Abstract
Gabaculine (3-amino-2,3-dihydrobenzoic acid) was an inhibitor of in vivo chlorophyll biosynthesis in lima bean (Phaseolus lunatus L. cv Henderson). When applied to roots of 9-day-old plants, 10 micromolar gabaculine was sufficient to terminate biosynthesis of new chlorophyll. The trifoliolate leaves which emerged after gabaculine treatment were yellow. Gabaculine-treated plants had slightly lower dry weights; yet, overall plant size showed very little change. Chlorophyll fluorescence induction kinetics and CO2 exchange measurements were used to monitor both immediate and long-term effects of gabaculine on photosynthesis. A lowered rate of the decline from the maximum level of fluorescence was observed after 10 hours for nitrate-supplemented plants, and all treated plants showed a slightly increased level of original fluorescence after 6 days. No change was observed in the rate of photosynthesis by unifoliolate leaves. The trifoliolate leaves, though not able to photosynthesize, were able to continue respiration. This suggested that heme biosynthesis for mitochondrial cytochromes was not abolished. In untreated lima bean, root nodules were induced by Rhizobium sp. 127E15. Following gabaculine treatment, root nodules formed, but were largely ineffective in nitrogen fixation. Nodule dry weight, nitrogen fixation activity, and leghemoglobin content were decreased by gabaculine.
Full text
PDF

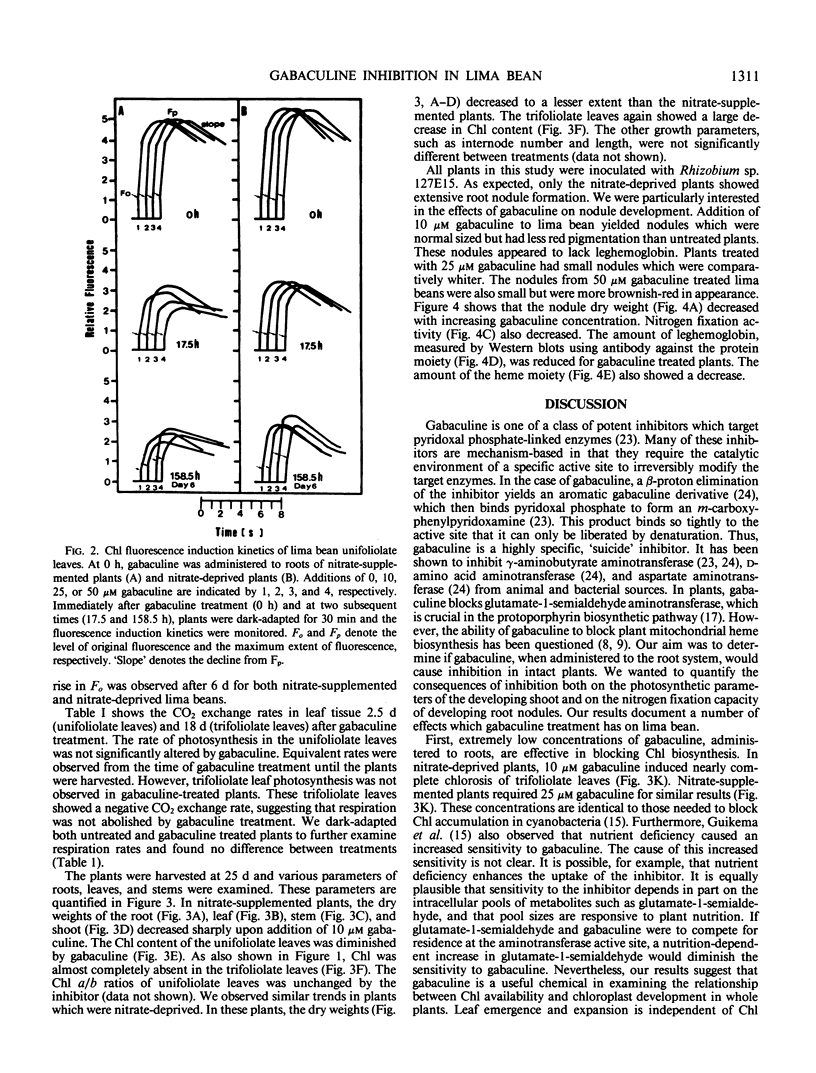
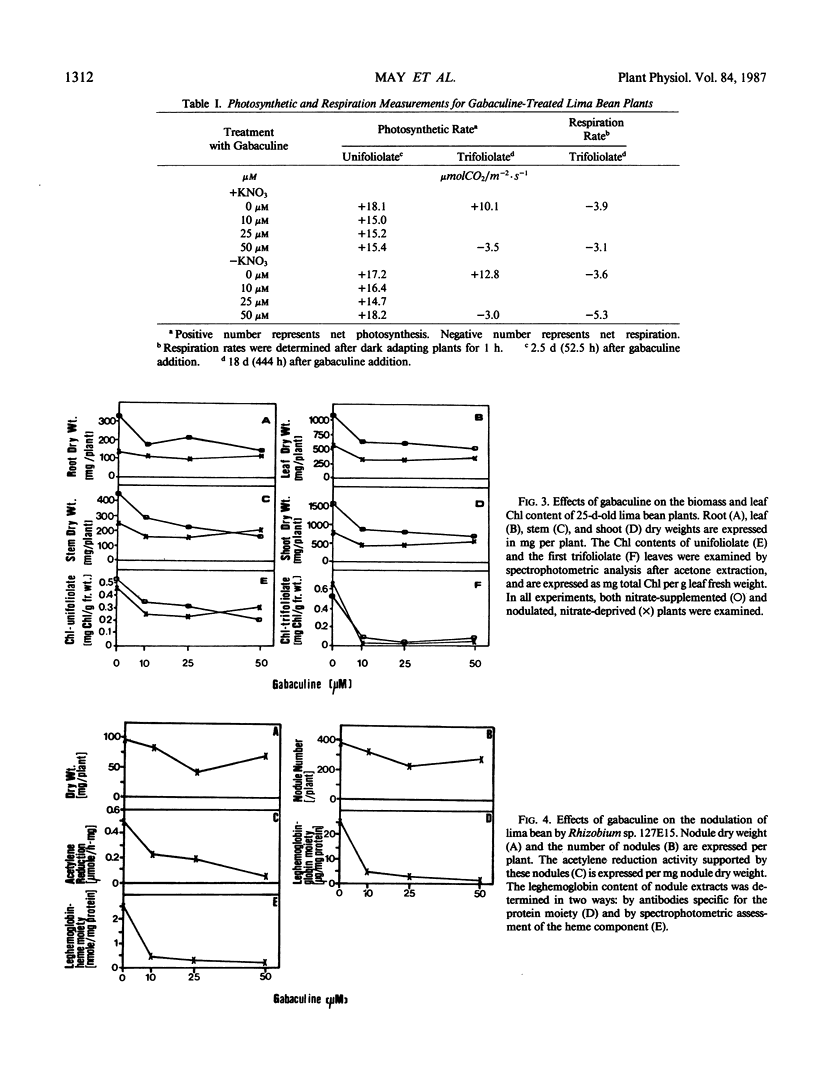

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L., Appleby C. A. Studies of the physiological role of leghaemoglobin in soybean root nodules. Biochim Biophys Acta. 1973 Jan 18;292(1):271–282. doi: 10.1016/0005-2728(73)90271-5. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Gardner G., Gorton H. L. Inhibition of phytochrome synthesis by gabaculine. Plant Physiol. 1985 Mar;77(3):540–543. doi: 10.1104/pp.77.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey C. A., Dilworth M. J. Haem biosynthesis from ( 14 C)- -aminolaevulinic acid in laboratory-grown and root nodule Rhizobium lupini. J Gen Microbiol. 1971 Dec;69(3):385–390. doi: 10.1099/00221287-69-3-385. [DOI] [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- Guerinot M. L., Chelm B. K. Bacterial delta-aminolevulinic acid synthase activity is not essential for leghemoglobin formation in the soybean/Bradyrhizobium japonicum symbiosis. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1837–1841. doi: 10.1073/pnas.83.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Freeman L., Fleming E. H. Effects of Gabaculine on Pigment Biosynthesis in Normal and Nutrient Deficient Cells of Anacystis nidulans. Plant Physiol. 1986 Sep;82(1):280–284. doi: 10.1104/pp.82.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. M., Pearson S. A., Smith A. J., Rogers L. J. Inhibition of chlorophyll synthesis in Hordeum vulgare by 3-amino 2,3-dihydrobenzoic acid (gabaculin). Biosci Rep. 1985 Sep;5(9):775–781. doi: 10.1007/BF01119876. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Manhart J. R., Wong P. P. Nitrate Effect on Nitrogen Fixation (Acetylene Reduction): ACTIVITIES OF LEGUME ROOT NODULES INDUCED BY RHIZOBIA WITH VARIED NITRATE REDUCTASE ACTIVITIES. Plant Physiol. 1980 Mar;65(3):502–505. doi: 10.1104/pp.65.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K. D., Avissar Y. J. Heme Synthesis in Soybean Root Nodules: I. On the Role of Bacteroid delta-Aminolevulinic Acid Synthase and delta-Aminolevulinic Acid Dehydrase in the Synthesis of the Heme of Leghemoglobin. Plant Physiol. 1977 Sep;60(3):433–436. doi: 10.1104/pp.60.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R. J. A rapid spectrophotometric assay for ferrochelatase activity in preparations containing much endogenous hemoglobin and its application to soybean root-nodule preparations. Anal Biochem. 1975 Sep;68(1):289–298. doi: 10.1016/0003-2697(75)90707-1. [DOI] [PubMed] [Google Scholar]
- Soper T. S., Manning J. M. Inactivation of pyridoxal phosphate enzymes by gabaculine. Correlation with enzymic exchange of beta-protons. J Biol Chem. 1982 Dec 10;257(23):13930–13936. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]



