Abstract
Cyclobutanone is a strained motif with broad applications, while direct assembly of the aromatic ring fused cyclobutanones beyond benzocyclobutenone (BCB) skeletons remains challenging. Herein, we report a Rh-catalyzed formal [3+2] annulation of diazo group tethered alkynes involving a 4-exo-dig carbocyclization process, providing a straightforward access to furan-fused cyclobutanones. DFT calculations disclose that, by comparison to the competitive 5-endo-dig process, 4-exo-dig carbocyclization is mainly due to lower angle strain of the key sp-hybridized vinyl cationic transition state in the cyclization step. Using less reactive catalysts Rh2(carboxylate)4 is critical for high selectivity, which is explained as catalyst-substrate hydrogen bonding interaction. This method is proved successful to direct access previously inaccessible and unknown furan-fused cyclobutanone scaffolds, which can participate in a variety of post-functionalization reactions as versatile synthetic blocks. In addition, preliminary antitumor activity study of these products indicates that some molecules exhibite significant anticancer potency against different human cancer cell lines.
Subject terms: Combinatorial libraries, Synthetic chemistry methodology, Reaction mechanisms, Reactive precursors
Aromatic ring fused cyclobutanone is a strained motif with broad applications. Here, the authors report a catalytic 4- exo-dig process, which proved successful to access furan-fused cyclobutanones that can serve as versatile synthetic blocks.
Introduction
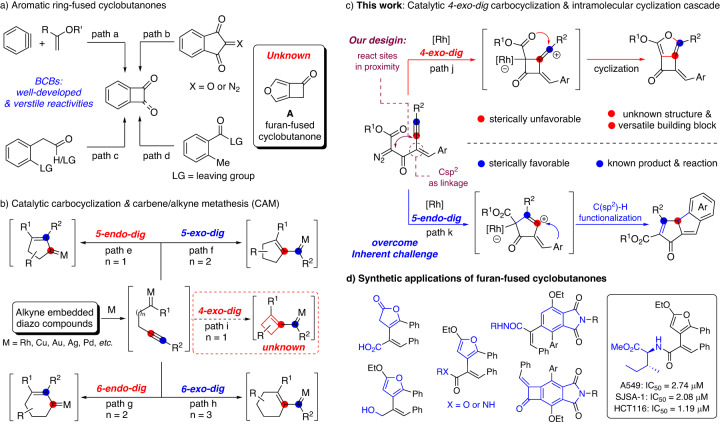
Cyclobutanone is a strained cyclic motif1–4 that is present in many natural products5–8, bioactive molecules9–11, and materials12,13. By virtue of its high tendency to release strain energy, four-membered cyclic ketone derivatives have made possible a myriad of synthetic methods through different catalytic ring-opening transformations1–4,14–16. Among these advances, benzocyclobutenones (BCBs)17,18, which possess higher ring strain than saturated cyclobutanones due to their rigid planar architectures, are versatile building blocks for the transition-metal-mediated C-C bond activation to access diverse skeletons that are nontrivial to synthesize otherwise19,20. Although many efforts have been devoted to the synthesis of BCB skeletons in the past few decades, including [2+2]-cycloadditions21,22, coupling reactions23,24, and few others25,26 (Fig. 1a, path a–d), the construction of cyclobutanones that are fused to nonbenzene aromatics remains an uncharted area in synthetic chemistry. For example, the furan-fused cyclobutanone A (Fig. 1a), which might provide an intriguing opportunity for the discovery of untapped properties and reactivities, is an unknown molecule and has not yet been prepared. Therefore, the development of practical methods for the directly assembly of these unique fused architectures with structural diversity from readily available materials has long been a demanding synthetic challenge.
Fig. 1. Catalytic cycloaddition & cyclization methods.
a Construction of aromatic ring-fused cyclobutanones & unknown structure of furan-fused cyclobutanone. b Catalytic carbocyclization & carbene/alkyne metathesis (CAM). c This study: catalytic 4-exo-dig carbocyclization process for the direct construction of furan-fused cyclobutanones vs competitive 5-endo-dig cyclization. d Synthetic applications of synthesized furan-fused cyclobutanones.
In the past two decades transition-metal complex catalyzed alkyne carbocyclization has emerged as a powerful method in organic synthesis for the direct construction of carbocyclic and heterocyclic structures from different functionalized alkyne precursors27–36. The carbene/alkyne metathesis (CAM)37,38 cascade reaction is one of the elegant cyclization protocols for the straightforward preparation of 5- and 6-membered carbocyclic structures through different types of exo- or endo-dig cyclizations (Fig. 1b, path e–h)39–52. However, only limited examples have been disclosed for the formation of small ring systems53–55, despite its potent capability in synthetic applications. The 4-exo-dig cyclization process is certainly the least-explored one, and no successful example has been disclosed thus far (Fig. 1b, path i), which might be due to the competitive 5-endo-dig pathway that was proposed to be more sterically favorable.
Inspired by these encouraging works and continuing our interests in catalytic alkyne functionalization38,56–58, we were curious about the feasibility of enabling an uncharted catalytic 4-exo-dig cyclization process for the direct construction of four-membered structures. To the best of our knowledge, the catalytic 4-exo-dig carbocyclization of alkynes for the construction of cyclobutanones has not been reported, although rare examples via radical type cyclization53 or coupling reactions54,55 have been disclosed for the synthesis of four-membered analogous.
Herein, we present our recent results in this direction, a Rh-catalyzed formal [3+2] annulation of diazo group tethered alkynes involving a 4-exo-dig carbocyclization process. This methodology provides direct and convenient access to the synthesis of furo[3,4-b]cyclobutanones via an intramolecular annulation of the in-situ formed zwitterionic intermediates rather than through a CAM process involving vinyl carbene species (Fig. 1c, path j). In our protocol, a vinylic sp2-hybridized carbon has been introduced as the key linkage of the diazo group and the triple bond, instead of using a flexible aliphatic chain, which could facilitate the 4-exo-dig carbocyclization by bringing the reacting centers in close proximity. Thus, one of the serious drawbacks, the inherent propensity of the competitive 5-endo-dig carbocyclization of alkynes towards thermodynamically stable tricyclic products through the endocyclic carbene intermediates, could be overcome (path k). Moreover, density functional theory (DFT) computation elucidates that the favorable 4-exo-dig cyclization is mainly attributed to the relatively lower angle strain of the key sp-hybridized vinyl cationic transition state in the cyclization step. The synthesized cyclobutanone-fused furan derivatives with imbedded furyl, carbonyl, and benzylidene motifs have not been previously reported and are potential versatile synthetic building blocks17,18. This practical method could be successfully applied for the late-stage modification of complex natural products and pharmaceutical molecules, and the synthetic application of these unique compounds has been demonstrated through a variety of ring-opening reactions and cycloadditions for the diversity-oriented-synthesis (Fig. 1d).
Results and discussion
Optimization of the reaction conditions
We began our investigation by pursuing the optimal reaction conditions for the catalytic 4-exo-dig carbocyclization process with enynone 1a, tethered with a diazo moiety by using a vinylic sp2-hybridized carbon linkage, as the model substrate (Table 1). Various metal catalysts were initially examined in dichloromethane (DCM) at 40 °C. Zinc chloride, ZnCl2, was found catalytically inactive in this reaction (entry 1), whereas, Ag(I) and Au(I) catalysts led to the decomposition of the material into a complex mixture (entries 2 and 3). When Cu(I) and Pd(II) complexes were introduced, the 4-exo-dig cyclized product 2a was formed in 15% and 21% yields, respectively (entries 4 and 5). The yields could be further improved by using the dirhodium complex, Rh2(OAc)4, as the catalyst, affording the furan-fused cyclobutanone 2a in 44% yield contaminated with tricyclic product 3a in 20% yield (entry 6). Encouraged by these results, a variety of dirhodium complexes were screened (entries 7–14), and the superior results were obtained with Rh2(OPiv)4, generating 2a in 54% yield (entry 9). Then, different solvents were investigated with Rh2(OPiv)4 as the metal catalyst (entries 15–18), showing that the yields could be enhanced to 81% by conducting the reaction in ethyl acetate (entry 18, EtOAc). Comparable high yields could be obtained with Rh2(OAc)4 as the catalyst under otherwise identical conditions (entry 19, 80% yield). Furthermore, the catalyst loading could be reduced to 1.0 mol% without any deleterious effect on the reaction yield and selectivity (entry 20). Using 4 Å MS as additive and Rh2(OPiv)4 as the catalyst in EtOAc, the best results, in terms of yield and selectivity, were obtained for the formation of 2a (entry 21, 88% NMR yield and 84% isolated yield). In addition, no reaction occurred in the absence of dirhodium catalyst, which indicated that the reaction initiated via the formation of metal carbene species.
Table 1.
Optimization of the reaction conditions
| Entry | Catalyst (x mol%) | Solvent | Yield (%)a | |
|---|---|---|---|---|
| 2a | 3a | |||
| 1b | ZnCl2 (20.0) | DCM | <5 | 6 |
| 2c | AgSbF6 (5.0) | DCM | – | – |
| 3c | JohnPhos(MeCN)AuSbF6 (5.0) | DCM | – | – |
| 4 | Cu(MeCN)4PF6 (5.0) | DCM | 15 | 30 |
| 5 | [PdCl(η3-C3H5)]2 (5.0) | DCM | 21 | <5 |
| 6 | Rh2(OAc)4 (2.0) | DCM | 44 | 20 |
| 7 | Rh2(Oct)4 (2.0) | DCM | 45 | 19 |
| 8 | Rh2(TPA)4 (2.0) | DCM | 41 | 15 |
| 9 | Rh2(OPiv)4 (2.0) | DCM | 54 | 10 |
| 10 | Rh2(esp)2 (2.0) | DCM | 43 | 9 |
| 11 | Rh2(pfb)4 (2.0) | DCM | 20 | 17 |
| 12 | Rh2(TFA)4 (2.0) | DCM | 16 | 14 |
| 13 | Rh2(tfacam)4 (2.0) | DCM | 20 | 65 |
| 14 | Rh2(cap)4 (2.0) | DCM | NR | NR |
| 15 | Rh2(OPiv)4 (2.0) | DCE | 74 | 13 |
| 16 | Rh2(OPiv)4 (2.0) | toluene | 47 | 12 |
| 17 | Rh2(OPiv)4 (2.0) | CH3CN | 71 | 10 |
| 18 | Rh2(OPiv)4 (2.0) | EtOAc | 81 | <5 |
| 19 | Rh2(OAc)4 (2.0) | EtOAc | 80 | <5 |
| 20 | Rh2(OPiv)4 (1.0) | EtOAc | 83 | <5 |
| 21d | Rh2(OPiv)4 (1.0) | EtOAc | 88 (84)e | <5 |
 | ||||
Reaction conditions: to a solution of metal catalyst (x mol%) in 1.0 mL solvent, was added 1a (0.1 mmol, 34.4 mg) in the same solvent (1.0 mL) slowly over 10 min via a syringe at 40 °C under an argon atmosphere, and the reaction mixture was stirred for additional 1.0 h under these conditions.
DCM dichloromethane, DCE dichloroethane, EtOAc ethyl acetate.
aYields were detected by proton NMR of the crude reaction mixture using 4-nitrobenzaldehyde as the internal standard.
bLow conversions (<20%) were observed, and most of the material was recovered.
cThe material 1a was decomposed into a complex mixture.
d100 mg 4 Å MS was added.
eIsolated yield.
Scope of the 4-exo-dig carbocyclization reaction
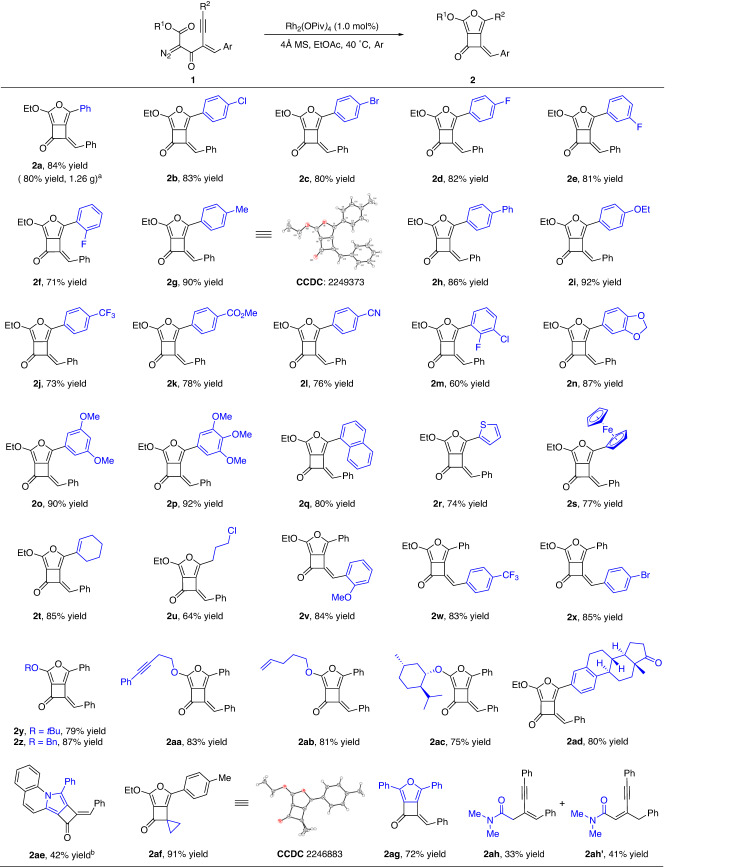
With the optimized reaction conditions in hand, we next examined the generality of this formal [3+2] annulation reaction with different alkyne-tethered diazo compounds. As shown in Fig. 2, different substitutions on the aryl ring attached to the alkyne had little effect on the reaction outcomes, leading to a range of furan-fused cyclobutanones bearing electron-neutral, -rich, or -deficient substituents on the different positions of the aromatic ring in high to excellent yields (2a–2p, 60–92%). Likewise, the 1-naphthyl, 1-thienyl, ferrocenyl, and cyclohexenyl groups substituted enynones underwent the reaction smoothly, delivering products 2q–2t in 74–85% yields. Notably, alkyl halide is also a compatible substituent under these conditions, forming the chlorinated product 2u in 64% yield. The substitution pattern on the styryl moiety was then examined, and the corresponding products were obtained in generally high yields (2v–2x, 83–85%) regardless of electronic or steric effects. The variation of the ester part had a negligible influence on the reactivity, giving the tert-butyl, benzyl, homopropargyl, and terminal alkenyl derivatives 2y–2ab in high yields. Furthermore, D-menthol and estrone derived substrates also worked very well under the optimal conditions, generating the corresponding products 2ac and 2ad 75% and 80% yields, respectively. Terminal alkyne and TMS substituted one did not give the desired product under current conditions, although all the materials were consumed. Notably, the pyrrole-fused cyclobutanone 2ae was formed in 42% yield when [1,2,3]triazolo[1,5-a]quinoline derivative was used as the carbene precursor in the presence of Rh2(esp)2 (1.0 mol%) in DCE at 80 °C (see note b in Fig. 2 for details). We could also replace the styryl moiety with the cyclopropane unit, delivering the product 2af in 91% yield. Rather than the ester substrates, the ketone analogous performed well under current conditions, forming the desired product 2ag in 72% isolated yield. The amide derivative did not form the desired cyclized product, but gave the eneyne products 2ah and 2ah’ in combined high yield, which formed via a Wolff rearrangement, nucleophilic addition with H2O, decarbonation, and isomerization sequence. The structures of products 2g and 2af were confirmed by single-crystal X-ray diffraction analysis. In addition, this reaction could be conducted on a gram scale (5.0 mmol), providing 1.27 g 2a in 80% yield (note a).
Fig. 2. Substrate scope for the synthesis of furan-fused cyclobutanones 2.
Reaction conditions: to a solution of Rh2(OPiv)4 (1.2 mg, 1.0 mol%), and 4 Å MS (100 mg) in EtOAc (1.0 mL), was added a solution of diazo compounds 1 (0.2 mmol) in EtOAc (1.0 mL) slowly over 10 min via a syringe under an argon atmosphere at 40 °C, and the reaction mixture was stirred for additional 1.0 h under these conditions. The yields are isolated yields. aThe reaction was carried out on a 5.0 mmol scale with 0.25 mol% of Rh2(OPiv)4 loading. bThe reaction was carried out in the presence of Rh2(esp)2 (1.0 mol%) in DCE at 80 °C.
Synthetic applications
The furan-fused cyclobutanone products obtained from this reaction were expected to serve as precursors to other versatile and useful synthetic building blocks owing to the rich ring-opening chemistry of this unique structure on the carbonyl group18,59. Thus, we performed a variety of ring-opening transformations of the strained cyclobutanone unit in the product 2a to demonstrate the utility of this method (Fig. 3). For example, addition of 2a with hydroxide ion followed by treatment with HCl gave lactone product 4 in 81% yields. Next, reduction with NaBH4, or addition with Grignard reagent, formed the primary and tertiary alcohols 5a and 5b in 94% and 90% yields, respectively. Furthermore, addition with alcohols directly provided ester products 6a and 6b in high yields. In addition, when trimethylsulfoxonium iodide was used as carbonic nucleophile, the ring-opening product 7 was generated in 50% yield in the presence of NaH; when diazoamide was used as the reagent, which forms the zwitterionic intermediate in situ in the presence of gold catalyst, the ring-opening product 8 was formed in 56% yield.
Fig. 3. Synthetic applications of furan-fused cyclobutanone 2a.
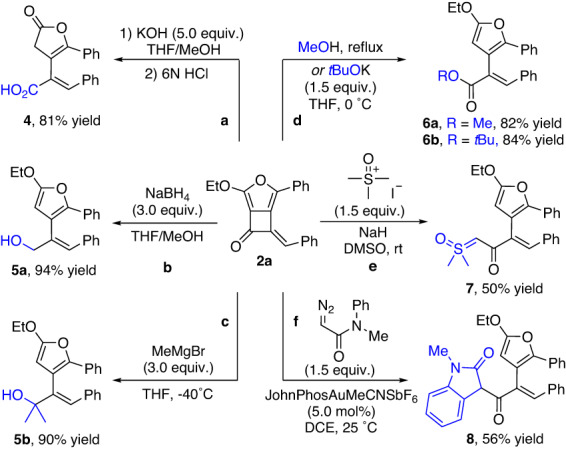
a Synthesis of 4. b Synthesis of 5a. c Synthesis of 5b. d Synthesis of 6a and 6b. e Synthesis of 7. f Synthesis of 8.
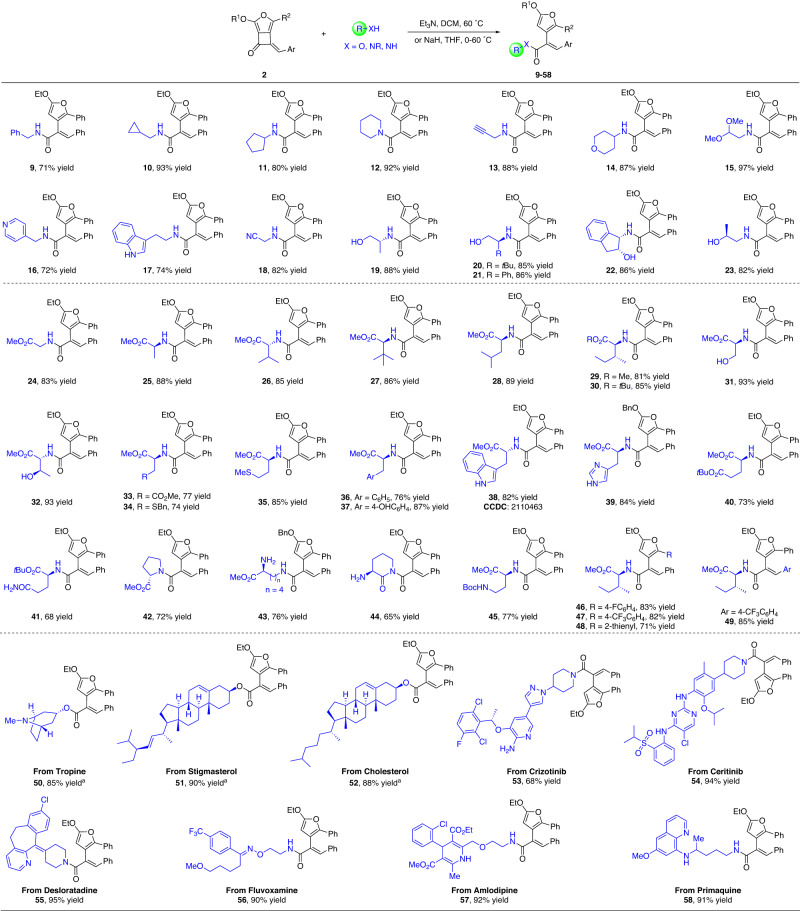
Encouraged by above results, we envisioned that these highly reactive fused cyclobutanone derivatives might be capable of late-stage functionalization (LSF) for different nucleophilic substrates under mild reaction conditions. After brief optimization, an extremely wide range of commercially available amines and alcohols, including those derived from natural products, underwent ring-opening addition to give the amide and ester products60, respectively (Fig. 4). Beyond the regular inert alkyl primary and secondary amines, which led to the amide products 9–12, in 71–92% yields, the mild conditions tolerated a wide range of functional groups, including terminal alkyne (13), ether (14), acetal (15), pyridyl (16), indolyl (17), and cyan (18) groups, forming the corresponding amides in high to excellent yields. Notably, a variety of chiral amino alcohols could be used as nucleophiles, selectively resulting the ring-opened amide products that contained a free hydroxyl group (19–23, in >82% yields). Moreover, a number of natural and synthetic amino acid derived esters were all suitable substrates, producing the furan-containing amino acid derivatives in good to high yields (24–49). In the case with L-ornithine methyl ester, an ester-amide exchange occurred with the ring-opening amide product, leading to valerolactam 44 in 65% yields. The broad scope of this mild ring-opening transformation inspired us to explore the feasibility of the current strategy to the late-stage modification of complex natural products and pharmaceutical molecules. To our delight, tropine, stigmasterol, and cholesterol all proceeded well under modified basic conditions, producing the corresponding ester derivatives (50–52) in >85% yields; meanwhile, the ring-opening reactions with bioactive molecules containing an amino group could be smoothly applied to the current conditions, generating the amide products in 68–95% yields (53–58). The structure of 38 was ambiguously assigned by X-ray crystallography. The successful application of the drugs and sterols mentioned above in this ring-opening reaction demonstrated the potent synthetic value of this method, which might be used as a potential click reaction in bioorthogonal chemistry.
Fig. 4. Synthesis of furan-embedded amide and ester derivatives.
Reaction conditions: 2 (0.1 mmol), amines or hydrochloride of amino acid esters (0.15 mmol, 1.5 equiv.), and Et3N (0.2 mmol, 2.0 equiv.) in DCM (2.0 mL), and stirring for 48 h in an oil bath at 60 °C, unless otherwise noted. a2 (0.1 mmol), alcohols (0.15 mmol, 1.5 equiv.), and NaH (0.15 mmol, 1.5 equiv.) in dry THF (2.0 mL) at 0 °C, then warmed to 60 °C slowly and stirred for 1.0 h. All yields are isolated yields.
Beyond the cyclobutanone unit, the presence of different combinations of functional groups renders these fused structures to be versatile synthetic building blocks. The furan motif, which is an electron-rich aromatic ring, could undergo a Diels-Alder reaction with electron-deficient alkenes followed by aromatization, providing a convenient access to poly-substituted benzocyclobutenones (BCBs), which would be difficult to access otherwise17,18,21–26. After a brief optimization, the tricyclic BCB derivatives 59–62 were obtained in 61–66% yields via an intermolecular Diels-Alder reaction of synthesized cyclobutanones 2 with N-substituted maleimides in a mixed solvent (toluene/DCE = 10/1) at 90 °C for 12 h (Fig. 5). The structure of product 59 was confirmed by X-ray crystallography. Furthermore, these obtained BCBs could also be subjected to the ring-opening transformation. For example, treatment of these products with piperidine in DCM at 60 °C, generated cinnamamide derivatives 63–69 in synthetically useful yields (Fig. 5).
Fig. 5. Synthesis of benzocyclobutenones and poly-substituted benzene derivatives.
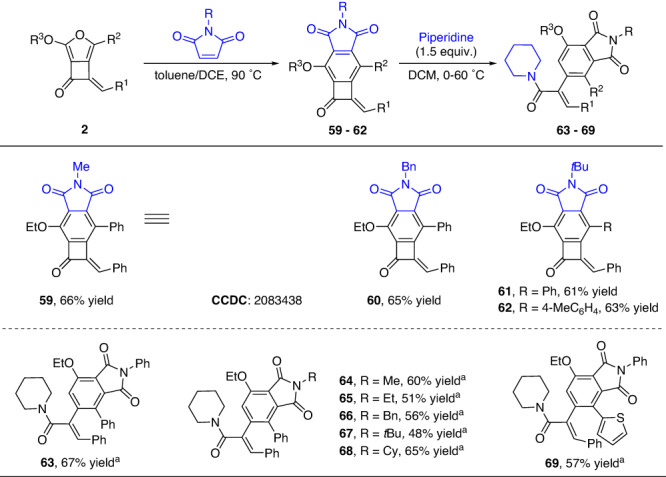
Reaction conditions: 2 (0.1 mmol), and maleimides (0.15 mmol, 1.5 equiv.) in a mixed solvent of toluene/DCE = 10/1 (1.0 mL) at 90 °C for 12 h. All yields are isolated yields. aBenzocyclobutenones (0.1 mmol), and piperidine (0.15 mmol, 1.5 equiv.) in DCM (2.0 mL), and stirring for 24 h in an oil bath at 60 °C.
Anticancer activity evaluation
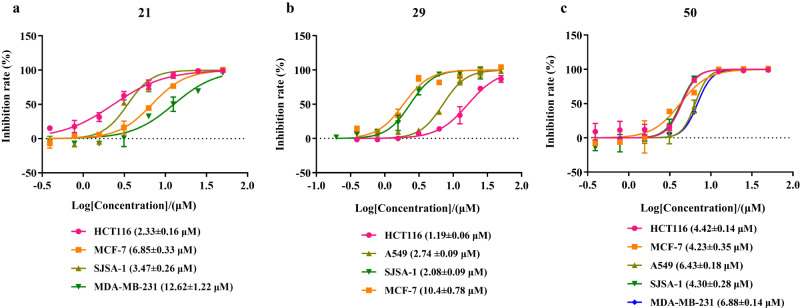
Moreover, we have selected a few of synthesized compounds (6a, 9–12, 14, 15, 18–21, 23–40, 42, 43, 46, 47, 49–55, 63–66, and 68) for the anticancer activity evaluation on cell viability via the CCK8 assay for HCT116 (colon cancer), MCF-7 (breast cancer), A549 (lung adenocarcinoma), SJSA-1 (osteosarcoma cancer), and MDA-MB-231(breast cancer) human cancer cell lines (see Supplementary Tables 1 and 2 in the Supplementary Information for details). The results show that compounds 21 (HCT116 cells, IC50 = 2.33 μM; MCF-7 cells, IC50 = 6.85 μM; SJSA-1 cells, IC50 = 3.47 μM), 29 (HCT116 cells, IC50 = 1.19 μM; A549 cells, IC50 = 2.74 μM; SJSA-1 cells, IC50 = 2.08 μM), and 50 (HCT116 cells, IC50 = 4.42 μM; MCF-7 cells, IC50 = 4.23 μM; A549 cells, IC50 = 6.43 μM; SJSA-1 cells, IC50 = 4.30 μM; MDA-MB-231 cells, IC50 = 6.88 μM) exhibited significant and broad anticancer potency (Fig. 6). A further structure−activity relationship study with various human cancer cell lines is ongoing in the laboratory.
Fig. 6. The cytotoxicity of the synthetic compounds in various human cell lines.
a The IC50 curves of compound 21 on the inhibition of various human tumor cells. b The IC50 curves of compound 29 on the inhibition of various human tumor cells. c The IC50 curves of compound 50 on the inhibition of various human tumor cells.
Mechanistic investigations
Control experiments were conducted to investigate the reaction mechanism of this intriguing process (Fig. 7). Initially, the reaction of 1af in the presence of excess amount of styrene under standard conditions was carried out, which intended to obtain the cyclopropanation product with possible carbene intermediate(s). However, only furan-fused cyclobutanone 2af was formed in 71% yield, and no cyclopropane product was observed by mass spectrometry (MS) analysis of the crude reaction mixture (Fig. 7a), which implies that the intramolecular cascade reaction might be more favorable even if the second carbene species was formed via the CAM process. On the contrary, the corresponding addition product with BnOH was isolated in 31% yield combined with 2af in 40% yield (Fig. 7b), and this cyclobutanone product 70 was confirmed by X-ray crystallography after converting to the ring-opening product 71 (CCDC 2249373, 2246883, 2110463, 2083438, and 2265899 contain the supplementary crystallographic data for 2g, 2v, 38, 59, and 71. These data can be obtained free of charge from the Cambridge crystallographic data centre via www.ccdc.cam.ac.uk/data_request/cif) (Fig. 7c). These results suggested potential carbene or cationic intermediates in this cascade reaction, and the density functional theory (DFT) calculations support the cationic intermediate (see Supplementary Fig. 236 in SI for details).
Fig. 7. Control experiments.
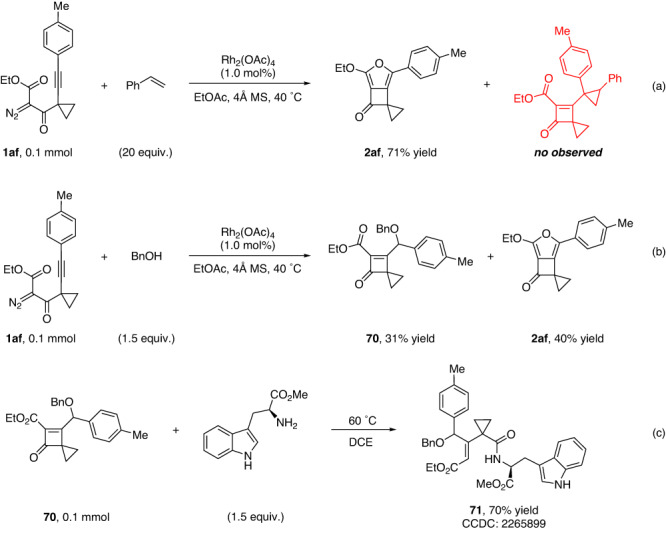
a Control reaction of 1af in the presence of styrene under standard conditions. b Control reaction of 1af in the presence of BnOH under standard conditions. c Ring-opening reaction of 70 with methyl L-tryptophanate.
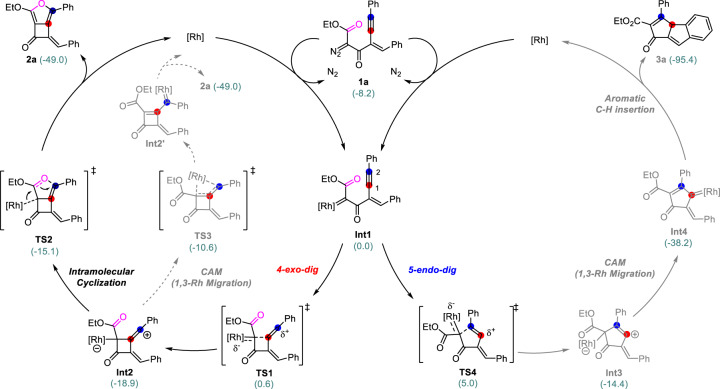
The catalyst effects on the regioselectivity of this reaction was studied through DFT calculations (Supplementary Data 1). To understand the intrinsic selectivity of this reaction, we computed the two competing pathways to 2a and 3a using Rh2(HCOO)4 as the model catalyst and 1a as the substrate, and all the structures were obtained in the gas phase. We proposed the reaction mechanisms (shown in Fig. 8), which have considered our calculation results and previous studies37–51. Initially, the acceptor/acceptor rhodium carbene intermediate Int1 is generated by extrusion of nitrogen from the diazo compound 1a in the presence of dirhodium complex (the overall activation free energy of this step is 16.5 kcal/mol, which should be the rate-determined step of this reaction, see Supplementary Fig. 232 in SI for details). Then Int1 undergoes electrophilic 4-exo-dig cyclization via TS1 to afford the vinyl cationic species Int2, requiring an activation free energy of 0.6 kcal/mol. After that, Int2 undergoes an intramolecular cyclization via TS2 to afford product 2a with an activation free energy of 3.8 kcal/mol. Another pathway to product 2a via carbene/alkyne metathesis (via the 1,3-Rh migration through a singlet transition state TS3 or intersystem crossing (ISC) process is disfavored, because TS3 is higher in energy than TS2 by 4.5 kcal/mol (see Supplementary Figs. 233 and 234 in SI for the full energy surface)44. The competing pathway leading to 3a starts from 5-endo-dig cyclization via TS4, which requires an activation free energy of 5.0 kcal/mol and converts Int1 into the vinyl cation Int3. Then, Int3 gives the final 6-5-5 tricyclic product 3a through a 1,3-Rh migration and an aromatic C-H insertion sequence via an endocyclic rhodium-carbene intermediate Int4 (See Supplementary Information for the full energy surface). As shown in Fig. 8, the first electrophilic cyclization steps via TS1 or TS4 determine the regioselectivity of this reaction, and the formation of 2a is favored over the formation of 3a because TS1 is lower in energy than TS4 by 4.4 kcal/mol.
Fig. 8. The reaction mechanism and DFT calculation for the 4-exo-dig and 5-endo-dig carbocyclization using Rh2(HCOO)4 as catalyst.
The numbers in the parentheses are the free energies in kcal/mol, computed at SMD(EtOAc)/BMK/def2-TZVPP//B3LYP/def2-SVP, all the structures are obtained in the gas phase.
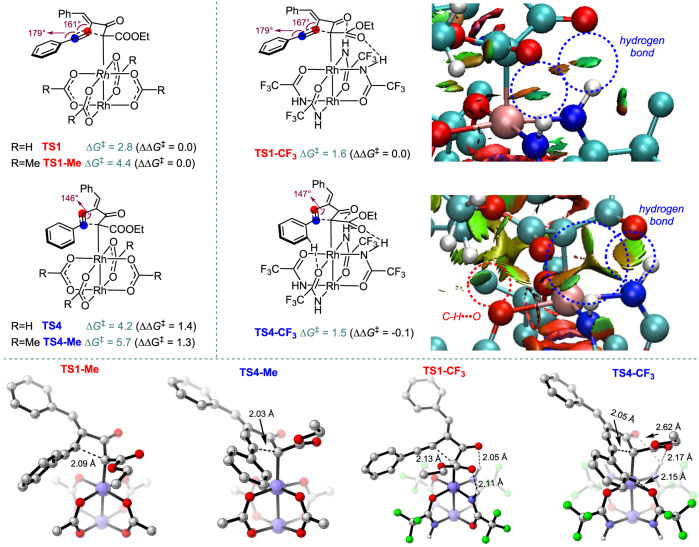
Then the catalyst effects on the regiochemistry were investigated by computing the activation energies of carbene complexes generated from 1a with catalysts of Rh2(HCOO)4, Rh2(OAc)4, and Rh2(tfacam)4, respectively (Fig. 9). Rh2(OAc)4 was chosen because of its comparable regioselectivity in EtOAc (2a:3a > 16:1) with the used Rh2(OPiv)4 (2a:3a > 17.6:1), and much less conformers compared to those from Rh2(OPiv)4, which has 12 rotated methyl group. Catalyst Rh2(tfacam)4 has different ligands and affords inverse selectivity (2a:3a = 1:3.6 in EtOAc), prompting us to investigate the reasons behind this phenomenon. Considering that solvation effects play an important role in affecting the regiochemistry (The axial effect has also been considered but showed insignificant effect on the regioselectivity of this reaction, see Supplementary Fig. 235 in SI for details)61, all the structures were obtained in solution rather than in the gas phase. The DFT calculations predict that, when Rh2(HCOO)4 is used as catalyst, 4-exo-dig cyclization (via TS1 requiring an activation free energy of 2.8 kcal/mol) is favored over the 5-endo-dig cyclization (via TS4 requiring 4.2 kcal/mol) by 1.4 kcal/mol, giving 2a as the major product. While using the real catalyst of Rh2(OAc)4, 4-exo-dig cyclization (via TS1-Me requiring an activation free energy of 4.4 kcal/mol) is favored over the 5-endo-dig cyclization (via TS4-Me requiring 5.6 kcal/mol) by 1.2 kcal/mol, indicating that 2a is the major product and the computed ratio of 2a/3a is 7.4:1. This is consistent with the experimental results, which gave the 4-membered ring structure 2a as the major product. Catalyst Rh2(OAc)4 is less reactive than Rh2(HCOO)4 for both cyclization reactions, which can be understood by considering the electron-donating effect of methyl groups in Rh2(OAc)4, making the carbene center less electrophilic (natural population analysis (NPA) charge of carbene center reduced from +0.063 in Int1 to +0.053 in Int1-Me). Here, 5-endo-dig cyclization forming the five-membered ring for both Rh2(HCOO)4 and Rh2(OAc)4 as catalysts are disfavored by 1.2–1.4 kcal/mol with respect to the 4-exo-dig cyclization forming four-membered ring, which is counter-intuitive, considering that the latter forming a smaller ring is usually disfavored than the former forming a five-membered ring. We attribute this to the formation of a strained vinyl cation62,63 in 5-endo-dig cyclization. The bond angles at the sp-hybridized vinyl cationic center in TS4 and TS4-Me are around 146°, which are far from the required bond angle of 180°.
Fig. 9. The key transition states in the regioselectivity of the real system and the NCI plots.
Free energies are in kcal/mol, computed at SMD(EtOAc)/BMK/def2-TZVPP//SMD(EtOAc)/B3LYP/def2-SVP, all the structures are obtained in solution.
However, when the catalyst Rh2(tfacam)4 is used, DFT calculations show that the activation free energies of both cyclization processes are decreased to only 1.5–1.6 kcal/mol, while using Rh2(HCOO)4 and Rh2(OAc)4 as the catalysts, the activation free energies are 2.8–5.6 kcal/mol. The hydrogen bonding interactions between the catalyst’s amide group and the substrate’s carbonyl group (supported by non-covalent interactions (NCI) analysis64) stabilize the transition states of TS1-CF3 and TS4-CF3, and lower activation free energies are then required. While for catalyst Rh2(HCOO)4 and Rh2(OAc)4, their carboxylate groups cannot form hydrogen bonding with substrate and higher activation free energies are needed. Therefore, the transition states for both 4-exo-dig and 5-endo-dig cyclizations using catalyst Rh2(tfacam)4 are earlier and have less energy difference (here is 0.1 kcal/mol) based on the Hammond postulate65. This is consistent with experimental observation of low regioselectivity. Low active catalysts of Rh2(HCOO)4 and Rh2(OAc)4 then can have later transition states and higher selectivity for 2a vs 3a.
In summary, we have disclosed a dirhodium-catalyzed 4-exo-dig carbocyclization/[3 + 2] annulation cascade reaction of alkyne-tethered diazo compounds, which provides a straightforward approach for the synthesis of structurally novel furan-fused cyclobutanone scaffolds that are nontrivial to construct otherwise. This reaction features a rare example of catalytic 4-exo-dig carbocyclization with high chemo- and regio-selectivity, in spite of many possible competitive pathways. Density functional theory (DFT) computation elucidated that the favorable 4-exo-dig carbocyclization is mainly attributed to the lower angle strain of the key sp-hybridized vinyl cationic transition state in the cyclization step in comparison to the competitive 5-endo-dig process. Calculations revealed that, compared to catalyst Rh2(tfacam)4, catalysts of Rh2(carboxylate)4 are less reactive/more selective for the reaction. The reason is due to the facts that, Rh2(tfacam)4 with amide ligand can form hydrogen bonding with the substrate to stabilize the regio-determining transition states (so the reactions become more reactive and less selective), while catalysts of Rh2(carboxylate)4 with carboxylate ligands, don’t have such stabilizing hydrogen bond interactions with the substrate (less reactive, more selective). The synthesized fused cyclobutanone derivatives, which are prepared with different combinations of functional groups, make possible a variety of ring-opening and cycloaddition reactions as versatile synthetic building blocks for diversity-oriented-synthesis. Further synthetic applications, including but not limited to novel transformations development and bioactive molecule discovery, could be expected in due course.
Methods
General procedure for the synthesis of furan-fused cyclobutanones 2
To a 10-mL oven-dried vial containing a magnetic stirring bar, Rh2(OPiv)4 (1.2 mg, 1.0 mol%), and 4 Å MS (100 mg) in EtOAc (1.0 mL), was added as a solution of diazo compounds 1 (0.2 mmol) in the EtOAc (1.0 mL) slowly via a syringe at 40 °C under argon atmosphere. After addition, the reaction mixture was stirred for additional 1.0 h under these conditions. Until consumption of the material (monitored by TLC), the reaction mixture was purified by column chromatography on silica gel without any additional treatment (Hexanes: EtOAc = 15:1 to 5:1) or recrystallized from MeOH to give the pure products 2.
General procedure for the synthesis of amide derivatives 9–49 and 53–58
To a 10-mL oven-dried vial containing a magnetic stirring bar, cyclobutanones 2 (0.1 mmol), amine or hydrochloride of amino acid ester (0.15 mmol, 1.5 equiv.), Et3N (0.2 mmol, 2.0 equiv.), and DCM (2.0 mL) were added sequentially. The vial was capped, and stirring for 48 h in an oil bath at 60 °C. After completion of the reaction (monitored by TLC), the solvent was evaporated under reduced pressure, and the residue was purified by flash chromatography on silica gel (Hexanes: EtOAc = 10:1 to 1:1) to afford a ring opening amide products.
General procedure for the synthesis of ester products 50–52
To a 10-mL oven-dried vial containing a magnetic stirring bar, and sodium hydride (60% dispersion in mineral oil, 0.2 mmol, 2.0 equiv.) in dry THF (2.0 mL), was added alcohol (0.15 mmol, 1.5 equiv.) dropwise under stirring at 0 °C under a nitrogen atmosphere. After the reaction mixture turned to clear, a solution of cyclobutanone 2 (0.1 mmol) in THF (1.0 mL) was added dropwise at room temperature, then the reaction was stirred at 60 °C for 0.5 h. Saturated aqueous NH4Cl (10 mL) was added to quench the reaction, the organic phase was separated, and the aqueous layer was extracted with EtOAc (3 × 5.0 mL). The combined organic layer was washed with brine, dried over anhydrous MgSO4, and concentrated in vacuo after filtration. The residue was purified by chromatography on silica gel (Hexanes: EtOAc = 30:1) to afford the ester products.
General procedure for the synthesis of benzocyclobutenones 59–62
To a 10-mL oven-dried vial containing a magnetic stirring bar, 2 (0.2 mmol), maleimide (0.3 mmol, 1.5 equiv.), and mixed solvent toluene/DCE (1.0/0.1 mL) were added in sequence, and the reaction mixture was stirred for 12 h at 90 °C. Upon completion, the mixture was cooled to room temperature, and the solvent was removed under reduced pressure. The residue was purified by silica gel chromatography (Hexanes: EtOAc = 10:1 to 5:1) to afford pure benzocyclobutenone products.
General procedure for the synthesis of poly-substituted benzene derivatives 63–69
To a 10-mL oven-dried vial containing a magnetic stirring bar, the above obtained benzene fused cyclobutanone derivative (0.1 mmol), piperidine (0.15 mmol, 1.5 equiv.), and DCM (2.0 mL) were added sequentially. The vial was capped, and stirring for 24 h in an oil bath at 60 °C. After completion of the reaction (monitored by TLC), the solvent was evaporated under reduced pressure, and the residue was purified by flash chromatography on silica gel (Hexanes: EtOAc = 10:1 to 1:1) to afford the ring opening products.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Support for this research from the National Natural Science Foundation of China (21971262), Guangdong Provincial Key R&D Programme (21202107201900002) and Guangdong Basic and Applied Basic Research (2021A1515010384) is greatly acknowledged.
Author contributions
Z.-X.Y. and X.-F.X. conceived and supervised the project, and wrote the manuscript. K.H. and Y.Z. designed the experiments. K.H., H.Y. and S.D. performed the experiments and analyzed the data. Z.-X.Y. and Y.Z. conducted the DFT calculation. Z.Z. and J.H. carried out the anticancer activity evaluation. W.H. supervised the project and revised the manuscript. All authors discussed the results and commented on the article.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Center, under deposition numbers CCDC 2249373 (2g), 2246883 (2af), 2110463 (38), and 2083438 (59), and 2265899 (71). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kemiao Hong, Yi Zhou.
Contributor Information
Zhi-Xiang Yu, Email: yuzx@pku.edu.cn.
Xinfang Xu, Email: xuxinfang@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-42032-9.
References
- 1.Fumagalli G, Stanton S, Bower JF. Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 2017;117:9404–9432. doi: 10.1021/acs.chemrev.6b00599. [DOI] [PubMed] [Google Scholar]
- 2.Mack DJ, Njardarson JT. Recent advances in the metal-catalyzed ring expansions of three- and four-membered rings. ACS Catal. 2013;3:272–286. [Google Scholar]
- 3.Ebner C, Carreira EM. Cyclopropanation strategies in recent total syntheses. Chem. Rev. 2017;117:11651–11679. doi: 10.1021/acs.chemrev.6b00798. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Yu S, Li X. Transition metal-catalysed couplings between arenes and strained or reactive rings: combination of C–H activation and ring scission. Chem. Soc. Rev. 2016;45:6462–6477. doi: 10.1039/c6cs00371k. [DOI] [PubMed] [Google Scholar]
- 5.Beniddir MA, Evanno L, Joseph D, Skiredj A, Poupon E. Emergence of diversity and stereochemical outcomes in the biosynthetic pathways of cyclobutane-centered marine alkaloid dimers. Nat. Prod. Rep. 2016;33:820–842. doi: 10.1039/c5np00159e. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Gao K, Bian M, Ding H. Recent advances in the total synthesis of cyclobutane-containing natural products. Org. Chem. Front. 2020;7:136–154. [Google Scholar]
- 7.Davey SG. Botanical bonanza. Nat. Rev. Chem. 2021;5:222. doi: 10.1038/s41570-021-00271-8. [DOI] [PubMed] [Google Scholar]
- 8.Tanino K, et al. Total synthesis of solanoeclepin A. Nat. Chem. 2011;3:484–488. doi: 10.1038/nchem.1044. [DOI] [PubMed] [Google Scholar]
- 9.Devi P, Rutledge PJ. Cyclobutanone analogues of β-lactam antibiotics: β-lactamase inhibitors with untapped potential? ChemBioChem. 2017;18:338–351. doi: 10.1002/cbic.201600529. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, et al. Biocatalytic reductive amination from discovery to commercial manufacturing applied to abrocitinib JAK1 inhibitor. Nat. Catal. 2021;4:775–782. [Google Scholar]
- 11.Fan Y-Y, Gao X-H, Yue J-M. Attractive natural products with strained cyclopropane and/or cyclobutene ring systems. Sci. China Chem. 2016;59:1126–1141. [Google Scholar]
- 12.Chen Z, et al. The cascade unzipping of ladderane reveals dynamic effects in mechanochemistry. Nat. Chem. 2020;12:302–309. doi: 10.1038/s41557-019-0396-5. [DOI] [PubMed] [Google Scholar]
- 13.Kleinmans R, et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature. 2022;605:477–482. doi: 10.1038/s41586-022-04636-x. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura S, Chu H, Felding J, Baran PS. Nineteen-step total synthesis of (+)-phorbol. Nature. 2016;532:90–93. doi: 10.1038/nature17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLeod MC, et al. Probing chemical space with alkaloid-inspired libraries. Nat. Chem. 2014;6:133–140. doi: 10.1038/nchem.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 2021;13:950–955. doi: 10.1038/s41557-021-00786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores-Gaspar A, Martin R. Recent advances in the synthesis and application of benzocyclobutenones and related compounds. Synthesis. 2013;45:563–580. [Google Scholar]
- 18.Xue Y, Dong G. Deconstructive synthesis of bridged and fused rings via transition metal-catalyzed “cut-and-sew” reactions of benzocyclobutenones and cyclobutanones. Acc. Chem. Res. 2022;55:2341–2354. doi: 10.1021/acs.accounts.2c00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami M, Ishida N. Cleavage of carbon−carbon σ-bonds of four-membered rings. Chem. Rev. 2021;121:264–299. doi: 10.1021/acs.chemrev.0c00569. [DOI] [PubMed] [Google Scholar]
- 20.Sietmann J, Wahl JM. Enantioselective desymmetrization of cyclobutanones: a speedway to molecular complexity. Angew. Chem. Int. Ed. 2020;59:6964–6974. doi: 10.1002/anie.201910767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, et al. Facile access to fused 2D/3D rings via intermolecular cascade dearomative [2+2] cycloaddition/rearrangement reactions of quinolines with alkenes. Nat. Catal. 2022;5:405–413. [Google Scholar]
- 22.Liu F, et al. Merging [2+2] cycloaddition with radical 1,4-addition: metal-free access to functionalized cyclobuta[a]naphthalen-4-ols. Angew. Chem. Int. Ed. 2017;56:15570–15574. doi: 10.1002/anie.201707615. [DOI] [PubMed] [Google Scholar]
- 23.Ye J, et al. Remote C-H alkylation and C-C bond cleavage enabled by an in situ generated palladacycle. Nat. Chem. 2017;9:361–368. doi: 10.1038/nchem.2631. [DOI] [PubMed] [Google Scholar]
- 24.Álvarez-Bercedo P, Flores-Gaspar A, Correa A, Martin R. Pd-Catalyzed intramolecular acylation of aryl bromides via C−H functionalization: a highly efficient synthesis of benzocyclobutenones. J. Am. Chem. Soc. 2010;132:466–467. doi: 10.1021/ja909811t. [DOI] [PubMed] [Google Scholar]
- 25.Hu S, Shima T, Hou Z. Hydrodeoxygenative cyclotetramerization of carbon monoxide by a trinuclear titanium polyhydride complex. J. Am. Chem. Soc. 2020;142:19889–19894. doi: 10.1021/jacs.0c10403. [DOI] [PubMed] [Google Scholar]
- 26.Garlets ZJ, Wertz BD, Liu W, Voight EA, Davies HML. Regio- and stereoselective rhodium(II)-catalyzed C–H functionalization of cyclobutanes. Chem. 2020;6:304–313. doi: 10.1016/j.chempr.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Zang W, Bird MJ, Hyland CJT, Shi M. Gold-catalyzed conversion of highly strained compounds. Chem. Rev. 2021;121:8685–8755. doi: 10.1021/acs.chemrev.0c00624. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Z, Wang Z, Wang Y, Zhang L. Au-Catalysed oxidative cyclisation. Chem. Soc. Rev. 2016;45:4448–4458. doi: 10.1039/c5cs00887e. [DOI] [PubMed] [Google Scholar]
- 29.Chintawar CC, Yadav AK, Kumar A, Sancheti SP, Patil NT. Divergent gold catalysis: unlocking molecular diversity through catalyst control. Chem. Rev. 2021;121:8478–8558. doi: 10.1021/acs.chemrev.0c00903. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y-M, Lackner AD, Toste FD. Development of catalysts and ligands for enantioselective gold catalysis. Acc. Chem. Res. 2014;47:889–901. doi: 10.1021/ar400188g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day DP, Chan PW. Gold-catalyzed cycloisomerizations of 1,n-diyne carbonates and esters. Adv. Synth. Catal. 2016;358:1368–1384. [Google Scholar]
- 32.Ye L-W, et al. Nitrene transfer and carbene transfer in gold catalysis. Chem. Rev. 2021;121:9039–9112. doi: 10.1021/acs.chemrev.0c00348. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Chen K, Zhu S. Transition-metal-catalyzed intramolecular nucleophilic addition of carbonyl groups to alkynes. Chem. 2018;4:1208–1262. [Google Scholar]
- 34.Campeau D, Rayo DFL, Mansour A, Muratov K, Gagosz F. Gold-catalyzed reactions of specially activated alkynes, allenes, and alkenes. Chem. Rev. 2021;121:8756–8867. doi: 10.1021/acs.chemrev.0c00788. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Hashmi ASK. 1,2-Migrations onto gold carbene centers. Chem. Rev. 2021;121:8948–8978. doi: 10.1021/acs.chemrev.0c00811. [DOI] [PubMed] [Google Scholar]
- 36.Mato M, Franchino A, Garcıa-Morales C, Echavarren AM. Gold-catalyzed synthesis of small rings. Chem. Rev. 2021;121:8613–8684. doi: 10.1021/acs.chemrev.0c00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres Ò, Pla-Quintana A. The rich reactivity of transition metal carbenes with alkynes. Tetrahedron Lett. 2016;57:3881–3891. [Google Scholar]
- 38.Pei C, Zhang C, Qian Y, Xu X. Catalytic carbene/alkyne metathesis (CAM): a versatile strategy for alkyne bifunctionalization. Org. Biomol. Chem. 2018;16:8677–8685. doi: 10.1039/c8ob02420k. [DOI] [PubMed] [Google Scholar]
- 39.Witham CA, Mauleón P, Shapiro ND, Sherry BD, Toste FD. Gold(I)-catalyzed oxidative rearrangements. J. Am. Chem. Soc. 2007;129:5838–5839. doi: 10.1021/ja071231+. [DOI] [PubMed] [Google Scholar]
- 40.Liu R, et al. Generation of rhodium(I) carbenes from ynamides and their reactions with alkynes and alkenes. J. Am. Chem. Soc. 2013;135:8201–8204. doi: 10.1021/ja4047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nösel P, et al. 1,6-Carbene transfer: gold-catalyzed oxidative diyne cyclizations. J. Am. Chem. Soc. 2013;135:15662–15666. doi: 10.1021/ja4085385. [DOI] [PubMed] [Google Scholar]
- 42.Le PQ, May JA. Hydrazone-initiated carbene/alkyne cascades to form polycyclic products: ring-fused cyclopropenes as mechanistic intermediates. J. Am. Chem. Soc. 2015;137:12219–12222. doi: 10.1021/jacs.5b08157. [DOI] [PubMed] [Google Scholar]
- 43.Shen W-B, et al. Divergent synthesis of N-heterocycles via controllable cyclization of azido-diynes catalyzed by copper and gold. Nat. Commun. 2017;8:1748. doi: 10.1038/s41467-017-01853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong K, et al. Transient-axial-chirality controlled asymmetric rhodium-carbene C(sp2)-H functionalization for the synthesis of chiral fluorenes. Nat. Commun. 2020;11:2363. doi: 10.1038/s41467-020-16098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang C, et al. Selective vinylogous reactivity of carbene intermediate in gold catalyzed alkyne carbocyclization: synthesis of indenols. ACS Catal. 2019;9:2440–2447. [Google Scholar]
- 46.Rao W, Koh MJ, Li D, Hirao H, Chan PWH. Gold-catalyzed cycloisomerization of 1,6-diyne carbonates and esters to 2,4a-dihydro−1H-fluorenes. J. Am. Chem. Soc. 2013;135:7926–7932. doi: 10.1021/ja4032727. [DOI] [PubMed] [Google Scholar]
- 47.Lauterbach T, et al. Gold-catalyzed carbenoid transfer reactions of diynes-pinacol rearrangement versus retro-buchner reaction. Adv. Synth. Catal. 2015;357:775–781. [Google Scholar]
- 48.Zheng Y, Bao M, Yao R, Qiu L, Xu X. Palladium-catalyzed carbene/alkyne metathesis with enynones as carbene precursors: synthesis of fused polyheterocycles. Chem. Commun. 2018;54:350–353. doi: 10.1039/c7cc08221e. [DOI] [PubMed] [Google Scholar]
- 49.Shu C, et al. Generation of endocyclic vinyl carbene complexes via gold catalyzed oxidative cyclization of terminal diynes: toward naphthoquinones and carbazolequinones. ACS Catal. 2019;9:1019–1025. [Google Scholar]
- 50.Zhang C, et al. Practical and modular construction of benzo[c]phenanthridine compounds. Sci. China Chem. 2022;65:1338–1346. [Google Scholar]
- 51.Zhang C, et al. Gold(I)-catalyzed intramolecular cyclization/intermolecular cycloaddition cascade as a fast track to polycarbocycles and mechanistic insights. Nat. Commun. 2021;12:1182. doi: 10.1038/s41467-021-21335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Díaz-Jiménez À, et al. Intramolecular interception of the remote position of vinylcarbene silver complex intermediates by C(sp3)-H bond insertion. Angew. Chem. Int. Ed. 2023;62:e202215163. doi: 10.1002/anie.202215163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacob C, et al. A general synthesis of azetidines by copper-catalysed photoinduced anti-Baldwin radical cyclization of ynamides. Nat. Commun. 2022;13:560. doi: 10.1038/s41467-022-28098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suffert J, Salem B, Klotz P. Cascade cyclization: carbopalladative cyclization followed by electrocyclic closure as a route to complex polycycles. J. Am. Chem. Soc. 2001;123:12107–12108. doi: 10.1021/ja0170495. [DOI] [PubMed] [Google Scholar]
- 55.Toyofuku M, Fujiwara S, Shin-ike T, Kuniyasu H, Kambe N. Palladium-catalyzed intramolecular selenocarbamoylation of alkynes with carbamoselenoates: formation of α-alkylidene-β-lactam framework. J. Am. Chem. Soc. 2005;127:9706–9707. doi: 10.1021/ja052175k. [DOI] [PubMed] [Google Scholar]
- 56.Bao M, Zhou S, Hu W, Xu X. Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization. Chin. Chem. Lett. 2022;33:4969–4979. [Google Scholar]
- 57.Zhou S, et al. Enantioselective oxidative multi-functionalization of terminal alkynes with nitrones and alcohols for expeditious assembly of chiral α-alkoxy-β-amino-ketones. J. Am. Chem. Soc. 2021;143:14703–14711. doi: 10.1021/jacs.1c06178. [DOI] [PubMed] [Google Scholar]
- 58.Dong G, et al. Asymmetric allylation by chiral organocatalyst-promoted formal hetero-ene reactions of alkylgold intermediates. Angew. Chem. Int. Ed. 2021;60:1992–1999. doi: 10.1002/anie.202012678. [DOI] [PubMed] [Google Scholar]
- 59.Bradley, J, Durst T, Williams AJ. Thermolysis of 2-benzylidenebenzocyclobutenols. J. Org. Chem. 1992;57:6575–6579. [Google Scholar]
- 60.Marichev KO, et al. Chiral donor−acceptor azetines as powerful reactants for synthesis of amino acid derivatives. Nat. Commun. 2019;10:5328. doi: 10.1038/s41467-019-13326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laconsay CJ, Pla-Quintana A, Tantillo DJ. Effects of axial solvent coordination to dirhodium complexes on the reactivity and selectivity in C−H insertion reactions: a computational study. Organometallics. 2021;40:4120–4132. [Google Scholar]
- 62.Gilmore K, Alabugin IV. Rules for ring closure cyclizations of alkynes: revisiting baldwin’s rules for ring closure. Chem. Rev. 2011;111:6513–6556. doi: 10.1021/cr200164y. [DOI] [PubMed] [Google Scholar]
- 63.Alcaide B, Almendros P, Campo TM, Fernández I. Fascinating reactivity in gold catalysis: synthesis of oxetenes through rare 4-exo-dig allene cyclization and infrequent β-hydride elimination. Chem. Commun. 2011;47:9054–9056. doi: 10.1039/c1cc13212a. [DOI] [PubMed] [Google Scholar]
- 64.Johnson ER, et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010;132:6498–6506. doi: 10.1021/ja100936w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammond GS. A correlation of reaction rates. J. Am. Chem. Soc. 1955;77:334–338. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Center, under deposition numbers CCDC 2249373 (2g), 2246883 (2af), 2110463 (38), and 2083438 (59), and 2265899 (71). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.