Abstract
Aims
This study aims to investigate the clinical and biochemical characteristics of patients with atrial fibrillation (AF) referred for ablation who develop arrhythmia‐induced cardiomyopathy (AiCM) as well as their long‐term outcomes after catheter ablation (CA).
Methods and results
A prospective multicentre study was conducted on consecutive AF patients who underwent CA. AiCM was defined as the development of heart failure in the presence of AF and an improvement of left ventricular fraction by at least 10% at 6 months after ablation. A subgroup of patients underwent peripheral and left atrial blood samples [galectin‐3, fatty acid‐binding protein 4 (FABP4), and soluble receptor for advanced glycation end products (sRAGE)] at the time of the procedure. Of the 769 patients who underwent AF ablation, 135 (17.56%) met the criteria for AiCM. Independent predictors of AiCM included persistent AF, male gender, left atrial volume, QRS width, active smoking, and chronic kidney disease (CKD). Biomarker analysis revealed that sRAGE, FABP4, and galectin‐3 levels were not predictive of AiCM development nor did they differ between groups or predict recurrence. There were no differences in AF recurrence between patients with and without AiCM (30.83% vs. 27.77%; P = 0.392) during a median follow‐up of 23.83 months (inter‐quartile range 9–36).
Conclusions
In the subset of patients referred for AF ablation, the development of AiCM was associated with persistent AF and CKD. Biomarker analysis was not different between groups nor predicted recurrence. Patients with AiCM benefited from ablation, with a significant improvement in left ventricular ejection fraction and similar AF recurrence rates to those without AiCM.
Keywords: Atrial fibrillation, Catheter ablation, Heart failure
Introduction
Atrial fibrillation (AF) is known to trigger a reversible dilated cardiomyopathy (CM) frequently referred to as arrhythmia‐induced CM (AiCM). It is still unclear why some patients are more prone to develop AiCM than others. AiCM has been reported to be present weeks or months to years after the onset of tachycardia, and the prevalence and factors that predispose or prevent AiCM are unknown. 1 , 2 Additionally, despite AF being the most prevalent arrhythmia, there are no clear data on the prevalence of AiCM in this population. A single study reported AiCM in 4% of patients referred for pulmonary vein (PV) isolation (PVI), although this number may be confounded by selection and referral biases. 3 Finally, although systemic inflammation has been recognized as a common pathobiological feature of heart failure (HF) and AF development, 4 , 5 , 6 up to date, biomarker differences among patients with AiCM vs. those without AiCM have not been reported. This could be of relevance for a better understanding of this entity and novel strategies for potential target therapies.
We aim to report the baseline clinical and biochemical profile of patients with AiCM as compared with patients without AiCM along with their clinical outcome after AF ablation.
Methods
An investigator‐initiated, prospective, non‐randomized study was conducted at three tertiary hospitals: University Clinical Hospital of Santiago de Compostela (Spain) and Antwerp University Hospital (UZA) (Belgium) between September 2016 and November 2021. Consecutive patients referred for point‐by‐point radiofrequency catheter ablation (CA) were included in the study.
Blood sample collection
All patients underwent general laboratory blood testing at admission. During the ablation procedure (after a night of fasting), peripheral blood sample was obtained from an ante‐cubital vein using an 18‐gauge butterfly cannula with a two‐syringe technique, discarding the first 5 mL of blood and using the second 5 mL for measurements. Blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes.
Plasma measurements
Fatty acid‐binding protein 4
After centrifugation at 1800 g for 10 min, the atrial and peripheral plasma samples were stored at −80°C until used. A magnetic Luminex multiplex test kit (R&D Systems, Minneapolis, MN, USA) was used. The manufacturer's instructions were followed when analysing plasma levels of fatty acid‐binding protein (FABP4). The sensitivity for FABP4 was 95.7.
Soluble receptor for advanced glycation end products and galectin‐3
Peripheral blood samples were collected in EDTA tubes, and after centrifugation at 1800 g for 10 min, the plasma samples were stored at −80°C until used. Plasma soluble receptor for advanced glycation end products (sRAGE) and galectin‐3 (Gal‐3) levels were determined using a commercially available enzyme‐linked immunosorbent assay (ELISA) kit according to the manufacturers' protocols (Quantikine, R&D Systems, Minneapolis, MN, USA, for sRAGE; BMS279‐4, eBioscience, Vienna, Austria, for Gal‐3).
Measurements were performed in duplicate, and the results were averaged. The intra‐assay and inter‐assay coefficients of variation values were <5% and <8%, respectively, for sRAGE. The intra‐assay and inter‐assay coefficients of variation values were 7.5% and 5.4%, respectively, for Gal‐3.
Ablation procedure and assessment of left atrial surface area
Patients underwent point‐by‐point radiofrequency CA (SMARTTOUCH®, Biosense Inc., Diamond Bar, CA, USA). The procedural endpoint was ipsilateral PVI. Assessment of left atrial (LA) surface area and LA fibrosis was based on bipolar voltage map, which was created simultaneously with LA surface reconstruction, guided by a three‐dimensional electroanatomical mapping system (CARTO 3, Biosense Webster) using a multipolar mapping catheter (Lasso or PentaRay, Biosense Webster). Bipolar voltage points were collected automatically with the use of the Confidense module. The settings of tissue proximity index or end filtering, local activation time, cycle length, and position stability were left to the operators' discretion.
Three different cut‐offs were used for low‐voltage zone (LVZ), according to the underlying rhythm. In sinus rhythm mapping, the LVZ cut‐off was <0.5 mV. If mapping was in AF, LVZ cut‐off was <0.24 mV and in atrial flutter (AFL) <0.3 mV. 7 LVZ was identified as an area of at least 1 cm2 containing ≥3 neighbouring points with ≤10 mm distance. The LVZ area was measured by manually encircling the area with a measurement tool and was expressed in cm2. Burden was calculated as the percentage of total LA surface area excluding the PV ostia and mitral valve area. All patients underwent ipsilateral wide‐area circumferential PVI with the use of contact force (CF)‐sensing irrigated tip ablation catheter (SMARTTOUCH®, Biosense Webster, Diamond Bar, CA, USA) and automatic ablation annotation module (VISITAG®, Biosense Webster, Diamond Bar, CA, USA).
Patient follow‐up
Oral anticoagulation (OA) was maintained for at least 3 months following the procedure (until the first ambulatory visit) and was subsequently continued in patients with a CHA2DS2‐VASc score ≥2. Antiarrhythmic drugs (AADs) were continued during the blanking period.
At the end of the blanking period, patients were encouraged to discontinue AAD. Ambulatory visits were systematically performed at 3, 6, and 12 months after the index procedure. Each visit comprised detailed history taking, physical examination, and 12‐lead electrocardiogram (ECG). Moreover, 24 h Holter recording was routinely performed at 3, 6, and 12 months. AF recurrence was defined as any episode of AF/atrial tachycardia (AT) lasting for more than 30 s. 8
Definitions
AiCM was defined as (i) development of unexplained HF [HF with reduced ejection fraction (HFrEF) or HF with mid‐range ejection fraction (HFmrEF)] in the presence of AF or clear evidence providing that AF contributed to the exacerbation of left ventricular (LV) dysfunction in patients with pre‐existing CM and (ii) ≥10% ejection fraction (EF) improvement of LV systolic EF 6 months after successful rhythm control, 9 , 10 including cardioversion or AF ablation.
At the time of the inclusion period, recognized guideline‐directed medical therapies (GDMTs) in the European Society of Cardiology guidelines for HFrEF were beta‐blockers, angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs). 11 Patients were categorized as being on GDMT if they had documented drug use between echocardiograms for at least 90 days.
Endpoint
The primary outcome of the study was (i) LVEF recovery at 6 months after cardiac ablation (CA), defined as a ≥10% increase in LVEF, and (ii) AF/AFL/AT recurrence‐free survival after CA considering a blanking period of 3 months.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Bivariate analysis was performed either with the Wilcoxon rank‐sum test or with Pearson's χ 2 test, where appropriate. Logistic regressions, Cox proportional hazards models and log‐rank test were used in order to test the existence of associations between independent and dependent variables. All analyses were programmed in R 4.1 and Stata 15. P < 0.05 was considered as the statistical significance reference.
Results
Baseline characteristics
The study population consisted of 769 consecutive patients. One hundred and thirty‐five (17.56%) patients met the predefined diagnostic criteria of AiCM. Three hundred and two patients underwent peripheral and LA blood sample collection for biomarker analysis (Table 1 ). The median follow‐up period was 23.83 months [inter‐quartile range (IQR) 9–36].
Table 1.
Demographic characteristics of the studied population
| AiCM | Non‐AiCM | P value | |
|---|---|---|---|
| N = 135 (17.56%) | N = 634 (82.44%) | ||
| Baseline characteristics | |||
| Age (mean ± SD) | 59.25 ± 9.89 | 60.61 ± 10.60 | 0.172 |
| Gender (male), n (%) | 75 (84.27%) | 282 (66.35%) | 0.001 |
| Persistent AF, n (%) | 123 (91.11%) | 401 (63.75%) | <0.001 |
| Pre‐procedural heart rate in SR (mean ± SD) | 63 ± 12.34 | 62 ± 10.94 | 0.357 |
| Pre‐procedural heart rate in AF (mean ± SD) | 90 ± 23.29 | 91 ± 23.37 | 0.715 |
| Left atrial volume index (mL/m2) | 37.32 ± 14.31 | 32.34 ± 6.33 | <0.001 |
| Arterial hypertension, n (%) | 76 (56.30%) | 285 (45.53%) | 0.023 |
| Type 2 diabetes mellitus, n (%) | 24 (17.78%) | 81 (12.92%) | 0.137 |
| Smokers, n (%) | 44 (32.60%) | 125 (19.94%) | 0.004 |
| Chronic kidney disease, n (%) | 13 (14.29%) | 28 (6.65%) | 0.015 |
| Pre‐procedural NYHA class | |||
| I | 526 (83%) | ||
| II | 84 (62%) | 101 (16%) | <0.001 |
| III | 49 (36%) | ||
| IV | 3 (2%) | ||
| Body mass index (mean ± SD) | 29.90 ± 4.58 | 29.04 ± 4.75 | 0.058 |
| Obstructive sleep apnoea syndrome, n (%) | 25 (18.52%) | 47 (7.52%) | <0.001 |
| QRS width (ms) | 105.24 ± 23.44 | 97.12 ± 18.15 | <0.001 |
| Low‐voltage zone % on voltage map (mean ± SD) | 11.28 ± 19.12 | 9.33 ± 16.67 | 0.320 |
| Biomarkers | N = 151 | N = 151 | |
| FABP4 peripheral blood (mean ± SD) | 22.40 ± 16.71 | 22.19 ± 18.01 | 0.936 |
| Galectin‐3 peripheral blood (mean ± SD) | 9.82 ± 6.39 | 10.62 ± 6.88 | 0.501 |
| sRAGE peripheral blood (mean ± SD) | 1220.22 ± 730.50 | 1697.59 ± 1506.424 | 0.199 |
| FABP4 left atrium (mean ± SD) | 20.24 ± 15.75 | 20.44 ± 17.29 | 0.939 |
| Galectin‐3 left atrium (mean ± SD) | 9.34 ± 5.69 | 10.21 ± 6.36 | 0.434 |
| sRAGE left atrium (mean ± SD) | 3089.95 ± 1320.29 | 3832.19 ± 2190.59 | 0.173 |
| Antiarrhythmic drugs | |||
| Pre‐procedural flecainide | 20 (14.93%) | 188 (29.29%) | <0.001 |
| Pre‐procedural amiodarone | 60 (44.78%) | 181 (28.50%) | <0.001 |
| Post‐procedural flecainide | 20 (14.93%) | 219 (34.49%) | <0.001 |
| Post‐procedural amiodarone | 50 (37.31%) | 114 (17.95%) | <0.001 |
AF, atrial fibrillation; AiCM, arrhythmia‐induced cardiomyopathy; FABP4, fatty acid‐binding protein 4; NYHA, New York Heart Association; SR, sinus rhythm; sRAGE, soluble receptor for advanced glycation end products.
In the non‐AiCM group, there were 25 patients with concomitant ischaemic heart disease, 4 patients with ostium secundum‐type atrial septal defect, and 30 patients with moderate or severe valvular heart disease (VHD), 18 aortic and 12 mitral. Six cases of hypertrophic cardiomyopathy; one patient with amyloidosis, one myocarditis, one sarcoidosis, one arrhythmogenic right ventricular CM and three ascending aortic aneurysms, one of 50 mm and two intervened by Bentall surgery.
In the AiCM cohort, we detected 15 cases of associated ischaemic heart disease, 17 cases of VHD (12 mitral and 5 aortic), 9 cases of dilated CM and 1 case of Becker muscular dystrophy. Both pre‐procedural New York Heart Association (NYHA) functional classification and basal heart rate are shown in Table 1 .
Guideline‐directed medical therapy
Regarding medical treatment, 236 (37.62%) of the non‐AiCM group were on GDMT as compared with 121 (89.50%) in the AiCM group. In the AiCM, 78 (57.72%) were under treatment with aldosterone receptor antagonists, whilst 69 (11%) in the non‐AiCM cohort. Furthermore, 70% of patients were receiving beta‐blockers at the time of the ablation [479 (75.05%) in the non‐AiCM patients and 128 (94.81%) in the AiCM patients]. Most patients were taking oral anticoagulants (direct oral anticoagulants 67.50% vs. vitamin K antagonists 30.10%).
Overall, optimal medical therapy (OMT) was followed in 78.48% of cases. We thoroughly assessed each treatment administered during ablation and identified the reasons for discontinuation of OMT as follows:
Beta‐blockers: five cases (3.36%) experienced bradycardia necessitating withdrawal, and one case had chronic obstructive pulmonary disease (COPD) exacerbation, which led to switching to calcium antagonist.
ACEIs: three cases (2.22%) were discontinued due to exacerbation of chronic renal failure.
MRAs: discontinuation was mainly due to hyperkalaemia, which occurred in five cases (3.36%) of patients.
Clinical and biochemical parameters associated with arrhythmia‐induced cardiomyopathy
Baseline characteristics of each group are shown in Table 1 . In multivariate analysis, persistent AF, active smoker status, chronic kidney disease (CKD), LA volume index (LAVi), and width of the QRS were found as independent predictors of AiCM (Table 2 ).
Table 2.
Univariate and multivariate logistic regression analyses for predicting the presence of AiCM
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% confidence interval | P value | OR | 95% confidence interval | P value | |
| Age | 0.98 | (0.97–1.00) | 0.172 | |||
| Gender (male) | 2.72 | (1.48–4.98) | 0.001 | 2.19 | (1.81–3.74) | 0.034 |
| Persistent AF | 5.83 | (3.15–7.78) | <0.001 | 3.85 | (1.85–6.16) | 0.007 |
| AHT | 1.54 | (1.06–2.24) | 0.024 | 0.86 | (0.47–1.6) | 0.624 |
| DM2 | 1.46 | (0.88–2.40) | 0.139 | |||
| Smokers | 1.67 | (1.23–2.29) | 0.001 | 1.76 | (1.06–3.55) | 0.037 |
| OSAS | 2.79 | (1.65–4.73) | <0.001 | 1.32 | (0.52–3.35) | 0.561 |
| CKD | 2.33 | (1.16–4.72) | 0.018 | 2.10 | (1.10–3.50) | 0.001 |
| LAVi | 1.10 | (1.06–1.14) | <0.001 | 1.08 | (1.03–1.14) | 0.002 |
| QRS width (ms) | 1.02 | (1.01–1.028) | <0.001 | 1.03 | (1.01–1.05) | 0.032 |
| LVZ % on voltage map | 1.00 | (0.99–1.02) | 0.321 | |||
| Biomarkers | ||||||
| FABP4 peripheral blood | 1.00 | (0.98–1.01) | 0.936 | |||
| Galectin‐3 peripheral blood | 0.98 | (0.93–1.04) | 0.501 | |||
| sRAGE peripheral blood | 0.99 | (0.99–1.00) | 0.190 | |||
| FABP4 left atrium | 0.99 | (0.98–1.01) | 0.939 | |||
| Galectin‐3 left atrium | 0.97 | (0.91–1.03) | 0.433 | |||
| sRAGE left atrium | 0.99 | (0.99–1.00) | 0.166 | |||
AF, atrial fibrillation; AHT, arterial hypertension; AiCM, arrhythmia‐induced cardiomyopathy; CKD, chronic kidney disease; DM2, diabetes mellitus 2; FABP4, fatty acid‐binding protein 4; LAVi, left atrial volume index; LVZ, low‐voltage zone; OR, odds ratio; OSAS, obstructive sleep apnoea syndrome; sRAGE, soluble receptor for advanced glycation end products.
We noted that patients who presented with AF on the day of ablation had higher heart rates than those with sinus rhythm in both groups. However, there were no statistically significant differences observed between the group that developed tachycardiomyopathy and the group that did not (see Table 1 ). None of the biomarkers analysed were associated with the presence of AiCM.
Ablation outcomes
The mean duration of the follow‐up was 23.35 ± 18 months (median 22.83 months; IQR 9–36), and 533 patients completed more than 12 months of follow‐up. During this period of time, AF recurred in 220 patients (28.53%) in the overall cohort after the initial ablation. Of those patients with recurrence, 70 (9.35%) underwent a redo procedure.
Arrhythmia‐free survival did not differ between the two groups after the first or last procedure. Following the blanking period, 41 (31.06%) and 173 (27.72%) patients experienced AF recurrence in the AiCM and non‐AiCM groups, respectively (P = 0.440). Results of both univariate and multivariate analyses are presented in Table 3 , and recurrence‐free survival between cohorts is shown in Figure 1 . Adherence to GDMT did not result in a significant difference in the recurrence of AF after ablation (Table 3 ). During the follow‐up period, as major adverse events, we registered one cardiovascular death in each group. In the non‐AiCM group, the incidence of HF requiring hospitalization, stroke, and all‐cause mortality was 0.15% (1 of 659), 0.61% (4 of 659), and 0.15% (1 of 659), respectively. On the other hand, in the AiCM cohort, there were no strokes detected and the incidence of HF hospitalization and all‐cause mortality was 0.30% (4 of 134) and 0.74% (1 of 134), respectively.
Table 3.
Univariate and multivariate Cox regression analyses for predicting the risk of recurrence after catheter ablation
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% confidence interval | P value | HR | 95% confidence interval | P value | |
| Age | 1.01 | (1.00–1.03) | 0.045 | |||
| Female sex | 1.35 | (1.01–1.80) | 0.040 | |||
| AHT | 1.24 | (0.94–1.64) | 0.131 | |||
| DM2 | 0.99 | (0.66–1.55) | 0.992 | |||
| Obesity | 1.02 | (0.77–1.36) | 0.870 | |||
| Persistent AF | 2.10 | (1.50–2.94) | <0.001 | 2.40 | (1.22–4.71) | 0.001 |
| LAVi | 1.00 | (0.99–1.02) | 0.126 | |||
| LVZ % on voltage map | 1.02 | (1.01–1.03) | <0.001 | 1.02 | (1.01–1.03) | 0.002 |
| AiCM | 1.26 | (0.89–1.79) | 0.189 | |||
| Previous admission for HF | 1.30 | (0.87–1.95) | 0.203 | |||
| COPD | 1.76 | (1.02–3.04) | 0.041 | 2.28 | (0.91–5.73) | 0.008 |
| GDMT | 0.85 | (0.32–1.29) | 0.756 | |||
| Biomarkers | ||||||
| FABP4 peripheral blood | 1.00 | (0.99–1.01) | 0.759 | |||
| Galectin‐3 peripheral blood | 1.00 | (0.97–1.03) | 0.816 | |||
| sRAGE peripheral blood | 1.00 | (1.00–1.00) | 0.016 | 1.00 | (0.99–1.00) | 0.838 |
| FABP4 left atrium | 1.00 | (0.99–1.01) | 0.787 | |||
| Galectin‐3 left atrium | 1.01 | (0.98–1.04) | 0.548 | |||
| sRAGE left atrium | 1.00 | (1.00–1.00) | 0.034 | 1.00 | (0.99–1.00) | 0.703 |
AF, atrial fibrillation; AHT, arterial hypertension; AiCM, arrhythmia‐induced cardiomyopathy; COPD, chronic obstructive pulmonary disease; DM2, diabetes mellitus 2; FABP4, fatty acid‐binding protein 4; GDMT, guideline‐directed medical therapy; HR, hazard ratio; LAVi, left atrial volume index; LVZ, low‐voltage zone; sRAGE, soluble receptor for advanced glycation end products.
Figure 1.
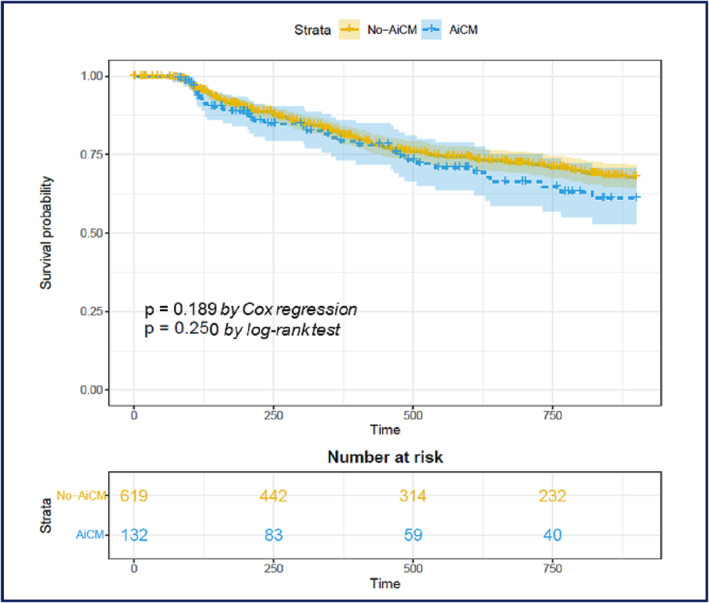
Kaplan–Meier curves showing atrial fibrillation recurrence‐free survival. AiCM, arrhythmia‐induced cardiomyopathy.
Improvement of left ventricular ejection fraction
The median increase in LVEF in patients with AiCM was 19.5% (IQR 12–28%) without global changes in the non‐AiCM group. The median LVEF previous to CA in the AiCM group was 35% (IQR 30–40%) and after the procedure 56% (IQR 51–60%) (Figure 2 ). In multivariate regression analysis, male sex and persistent AF were associated with an LVEF increase of ≥10%, whereas AF recurrence, age, and biomarkers were not (Table 4 ).
Figure 2.
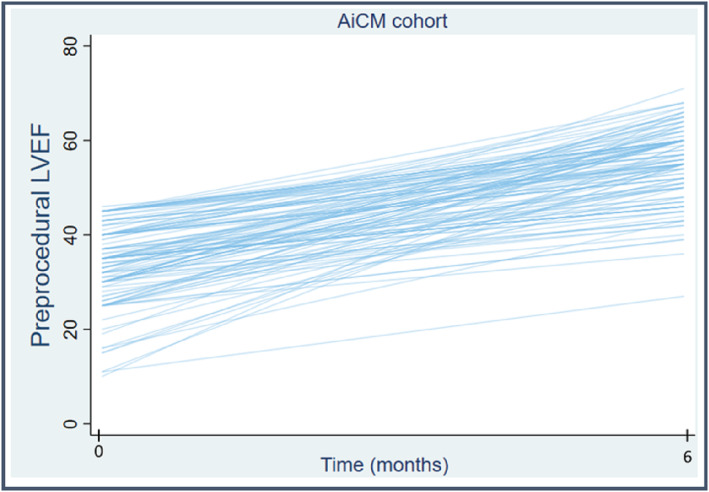
Improvement of left ventricular ejection fraction (LVEF) after catheter ablation in patients with arrhythmia‐induced cardiomyopathy (AiCM).
Table 4.
Whole‐cohort univariate and multivariate logistic regression analyses for predicting a recovery in LVEF of ≥10%
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% confidence interval | P value | OR | 95% confidence interval | P value | |
| Age | 1.01 | (0.99–1.02) | 0.516 | |||
| Male sex | 1.84 | (1.26–2.70) | 0.002 | 3.40 | (1.48–7.79) | 0.004 |
| AHT | 1.41 | (1.02–1.95) | 0.040 | 1.52 | (0.81–2.84) | 0.190 |
| DM2 | 1.38 | (0.89–2.16) | 0.157 | |||
| Obesity | 1.19 | (0.86–1.66) | 0.297 | |||
| CKD | 1.75 | (0.85–3.61) | 0.129 | |||
| Persistent AF | 2.50 | (1.68–3.74) | <0.001 | 3.10 | (1.27–7.55) | 0.013 |
| OSAS | 2.69 | (1.38–3.74) | 0.001 | |||
| LAVi | 1.10 | (1.06–1.14) | <0.001 | |||
| LVZ % on voltage map | 1.00 | (0.99–1.01) | 0.950 | |||
| First recurrence after blanking | 1.12 | (0.78–1.60) | 0.531 | |||
| Biomarkers | ||||||
| FABP4 peripheral blood | 1.00 | (0.98–1.02) | 0.936 | |||
| Galectin‐3 peripheral blood | 0.98 | (0.93–1.03) | 0.497 | |||
| sRAGE peripheral blood | 0.99 | (0.99–1.00) | 0.200 | |||
| FABP4 left atrium | 0.99 | (0.98–1.02) | 0.937 | |||
| Galectin‐3 left atrium | 0.97 | (0.91–1.04) | 0.428 | |||
| sRAGE left atrium | 0.99 | (0.99–1.00) | 0.168 | |||
AF, atrial fibrillation; AHT, arterial hypertension; CKD, chronic kidney disease; DM2, diabetes mellitus 2; FABP4, fatty acid‐binding protein 4; LAVi, left atrial volume index; LVEF, left ventricular ejection fraction; LVZ, low‐voltage zone; OR, odds ratio; OSAS, obstructive sleep apnoea syndrome; sRAGE, soluble receptor for advanced glycation end products.
Figure 3 illustrates an example of a patient with severely depressed LVEF and complete normalization of LV systolic function after ablation.
Figure 3.
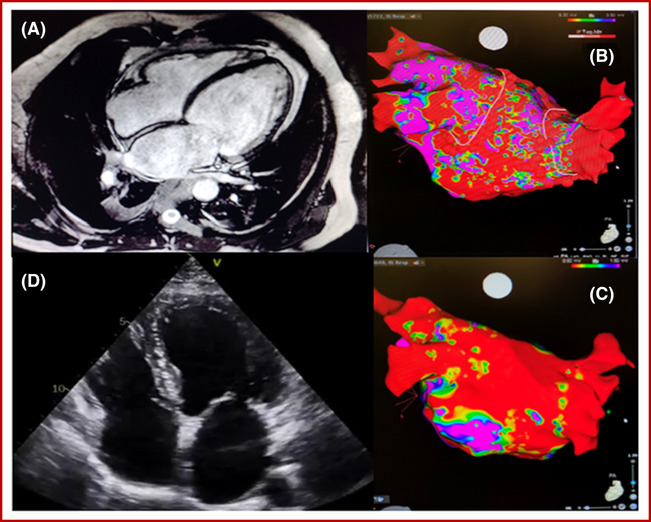
Example of a 60‐year‐old patient who had been admitted for HF with severely depressed left ventricular ejection fraction and experienced complete normalization of left ventricular systolic function after ablation. Despite long‐standing persistent atrial fibrillation and 60% of low‐voltage zone on the bipolar voltage map, he maintained sinus rhythm during 2 years of follow‐up.
Discussion
The present study aims to deepen in the characterization of AiCM patients by performing a clinical and biomarker investigation at baseline along with clinical outcomes after CA (Figure 4 ). Our analysis, performed in a large cohort of patients, confirms that patients with AiCM benefit from AF ablation with significant improvement in LVEF. We also report a similar efficacy of AF ablation in patients with and without AiCM. Contrary to our hypothesis, biomarker analysis (including peripheral Gal‐3, FABP4, and sRAGE levels) did not identify patients with AiCM nor patients at higher risk of recurrence after ablation.
Figure 4.
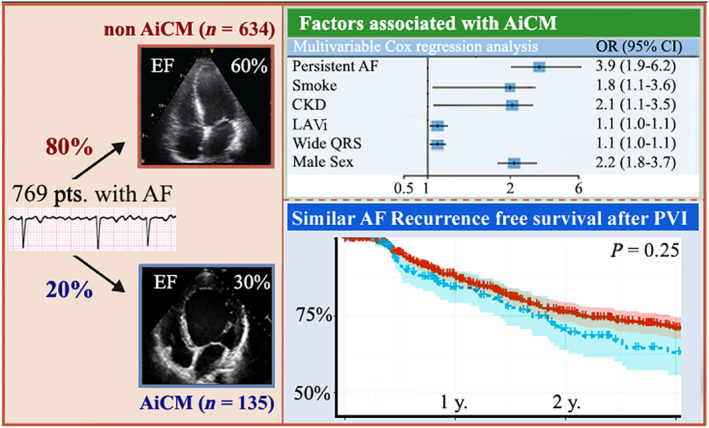
Central illustration. This figure summarizes the key points of our study. It includes the results of our analysis to identify the independent predictors for arrhythmia‐induced cardiomyopathy (AiCM) development. The Kaplan–Meier curves represent that no differences in the rate of recurrence were found during long‐term follow‐up after atrial fibrillation (AF) ablation between both groups. CI, confidence interval; CKD, chronic kidney disease; EF, ejection fraction; LAVi, left atrial volume index; OR, odds ratio; PVI, pulmonary vein isolation.
Our results open the search for other biomarkers of AiCM and reinforce the efficacy of CA as a useful strategy not only in the global population of patients with AF but also in patients with AiCM.
Background
The AF epidemic has been closely linked to a concomitant rise in HF morbidity and mortality. Cohort studies suggest that the estimated incidence of HF among patients with AF is 1.58–4.4 per 100 person‐years. 12 Additionally, development of HF among patients with AF in the Framingham Heart Study was associated with two‐fold to three‐fold increase in mortality. 13 AiCM is an important reversible cause of HF that is likely underdiagnosed in today's clinical practice. Actually, the true prevalence of AiCM is still unknown and is likely underestimated due in part to the challenges in diagnosis. In our study population, it represents almost 18% of the patients referred for ablation. However, despite this growing incidence and recent advances, significant knowledge gaps exist in our understanding of the mechanisms and prognosis of AiCM.
Predictors of arrhythmia‐induced cardiomyopathy
It has been advocated that the development of AiCM may be partially related to the common risk factors between AF and HF, including age, obesity, diabetes mellitus, hypertension, sleep apnoea, or coronary artery disease. 14 In our study population, active smoker status, presence of CKD, persistent AF, QRS width, and LAVi were the clinical variables able to predict the presence of AiCM. Although there is scarce information regarding markers for AiCM, the most established risk factor is the presence of persistent AF. Several studies have reported the association between persistent AF and new‐onset HF. For instance, in the ORBIT‐AF registry, 12 persistent AF predicted new‐onset HF compared with paroxysmal AF. Likely, resting heart rate in AF is probably a poor indicator of overall heart rate. 15 Interestingly, QRS width, a marker of underlying CM that increases the susceptibility of AiCM, resulted as an independent predictor.
This may be in line with the results of the CABANA trial that reported that late gadolinium enhancement (LGE) was present in 36% of patients with persistent AF and idiopathic CM. 16 These factors, QRS width and AF burden, could explain, at least partially, the increase in LAVi. Nevertheless, LAVi was independently associated with AiCM in multivariable analysis, reflecting that some other factors may be involved in LA dilatation. Concerning CKD, there are several mechanisms connecting AF and CKD. As such, elevated levels of inflammatory markers have been reported in the early stages of CKD, which becomes more significant as the disease progresses. 17 Activation of the renin–angiotensin–aldosterone system (RAAS) is another important link between AF and CKD. 18 Further investigations are needed to define first the precise role of CKD in AiCM and, second, to determine whether the inhibition of RAAS activation may have an important role in reducing the progression of AF to manifest AiCM.
Role of biomarkers
We have hypothesized that differences in serum biomarkers between patients with and without AiCM may be present. Our first hypothesis relied on the role of obesity in AiCM, as it is characterized by a systemic pro‐inflammatory state. 19 Proteomic studies have identified a FABP4, also known as adipocyte protein 2 (aP2), as a predictor of metabolic disorders and a new biomarker for AF risk. 20 Accordingly, FABP4 has been reported to contribute to structural heart disease and cardiac contractile dysfunction, explaining the relationship between FABP4 and AF perpetuation. 21
However, in the present study, we did not find significant difference in FABP4 levels nor in leptin concentrations, which could point towards an alternative pathway mechanism involved in the pathophysiology of AiCM different to epicardial adipose tissue. 22 , 23
In addition to inflammation, fibrosis has been shown to induce an arrhythmogenic substrate by inducing new micro re‐entry circuits, electrical heterogeneity, and alterations in atrial refractory periods. 24 Gal‐3 represents a pivotal actor of cardiac fibrosis, is highly expressed in fibrotic tissues, and is up‐regulated in chronic inflammatory and fibrotic conditions in human. It also seems to be an independent predictor of AF recurrence after ablation 25 , 26 and was proposed to serve as therapeutic target for AF treatment. Takemoto et al. 27 reported that Gal‐3 inhibition decreased AF inducibility. Nevertheless, contrary to our hypothesis, there was no association between AiCM and Gal‐3 levels. Although we do not have an explanation for this finding, it needs to be said that Gal‐3 is not a specific of cardiac fibrosis nor distinguish between atrial or ventricular fibrosis.
Finally, advanced glycation end products (AGEs) and its cell receptor RAGE (receptor for AGE) and soluble receptor (sRAGE) are involved in the pathogenesis of AF. In addition, some studies have shown that levels of sRAGE rise as the degree of HF worsens 28 , 29 and that sRAGE levels increase in renal disease. 30 Other studies have reported contradictory findings regarding changes in sRAGE with extent of disease. 31 Relevantly, sRAGE levels may be modulated by drug treatments such as statins, 32 calcium channel blockers, ARBs, thiazolidines, and ACEIs, 33 , 34 , 35 which could have had influence of this result.
In summary, further studies are needed to confirm the role of AGEs in the characterization of patients with AiCM.
Outcomes
There is relative paucity of information regarding the outcome of CA in patients with AiCM with contradictory results. Yamashita et al. 36 reported that the outcome after CA was superior in the AiCM cohort (89% vs. 72%; P = 0.030) with fewer CA procedures as compared with the non‐AiCM cohort. In contrast, Calvo et al. 37 compared the outcome of CA in AiCM vs. non‐AiCM and reported arrhythmia‐free survival rates of 40% and 60% respectively at 2 years, without differences in those with or without AiCM. In the current study, recurrence rate was comparable in the two study groups. A possible explanation may be that AiCM patients have a reversible LV dysfunction and do not represent such a dissimilar population as compared with patients without AiCM. Alternatively, AF in the setting of AiCM may be detected and treated in earlier stage and more aggressively due to the more severe clinical consequence, which could have resulted in an improved clinical outcome after CA comparable with non‐AiCM patients.
Improvement of left ventricular ejection fraction
The mechanisms of LVEF improvement after AF ablation remain unclear. Postulated mechanisms for reverse LV remodelling include improved atrioventricular synchrony, regularization of rhythm‐enhancing haemodynamics, or reversal of tachycardia‐mediated CM. 38 In the setting of AF CA, improvement in LVEF in patients with HF has been widely discussed. 39 , 40 , 41 For instance, the CASTLE‐AF study 42 described a median improvement in LVEF in paroxysmal AF of 7.3% at 60 months and 10.1% in persistent AF. In our cohort, in patients with suspected AiCM, the improvement was even superior [16% (IQR 5.5–27)] without discerning differences based on AF pattern.
Nevertheless, there is scarce information regarding the predictors of LVEF improvement. Ukita et al. 43 found in 401 patients with persistent AF and HFrEF the presence of LV end‐diastolic diameter (LVEDD) <53 mm pre‐ablation as the only marker for improvement.
In our sample, male sex and previous admission for HF were the only identifiable factors associated with LVEF increase. Proposed reasons for this gender effect could be the presence of more atrial fibrosis [13 ± 8 vs. 8 ± 5 (P = 0.018)] and older age [64 ± 5 vs. 58 ± 4 (P < 0.001)] in females as compared with males, findings that are in line with previous studies. 44 , 45
Conclusions
Persistent AF and CKD may play a key role in the development of AiCM. Biomarker analysis including peripheral Gal‐3, FABP4, and sRAGE levels did not differ between groups nor predicted recurrence during long‐term follow‐up. Importantly, patients with AiCM benefit from AF ablation, with a significant improvement in LVEF and without differences in the rate of recurrence as compared with patients without AiCM.
Limitations
The main limitation of our study is that the diagnostic criteria for AiCM have not been well established and standardized. The lack of association with some clinical variables or biomarkers could be due to a lack of statistical power, and potentially, if a larger number of patients were included, the conclusion could have been different. Nonetheless, to the best of our knowledge, it represents the largest study analysing a widespread panel of biomarkers in the subset of patients with AiCM. The study has also the inherent limitations of a non‐randomized study with a limited number of patients in two centres. The population of female patients represents a minority as compared with males (30.81%); however, although the results may not be generalizable to female patients, the percentage of females is similar or superior to previous studies. 36 , 41 We were able to assess NYHA functional classification of patients only at the pre‐procedural stage and not during the follow‐up period, so conclusions in terms of improvement in functional status are lacking. Finally, the outcomes could have potentially differed if the current GDMT including sodium–glucose cotransporter 2 (SGLT2) inhibitors and higher ARNI prescription had been implemented.
Conflict of interest
None declared.
Funding
This study was supported by projects (PI19/01330) integrated in the Plan Estatal de I+D+I 2016–2019 and cofounded by ‘Instituto de Salud Carlos III (ISCIII)—Subdirección General de Evaluación y Fomento de la Investigación del Fondo Europeo de Desarrollo Regional (FEDER)’. S.E. and M.R.‐M. were a recipient of a Sociedade Galega de Cardioloxía (SOGACAR) research grant. CIBERCV is a project from Carlos III Health Institute.
González‐Ferrero, T. , Bergonti, M. , López‐Canoa, J. N. , Arias, F. G.‐R. , Eiras Penas, S. , Spera, F. , González‐Maestro, A. , Minguito‐Carazo, C. , Martínez‐Sande, J. L. , González‐Melchor, L. , García‐Seara, F. J. , Fernández‐López, J. A. , Álvarez‐Castro, E. , González‐Juanatey, J. R. , Heidbuchel, H. , Sarkozy, A. , and Rodríguez‐Mañero, M. (2023) Atrial fibrillation ablation in patients with arrhythmia‐induced cardiomyopathy: a prospective multicentre study. ESC Heart Failure, 10: 3055–3066. 10.1002/ehf2.14448.
References
- 1. Watanabe H, Okamura K, Chinushi M, Chinushi M, Furushima H, Tanabe Y, Kodama M, Aizawa Y. Clinical characteristics, treatment, and outcome of tachycardia induced cardiomyopathy. Int Heart J. 2008; 49: 39–47. [DOI] [PubMed] [Google Scholar]
- 2. Redfield MM, Kay GN, Jenkins LS, Mianulli M, Jensen DN, Ellenbogen KA, APT investigators . Tachycardia‐related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin Proc. 2000; 75: 790–795. [DOI] [PubMed] [Google Scholar]
- 3. Gentlesk PJ, Sauer WH, Gerstenfeld EP, Lin D, Dixit S, PA‐C EZ, Callans D, Marchlinski FE. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2007; 18: 9–14. [DOI] [PubMed] [Google Scholar]
- 4. Clementy N, Piver E, Bisson A, Andre C, Bernard A, Pierre B, Fauchier L, Babuty D. Galectin‐3 in atrial fibrillation: mechanisms and therapeutic implications. Int J Mol Sci. 2018; 19: 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hudson BI, Dong C, Gardener H, Elkind MS, Wright CB, Goldberg R, Sacco RL, Rundek T. Serum levels of soluble receptor for advanced glycation end‐products and metabolic syndrome: the Northern Manhattan Study. Metabolism. 2014; 63: 1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dou HX, Wang T, Su HX, Gao DD, Xu YC, Li YX, Wang HY. Exogenous FABP4 interferes with differentiation, promotes lipolysis and inflammation in adipocytes. Endocrine. 2020; 67: 587–596. [DOI] [PubMed] [Google Scholar]
- 7. Rodríguez‐Mañero M, Valderrábano M, Baluja A, Kreidieh O, Martínez‐Sande JL, García‐Seara J, Saenen J, Iglesias‐Álvarez D, Bories W, Villamayor‐Blanco LM, Pereira‐Vázquez M. Validating left atrial low voltage areas during atrial fibrillation and atrial flutter using multielectrode automated electroanatomic mapping. JACC Clin Electrophysiol. 2018; 4: 1541–1552. [DOI] [PubMed] [Google Scholar]
- 8. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017; 14: e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heidenreich P, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol. 2022; 79: e263–e421. [DOI] [PubMed] [Google Scholar]
- 10. Qin D, Mansour MC, Ruskin JN, Heist EK. Atrial fibrillation‐mediated cardiomyopathy. Circ Arrhythm Electrophysiol. 2019; 12: e007809. [DOI] [PubMed] [Google Scholar]
- 11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022; 24: 4–131. [DOI] [PubMed] [Google Scholar]
- 12. Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, Piccini JP, Fonarow GC, ORBIT‐AF Investigators and Patients . Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail. 2017; 5: 44–52. [DOI] [PubMed] [Google Scholar]
- 13. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016; 133: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016; 68: 2217–2228. [DOI] [PubMed] [Google Scholar]
- 15. Ellis ER, Josephson ME. Heart failure and tachycardia‐induced cardiomyopathy. Curr Heart Fail Rep. 2013; 10: 296–306 PMID: 23963583. [DOI] [PubMed] [Google Scholar]
- 16. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling LH, Kalman JM, Kistler PM. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA‐MRI study. J Am Coll Cardiol. 2017; 70: 1949–1961. [DOI] [PubMed] [Google Scholar]
- 17. Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ, Ball S, Townend JN, Baigent C. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004; 43: 244–253. [DOI] [PubMed] [Google Scholar]
- 18. Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, Warnock DG, Muntner P. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circ Arrhythm Electrophysiol. 2011; 4: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Javed S, Gupta D, Lip GYH. Obesity and atrial fibrillation: making inroads through fat. Eur Heart J Cardiovasc Pharmacother. 2021; 7: 59–67. [DOI] [PubMed] [Google Scholar]
- 20. Furuhashi M, Saitoh S, Shimamoto K, Miura T. Fatty acid‐binding protein 4 (FABP4): pathophysiological insights and potent clinical biomarker of metabolic and cardiovascular diseases. Clin Med Insights Cardiol. 2015; 8: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lamounier‐Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck WH, Ehrhart‐Bornstein M, Bornstein SR, Morano I. Adipocyte fatty acid‐binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res. 2009; 105: 326–334. [DOI] [PubMed] [Google Scholar]
- 22. Lopez‐Canoa JN, Baluja A, Couselo‐Seijas M, Naveira AB, Gonzalez‐Melchor L, Rozados A, Martínez‐Sande L, García‐Seara J, Fernandez‐Lopez XA, Fernandez AL, Gonzalez‐Juanatey JR, Eiras S, Rodriguez‐Mañero M. Plasma FABP4 levels are associated with left atrial fat volume in persistent atrial fibrillation and predict recurrence after catheter ablation. Int J Cardiol. 2019; 292: 131–135. [DOI] [PubMed] [Google Scholar]
- 23. Gan L, Liu Z, Cao W, Zhang Z, Sun C. FABP4 reversed the regulation of leptin on mitochondrial fatty acid oxidation in mice adipocytes. Sci Rep. 2015; 5: 13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen MN, Kiriazis H, Gao XM, Du XJ. Cardiac fibrosis and arrhythmogenesis. Compr Physiol. 2017; 7: 1009–1049. [DOI] [PubMed] [Google Scholar]
- 25. Kornej J, Hindricks G, Shoemaker MB, Husser D, Arya A, Sommer P, Rolf S, Saavedra P, Kanagasundram A, Patrick Whalen S, Montgomery J, Ellis CR, Darbar D, Bollmann A. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol. 2015; 104: 871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu XY, Li SN, Wen SN, Nie JG, Deng WN, Bai R, Liu N, Tang RB, Zhang T, Du X, Dong JZ, Ma CS. Plasma galectin‐3 predicts clinical outcomes after catheter ablation in persistent atrial fibrillation patients without structural heart disease. Europace. 2015; 17: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 27. Takemoto Y, Ramirez RJ, Yokokawa M, Kaur K, Ponce‐Balbuena D, Sinno MC, Willis BC, Ghanbari H, Ennis SR, Guerrero‐Serna G, Henzi BC, Latchamsetty R, Ramos‐Mondragon R, Musa H, Martins RP, Pandit SV, Noujaim SF, Crawford T, Jongnarangsin K, Pelosi F, Bogun F, Chugh A, Berenfeld O, Morady F, Oral H, Jalife J. Galectin‐3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. JACC Basic Transl Sci. 2016; 1: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raposeiras‐Roubín S, Rodiño‐Janeiro BK, Grigorian‐Shamagian L, Moure‐González M, Seoane‐Blanco A, Varela‐Román A, Alvarez E, González‐Juanatey JR. Soluble receptor of advanced glycation end products levels are related to ischaemic aetiology and extent of coronary disease in chronic heart failure patients, independent of advanced glycation end products levels: new roles for soluble RAGE. Eur J Heart Fail. 2010; 12: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 29. Falcone C, Emanuele E, D'Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005; 25: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 30. Kalousová M, Hodková M, Kazderová M, Fialová J, Tesar V, Dusilová‐Sulková S, Zima T. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006; 47: 406–411. [DOI] [PubMed] [Google Scholar]
- 31. Colhoun HM, Betteridge DJ, Durrington P, Hitman G, Neil A, Livingstone S, Charlton‐Menys V, Bao W, Demicco DA, Preston GM, Deshmukh H, Tan K, Fuller JH. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011; 60: 2379–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santilli F, Bucciarelli L, Noto D, Cefalù AB, Davì V, Ferrante E, Pettinella C, Averna MR, Ciabattoni G, Davì G. Decreased plasma soluble RAGE in patients with hypercholesterolemia: effects of statins. Free Radic Biol Med. 2007; 43: 1255–1262 PMID: 17893038. [DOI] [PubMed] [Google Scholar]
- 33. Grossin N, Boulanger E, Wautier MP, Wautier JL. The different isoforms of the receptor for advanced glycation end products are modulated by pharmacological agents. Clin Hemorheol Microcirc. 2010; 45: 143–153. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Koide H, Ueda Y, Takeuchi M, Yamagishi S. Calcium channel blocker inhibition of AGE and RAGE axis limits renal injury in nondiabetic patients with stage I or II chronic kidney disease. Clin Cardiol. 2011; 34: 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lanati N, Emanuele E, Brondino N, Geroldi D. Soluble RAGE‐modulating drugs: state‐of‐the‐art and future perspectives for targeting vascular inflammation. Curr Vasc Pharmacol. 2010; 8: 86–92. [DOI] [PubMed] [Google Scholar]
- 36. Yamashita S, Tokuda M, Matsuo S, Mahida S, Hachisuka EO, Sato H, Ikewaki H, Oseto H, Yokoyama M, Isogai R, Tokutake K, Yokoyama K, Narui R, Kato M, Tanigawa S, Sugimoto K, Yoshimura M, Yamane T. Comparison of atrial arrhythmia recurrence after persistent atrial fibrillation ablation between patients with or without tachycardia‐induced cardiomyopathy. J Cardiovasc Electrophysiol. 2019; 30: 2310–2318. [DOI] [PubMed] [Google Scholar]
- 37. Calvo N, Bisbal F, Guiu E, Ramos P, Nadal M, Tolosana JM, Arbelo E, Berruezo A, Sitges M, Brugada J, Mont L. Impact of atrial fibrillation‐induced tachycardiomyopathy in patients undergoing pulmonary vein isolation. Int J Cardiol. 2013; 168: 4093–4097. [DOI] [PubMed] [Google Scholar]
- 38. Eysenck W, Saba M. Rhythm control in heart failure patients with atrial fibrillation. Arrhythmia Electrophysiol Rev. 2020; 9: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. AlTurki A, Proietti R, Dawas A, Alturki H, Huynh T, Essebag V. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta‐analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019; 19: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ganesan AN, Nandal S, Lüker J, Pathak RK, Mahajan R, Twomey D, Lau DH, Sanders P. Catheter ablation of atrial fibrillation in patients with concomitant left ventricular impairment: a systematic review of efficacy and effect on ejection fraction. Heart Lung Circ. 2015; 24: 270–280. [DOI] [PubMed] [Google Scholar]
- 41. Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, Potenza D, Massaro R, Wazni O, Schweikert R, Saliba W, Wang P, Al‐Ahmad A, Beheiry S, Santarelli P, Starling RC, Dello Russo A, Pelargonio G, Brachmann J, Schibgilla V, Bonso A, Casella M, Raviele A, Haïssaguerre M, Natale A, PABA‐CHF Investigators . Pulmonary‐vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008; 359: 1778–1785. [DOI] [PubMed] [Google Scholar]
- 42. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D, CASTLE‐AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018; 378: 417–427. [DOI] [PubMed] [Google Scholar]
- 43. Ukita K, Egami Y, Nakamura H, Matsuhiro Y, Yasumoto K, Tsuda M, Okamoto N, Tanaka A, Matsunaga‐Lee Y, Yano M, Shutta R, Sakata Y, Nishino M, Tanouchi J. Predictors of improvement of left ventricular systolic function after catheter ablation of persistent atrial fibrillation in patients with heart failure with reduced ejection fraction. Heart Vessels. 2021; 36: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 44. Patel N, Deshmukh A, Thakkar B, Coffey JO, Agnihotri K, Patel A, Ainani N, Nalluri N, Patel N, Patel N, Patel N, Badheka AO, Kowalski M, Hendel R, Viles‐Gonzalez J, Noseworthy PA, Asirvatham S, Lo K, Myerburg RJ, Mitrani RD. Gender, race, and health insurance status in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol. 2016; 117: 1117–1126. [DOI] [PubMed] [Google Scholar]
- 45. Linde C, Bongiorni MG, Birgersdotter‐Green U, Curtis AB, Deisenhofer I, Furokawa T, Gillis AM, Haugaa KH, Lip GYH, Van Gelder I, Malik M, Poole J, Potpara T, Savelieva I, Sarkozy A, ESC Scientific Document Group . Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. 2018; 20: 1565–1565. [DOI] [PubMed] [Google Scholar]


