linear regression (for continuous variables) or logistic regression (for categorical variables). To determine the independent predictors of primary and secondary endpoints, appropriate univariate and multivariate techniques will be applied. Kaplan–Meier curves will graphically demonstrate the occurrence of secondary clinical endpoints. Subgroup analyses have been planned, including division of the studied population based on the LVEF, treatment with vs. without anthracyclines, or treatment with vs. without anti‐HER‐2 drugs. All pre‐specified subgroups of interest for the occurrence of primary or secondary endpoints are summarized in Supporting Information, Table S1 .
Outcome assessment
At the screening visit and completion of the study, the patient will undergo TTE, which, according to the recent ESC guidelines on cardio‐oncology, has been defined as the first‐line modality in assessment of cardiac function in oncological patients. 2 The assessment of LVEF with the use of 3D echocardiography will be preferred in all patients, although in selected cases, a two‐dimensional LVEF assessment will be accepted. In every patient, the assessment of global longitudinal strain will be recommended. The investigator performing the echocardiography will be blinded to the patient's randomization scheme. All images will be sent to the core laboratory. The quality of life will be assessed with the 36‐Item Short Form Survey (SF‐36) questionnaire at the screening visit and at the completion of the study. 16 , 17
Laboratory tests
The details of the blood tests performed during each study visit are presented in Table 4 . All blood samples will be performed at fasting, on the day of the study visits, and analysed in the local laboratories of the three participating oncology centres. The blood sample for genetic testing in patients who sign an additional consent for genetic evaluation will be collected only on the screening visit and stored locally for the eventual transfer into the biobank of the Maria Sklodowska‐Curie Institute—Oncology Center (MSCI), Gliwice, Poland, for further analyses. High‐sensitivity cardiac troponin (with a preference to measure cardiac troponin T over troponin I due to previously described variability in the results of the latter), as well as N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), will be assessed using assays at the local laboratories of the participating oncology centres during study visits and at any time when deemed necessary by the physician‐in‐charge or as supported by the local treatment protocols established by the participating centres and with reference to the ESC guidelines on cardio‐oncology. 2 , 18 The investigators will be blinded to the patients' randomization results.
Table 4.
The laboratory tests performed during each study visit in the MAINSTREAM trial
| Blood laboratory tests performed during the consecutive visits in the MAINSTREAM trial | |||
|---|---|---|---|
| Laboratory test | Screening visit (Visit No. 1) | Run‐in visit and randomization visit (Visit Nos. 2 and 3) | Visit Nos. 4–6 (visits at 3, 12, and 24 months from the randomization visit) |
| Creatinine, eGFR, and K+ | X | X | X |
| FBC, ALT, AST, BNP, NT‐proBNP, and cTn (cTnT preferred) | X | X | |
| Bilirubin, glucose, Na+, HDL, LDL, TGs, and TCh | X | ||
| Blood sample for genetic testing | X | ||
| Pregnancy test—β‐hCG | X | X | |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; cTn, cardiac troponin; cTnT, cardiac troponin T; eGFR, estimated glomerular filtration rate based on the Modification of Diet in Renal Disease (MDRD) equation; FBC, full blood count; HDL, high‐density lipoprotein; K+, potassium; LDL, low‐density lipoprotein; Na+, sodium; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TCh, total cholesterol; TGs, triglycerides; β‐hCG, β‐human chorionic gonadotropin.
Intervention in case of HF development
Based on the prior studies, it is estimated that ~20% of patients from the placebo arm and 10% of study drug arm might develop a certain degree of cardiotoxicity and require introduction of cardioprotective agents. The diagnosis of CTRCD should be based on the assessment of TTE and/or high‐sensitivity cardiac troponin, as well as NT‐proBNP. 2 The intervention in case of development of either symptomatic or asymptomatic CTRCD should be in line with current ESC guidelines on cardio‐oncology and may include discontinuation or temporary interruption of chemotherapy, or continuation of treatment with thorough patient monitoring, while the study drug/placebo might be terminated or continued, depending on the severity of CTRCD. 2
Results
The study is set to recruit the first patients in the coming months. The estimated completion of the study and publication of the results are expected in December 2027, providing successful recruitment of the participants.
Discussion
The number of patients with concomitant cardiovascular disease and malignancies, including breast cancer, has been growing, and the forecasts predict their further increase. 19 , 20 Constituting more than 10% of all cancers, breast cancer is the leading cause of death in women aged 20–50 years. 21 , 22
The treatment strategies, depending on the stage of cancer, the patient's comorbidities, and further factors, include a combination of surgery, radiotherapy, and systemic treatment, with reports indicating that ~95% of patients receive pre‐surgical or post‐surgical additional treatment. 23 The cornerstones of chemotherapy are still anthracyclines and monoclonal antibodies targeted against HER‐2 molecules, while the other drug groups are used in the lower percentages of patients with breast cancer. It should be noted that each of these treatment modalities may exert adverse cardiovascular effects, and such risk could rise exponentially when the drugs are used in combination. 24 , 25
In recent years, consensus documents, position papers, and the first cardio‐oncology guidelines were published, which all aim to identify patients at risk of cardiotoxicity, introduce cardioprotective measures, and treat the occurring complications. 2 , 26 , 27 , 28 However, the recommendations have been mostly based on the experiences of the Guidelines' authors, rather than on the randomized data, which are still scarce. A possible explanation is that to date, no therapy has proven irrefutably successful in significantly mitigating the risk of cardiotoxicity.
Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA) and Carvedilol for Prevention of Chemotherapy‐Related Cardiotoxicity (CECCY) were the two largest randomized controlled trials conducted to date, which investigated whether a cardioprotective benefit could be achieved by addition of parallel treatment with chemotherapy and drugs augmenting the RAA axis. In PRADA, 130 patients with early breast cancer treated with anthracyclines were randomized to either candesartan or metoprolol. Treatment with candesartan but not with the latter was associated with a subtle but statistically significant decrease in LVEF reduction, while treatment with metoprolol was associated with smaller increases in cardiac troponin T and I levels. 9 However, in the long‐term analysis, no differences in LVEF were observed. 8
In contrast, the placebo‐controlled CECCY trial demonstrated that carvedilol, a non‐cardioselective beta‐blocker, did not reduce the occurrence of cardiotoxicity (defined as the proportion of patients with a ≥10% reduction in LVEF). However, the trial proved that analogous to the effect of metoprolol in the PRADA trial, the use of carvedilol was associated with attenuation of the increase in cardiac troponin I during anthracycline treatment. 10
The results of the two aforementioned trials confirm that inhibition of the RAA axis may lead to at least a transient improvement in cardiac contractility and a reduction in myocardial damage markers' levels. Preclinical studies have shown that activation of natriuretic peptide cellular pathways reduces the risk of developing anthracycline‐induced cardiomyopathy in rodents, while deprivation of the angiotensin II receptor type 1a gene minimizes the risk of anthracycline‐induced cardiotoxicity. 29 Thus, there is preclinical rationale to consider sacubitril/valsartan a possible strategy of choice in the prevention of CTRCD. First of all, the drug acts via the pathways of natriuretic peptides, as well as by the inhibition of the RAA axis—and therefore has been associated with the most notable improvement in LVEF and exercise capacity in patients with HFrEF or HFmrEF among any RAA‐inhibiting drugs. Second, the development of CTRCD usually involves a reduction of LVEF, suggesting that the drug more effectively mitigating the risk in HFrEF might result in a more pronounced clinical and imaging improvement in subjects with CTRCD. 30 , 31
Although limited, the retrospective studies have yielded promising results supporting administration of sacubitril/valsartan in patients with CTRCD. In a single‐centre analysis, patients in whom LVEF had been reduced by >10% to <53% due to oncological treatment, and who were treated with sacubitril/valsartan, experienced a reduction in median NT‐proBNP and an increase in the median distance of the 6 min walk test as well as in the mean LVEF. 13 In the Spanish registry, the use of sacubitril/valsartan in cancer patients with a symptomatic decrease of LVEF to <40% caused by oncological therapy was associated with a significant increase in LVEF and improvement in New York Heart Association (NYHA) class, along with a reduction in NT‐proBNP levels and left ventricular dimensions. 14
Finally, the issue of baseline CTRCD risk should be elaborated. The majority of patients included in the MAINSTREAM trial will be in the intermediate or low risk, as defined by the HFA‐ICOS stratification risk score recommended by the ESC cardio‐oncology guidelines. Such representation of patients could potentially reduce the magnitude of CTRCD occurrence and, possibly, the possibility of observing benefit of cardioprotective measures. However, it must be noted that real‐life patients with breast cancer are usually classified as either low or moderate risk of cardiotoxicity. In a recent paper of more than 350 patients with breast cancer, who were treated with anthracyclines or anti‐HER‐2 agents, 51% of the anthracycline group were at low risk and 43% at medium risk of CTRCD, while in the group treated with anti‐HER‐2 agents, these percentages were respectively 27% and 58%. The percentages of patients in the high‐risk group were 6% and 15% respectively for both treatment modalities. 32 In another manuscript, in more than 900 patients with HER‐2‐positive early breast cancer, the percentage of patients in the low and moderate risks according to HFA‐ICOS score was respectively 43% and 49%. 33
In conclusion, the optimal management of cancer patients undergoing chemotherapy, who are at risk of developing cardiotoxicity, is still debatable. There is increasing evidence of benefit from introduction of sacubitril/valsartan in this population. The randomized MAINSTREAM trial will evaluate efficacy and safety of sacubitril/valsartan in the prevention of cardiotoxicity in patients with early breast cancer, who undergo treatment with anthracyclines with or without treatment directed against HER‐2.
Conflict of interest
None declared.
Funding
This non‐commercial research trial is funded by the scientific grant of the Medical Research Agency (Agencja Badań Medycznych; Grant No. 2021/ABM/03/00008).
Supporting information
Table S1. Prespecified sub‐groups for analysis of the occurrence of primary and secondary endpoints.
Tajstra, M. , Dyrbuś, M. , Rutkowski, T. , Składowski, K. , Sosnowska‐Pasiarska, B. , Góźdź, S. , Radecka, B. , Staszewski, M. , Majsnerowska, A. , Myrda, K. , Nowowiejska‐Wiewióra, A. , Skoczylas, I. , Rymkiewicz, I. , Niklewski, T. , Nowak, J. , Przybyłowski, P. , Gąsior, M. , and Jarząb, M. (2023) Sacubitril/valsartan for cardioprotection in breast cancer (MAINSTREAM): design and rationale of the randomized trial. ESC Heart Failure, 10: 3174–3183. 10.1002/ehf2.14466.
Contributor Information
Mateusz Tajstra, Email: mateusztajstra@wp.pl.
Maciej Dyrbuś, Email: mdyrbus@op.pl.
References
- 1. Breast cancer statistics [Internet]. https://www.wcrf.org/cancer‐trends/breast‐cancer‐statistics/. Accessed 07 June 2023.
- 2. Lyon AR, López‐Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler‐Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH, ESC Scientific Document Group . 2022 ESC guidelines on cardio‐oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio‐Oncology Society (IC‐OS). Eur Heart J [Internet]. 2022; 43: 4229–4361. http://www.ncbi.nlm.nih.gov/pubmed/36017568. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 3. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation [Internet]. 2015; 131: 1981–1988. http://www.ncbi.nlm.nih.gov/pubmed/25948538. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 4. Mohan N, Jiang J, Dokmanovic M, Wu WJ. Trastuzumab‐mediated cardiotoxicity: current understanding, challenges, and frontiers. Antib Ther [Internet]. 2018; 1: 13–17. http://www.ncbi.nlm.nih.gov/pubmed/30215054. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padegimas A, Clasen S, Ky B. Cardioprotective strategies to prevent breast cancer therapy‐induced cardiotoxicity. Trends Cardiovasc Med [Internet]. 2020; 30: 22–28. http://www.ncbi.nlm.nih.gov/pubmed/30745071. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onitilo AA, Engel JM, Stankowski RV. Cardiovascular toxicity associated with adjuvant trastuzumab therapy: prevalence, patient characteristics, and risk factors. Ther Adv Drug Saf [Internet]. 2014; 5: 154–166. http://www.ncbi.nlm.nih.gov/pubmed/25083270. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long‐term observational study of outcome in 120 patients. Ann Oncol Off J Eur Soc Med Oncol [Internet]. 2002; 13: 699–709. http://www.ncbi.nlm.nih.gov/pubmed/12075737. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 8. Heck SL, Mecinaj A, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, Røsjø H, Steine K, Geisler J, Gulati G, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): extended follow‐up of a 2 × 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Circulation [Internet]. 2021; 143: 2431–2440. http://www.ncbi.nlm.nih.gov/pubmed/33993702. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz‐Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff‐Brenkenhoff F, Bratland Å, Storås TH, Hagve TA, Røsjø H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo‐controlled, double‐blind clinical trial of candesartan and metoprolol. Eur Heart J [Internet]. 2016; 37: 1671–1680. http://www.ncbi.nlm.nih.gov/pubmed/26903532. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avila MS, Ayub‐Ferreira SM, de Barros Wanderley MR, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, Higuchi‐dos‐Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M, Ferrari MSM, de Paula Costa RL, Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS, Bocchi EA. Carvedilol for prevention of chemotherapy‐related cardiotoxicity: the CECCY trial. JACC Adv. 2018; 71: 2281–2290. http://www.ncbi.nlm.nih.gov/pubmed/29540327. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 11. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol [Internet]. 2022; 79: 1757–1780. http://www.ncbi.nlm.nih.gov/pubmed/35379504. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 12. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J [Internet]. 2021; 42: 3599–3726. http://www.ncbi.nlm.nih.gov/pubmed/34447992. Accessed 07 June 2023.34447992 [Google Scholar]
- 13. Gregorietti V, Fernandez TL, Costa D, Chahla EO, Daniele AJ. Use of sacubitril/valsartan in patients with cardio toxicity and heart failure due to chemotherapy. Cardio‐oncology (London, England). 2020; 6: 24. http://www.ncbi.nlm.nih.gov/pubmed/33292750. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martín‐Garcia A, López‐Fernández T, Mitroi C, Chaparro‐Muñoz M, Moliner P, Martin‐Garcia AC, Martinez‐Monzonis A, Castro A, Lopez‐Sendon JL, Sanchez PL. Effectiveness of sacubitril–valsartan in cancer patients with heart failure. ESC Hear Fail [Internet]. 2020; 7: 763–767. http://www.ncbi.nlm.nih.gov/pubmed/32022485. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jassem EJ, Krzakowski M, Jassem AJ, Krzakowski M, Bobek‐Billewicz B, Duchnowska R, Jeziorski A, Olszewski W, Senkus‐Konefka E, Tchórzewska‐Korba H, Wysocki P. Breast cancer. Oncol Clin Pract. 2020; 16: 207–260. [Google Scholar]
- 16. Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ [Internet]. 1993; 306: 1440–1444. http://www.ncbi.nlm.nih.gov/pubmed/8518640. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim I‐C, Youn J‐C, Jang SY, Lee SE, Cho H‐J, Choi J‐O, Lee JH, Kim KH, Lee SH, Kim KH, Lee JM, Yoo BS, the SPARK study group . Physician adherence and patient‐reported outcomes in heart failure with reduced ejection fraction in the era of angiotensin receptor‐neprilysin inhibitor therapy. Sci Rep [Internet]. 2022; 12: 7730. http://www.ncbi.nlm.nih.gov/pubmed/35545653. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venge P, James S, Jansson L, Lindahl B. Clinical performance of two highly sensitive cardiac troponin I assays. Clin Chem [Internet]. 2009; 55: 109–116. http://www.ncbi.nlm.nih.gov/pubmed/18988756. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 19. Herrmann J, López‐Fernández T, Lyon AR. Year in cardiovascular medicine: cardio‐oncology 2020‐21. Eur Heart J [Internet]. 2022. http://www.ncbi.nlm.nih.gov/pubmed/34974609. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 20. Lenihan DJ, Fradley MG, Dent S, Brezden‐Masley C, Carver J, Filho RK, Neilan TG, Blaes A, Melloni C, Herrmann J, Armenian S, Thavendiranathan P, Armstrong GT, Ky B, Hajjar L. Proceedings from the Global Cardio‐Oncology Summit: the top 10 priorities to actualize for cardiooncology. JACC CardioOncology [Internet]. 2019; 1: 256–272. http://www.ncbi.nlm.nih.gov/pubmed/34396188. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin [Internet]. 2019; 69: 7–34. http://www.ncbi.nlm.nih.gov/pubmed/30620402. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 22. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin [Internet]. 2021; 71: 209–249. http://www.ncbi.nlm.nih.gov/pubmed/33538338. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 23. Almuwaqqat Z, Meisel JL, Barac A, Parashar S. Breast cancer and heart failure. Heart Fail Clin [Internet]. 2019. Jan; 15: 65–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30449381. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 24. Fu Z, Lin Z, Yang M, Li C. Cardiac toxicity from adjuvant targeting treatment for breast cancer post‐surgery. Front Oncol [Internet]. 2022; 12: 706861. http://www.ncbi.nlm.nih.gov/pubmed/35402243. Accessed 07 June 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papageorgiou C, Andrikopoulou A, Dimopoulos M‐A, Zagouri F. Cardiovascular toxicity of breast cancer treatment: an update. Cancer Chemother Pharmacol [Internet]. 2021; 88: 15–24. http://www.ncbi.nlm.nih.gov/pubmed/33864486. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 26. Herrmann J, Lenihan D (Co‐chair), Armenian S, Barac A, Blaes A, Cardinale D, Carver J, Dent S, Ky B, Lyon AR, López‐Fernández T, Fradley MG, Ganatra S, Curigliano G, Mitchell JD, Minotti G, Lang NN, Liu JE, Neilan TG, Nohria A, O'Quinn R, Pusic I, Porter C, Reynolds KL, Ruddy KJ, Thavendiranathan P, Valent P. Defining cardiovascular toxicities of cancer therapies: an International Cardio‐Oncology Society (IC‐OS) consensus statement. Eur Heart J. 2022; 43: 280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P, Patel A, DeCara J, Mitchell J, Harrison E, Moslehi J, Witteles R, Calabro MG, Orecchia R, de Azambuja E, Zamorano JL, Krone R, Iakobishvili Z, Carver J, Armenian S, Ky B, Cardinale D, Cipolla CM, Dent S, Jordan K. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020; 31: 171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyon AR, Dent S, Stanway S, Earl H, Brezden‐Masley C, Cohen‐Solal A, Tocchetti CG, Moslehi JJ, Groarke JD, Bergler‐Klein J, Khoo V. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio‐Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio‐Oncology Society. Eur J Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toko H, Oka T, Zou Y, Sakamoto M, Mizukami M, Sano M, Yamamoto R, Sugaya T, Komuro I. Angiotensin II type 1a receptor mediates doxorubicin‐induced cardiomyopathy. Hypertens Res [Internet]. 2002; 25: 597–603. http://www.ncbi.nlm.nih.gov/pubmed/12358147. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 30. Zhang R, Sun X, Li Y, He W, Zhu H, Liu B, Zhang A. The efficacy and safety of sacubitril/valsartan in heart failure patients: a review. J Cardiovasc Pharmacol Ther [Internet]; 27: 10742484211058680. http://www.ncbi.nlm.nih.gov/pubmed/34994233. Accessed 07 June 2023. [Google Scholar]
- 31. Zheng C, Dai H, Huang J, Lin M, Zheng Q, Tang P, Xiao J, Zhang Y. The efficacy and safety of sacubitril/valsartan in the treatment of chronic heart failure: a meta‐analysis. Am J Transl Res [Internet]. 2021; 13: 12114–12128. http://www.ncbi.nlm.nih.gov/pubmed/34956440. Accessed 07 June 2023. [PMC free article] [PubMed] [Google Scholar]
- 32. Tini G, Cuomo A, Battistoni A, Sarocchi M, Mercurio V, Ameri P, Volpe M, Porto I, Tocchetti CG, Spallarossa P. Baseline cardio‐oncologic risk assessment in breast cancer women and occurrence of cardiovascular events: the HFA/ICOS risk tool in real‐world practice. Int J Cardiol [Internet]. 2022. Feb; 15: 134–137. http://www.ncbi.nlm.nih.gov/pubmed/34848212. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
- 33. Battisti NML, Andres MS, Lee KA, Ramalingam S, Nash T, Mappouridou S, Senthivel N, Asavisanu K, Obeid M, Tripodaki ES, Angelis V, Fleming E, Goode EF, John S, Rosen SD, Allen M, Stanway S, Lyon AR, Ring A. Incidence of cardiotoxicity and validation of the Heart Failure Association‐International Cardio‐Oncology Society risk stratification tool in patients treated with trastuzumab for HER2‐positive early breast cancer. Breast Cancer Res Treat [Internet]. 2021; 188: 149–163. http://www.ncbi.nlm.nih.gov/pubmed/33818652. Accessed 07 June 2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Prespecified sub‐groups for analysis of the occurrence of primary and secondary endpoints.


