Abstract
Although the average life span differs between males and females, little is known about differences in clinical features and short and long-term outcomes between elderly male and female gastric cancer patients. This study was designed to clarify these issues to identify the possibility for sex-based treatment strategies in elderly gastric cancer patients. This study included 295 consecutive elderly gastric cancer patients (75 years or older) who underwent curative gastrectomy between 1997 and 2016. We defined postoperative complications as Clavien–Dindo classification grade II or higher. Comorbidities were present in 67% of all patients. Males tended to have more comorbidities than females (P = 0.077). Male patients had significantly more upper gastric cancers (P = 0.001), a higher incidence of postoperative complications (P = 0.045), and poorer prognoses than females (P = 0.003). Multivariate analysis revealed that being male was an independent risk factor for postoperative complications (Odds ratio 2.5, P = 0.045) and a poor prognostic factor (Hazard ratio 1.81, P = 0.008). Patients who underwent limited surgery without postoperative complications tended to have a better prognosis than patients receiving standard surgery with postoperative complications (3-year overall survival: 78% vs. 55%, P = 0.156). Male was an independent risk factor for postoperative complications and an independent poor prognostic factor in elderly gastric cancer patients. To avoid postoperative complications, the limited surgery might be justified for high-risk elderly male patients.
Subject terms: Cancer, Gastroenterology, Risk factors
Introduction
Remarkably, aging societies are increasing worldwide, particularly in developed countries1–3. In Japan, the incidence of elderly persons 65 years or older is 28.9%, which is considerably higher than the average incidence of 8.9% globally. Elderly gastric cancer patients are also rising because of an aging society4. Elderly patients have frailties across multiple organ systems5, which is a risk factor for postoperative complications following gastrectomy6,7. Because several studies have already identified that postoperative complications are a poor prognostic factor for patients with gastric cancer8–11, establishing appropriate treatment strategies for elderly gastric cancer patients is pivotal to improving short and long-term outcomes.
The average life span differs between male and female elderly persons. Specifically, the average life expectancy of males is 6 years shorter than females (male vs. female: 82 vs. 88 years old) in Japan12. However, little is known about differences in clinical features and short and long-term outcomes between males and females following gastrectomy. In this study, we investigated these parameters to clarify these issues.
We hypothesized that the prognosis may be improved by reducing complications in male, which was an independent risk factor for complications. Thus, we proposed that limited surgery could be an effective strategy, especially for patients with comorbidities.
Materials and methods
Patients and procedures
The study was institutionally approved by the Kyoto Prefectural University of Medicine (the approved number from the review board, ERB-C-67-5), and each participant provided written informed consent. All methods were performed based on the Declaration of Helsinki. A total of 291 patients aged 75 years or older who underwent curative gastrectomy at Kyoto Prefectural University of Medicine between 1997 and 2016 were included in the study. The clinical characteristics of the patients are shown in Table 1. In this study, we retrospectively analyzed clinicopathological features and early and long-term outcomes.
Table 1.
Comparison of clinicopathological factors between female and male patients.
| All | Female | Male | P-value | ||||
|---|---|---|---|---|---|---|---|
| n = 295 | n = 114 | n = 181 | |||||
| Age (years) | 0.207 | ||||||
| ≥ 85 | 34 | 12% | 13 | 11% | 21 | 12% | |
| < 85 | 261 | 89% | 101 | 89% | 160 | 88% | |
| BMI (kg/m2) | 0.766 | ||||||
| ≥ 25 | 59 | 20% | 24 | 21% | 35 | 19% | |
| < 25 | 236 | 80% | 90 | 79% | 146 | 81% | |
| Histological type | 0.003 | ||||||
| Undifferentiated | 111 | 38% | 55 | 48% | 56 | 31% | |
| Differentiated | 184 | 62% | 59 | 52% | 125 | 69% | |
| Lymphatic invasion | 0.233 | ||||||
| Positive | 142 | 48% | 60 | 53% | 82 | 45% | |
| Negative | 153 | 52% | 54 | 47% | 99 | 55% | |
| Venous invasions | 0.473 | ||||||
| Positive | 160 | 54% | 65 | 57% | 95 | 53% | |
| Negative | 135 | 46% | 49 | 43% | 86 | 48% | |
| Tumor location | 0.001 | ||||||
| U | 80 | 27% | 19 | 17% | 61 | 34% | |
| M and L | 215 | 73% | 95 | 83% | 120 | 66% | |
| Pathological N status | 0.011 | ||||||
| N0 | 195 | 66% | 83 | 73% | 112 | 62% | |
| N1 | 41 | 14% | 7 | 6% | 34 | 19% | |
| N2 | 30 | 10% | 14 | 12% | 16 | 9% | |
| N3 | 29 | 10% | 10 | 9% | 19 | 1% | |
| Pathological T status | 0.476 | ||||||
| T1 | 149 | 51% | 63 | 55% | 86 | 48% | |
| T2 | 41 | 14% | 20 | 18% | 21 | 12% | |
| T3 | 67 | 23% | 14 | 12% | 53 | 29% | |
| T4 | 38 | 13% | 17 | 15% | 21 | 12% | |
| Tumor size (mm) | 0.358 | ||||||
| ≥ 60 | 86 | 29% | 37 | 33% | 49 | 27% | |
| < 60 | 209 | 71% | 77 | 68% | 132 | 73% | |
| Surgical approach | 0.698 | ||||||
| Open | 204 | 69% | 77 | 68% | 127 | 70% | |
| Laparoscopic | 91 | 31% | 37 | 33% | 54 | 30% | |
| Surgical procedure | 0.027 | ||||||
| Total | 92 | 31% | 25 | 22% | 67 | 37% | |
| Distal | 188 | 64% | 83 | 73% | 105 | 58% | |
| Proximal | 15 | 5% | 6 | 5% | 9 | 5% | |
| Complications | 0.045 | ||||||
| ≥ Grade II | 45 | 15% | 11 | 10% | 34 | 19% | |
| < Grade II | 250 | 85% | 103 | 90% | 147 | 81% | |
| Comorbidities | 0.077 | ||||||
| Positive | 197 | 67% | 69 | 61% | 128 | 71% | |
| Negative | 98 | 33% | 45 | 40% | 53 | 29% | |
The postoperative follow-up program at our institution comprises a regular physical examination, laboratory blood tests, and chest X-rays every 3 or 6 months. Endoscopy and ultrasonography, or computed tomography, were performed annually for the first 5 years, if possible. All enrolled patients underwent pathological or macroscopic curative resection (R0). Histological types were classified as differentiated (papillary adenocarcinoma, or moderately or well-differentiated adenocarcinoma) or undifferentiated (poorly differentiated or undifferentiated adenocarcinoma, signet-ring cell carcinoma, or mucinous adenocarcinoma) based on the 15th edition of the Japanese Classification of Gastric Carcinoma13. We defined patients with postoperative complications as grade II or higher according to the Clavien–Dindo classification system. Additionally, the comorbidities of these patients were classified based on the American Society of Anesthesiologists (ASA) classification. Specifically, we included those with an ASA grade of III and above, necessitating therapeutic interventions.
Definition of standard surgery and limited surgery
We defined standard surgery as D1 or D1 + lymphadenectomy for clinical T1 and N0 tumors and D2 or D2 + lymphadenectomy with more advanced tumors. In D2 dissections, peri-gastric lymph nodes and all second-tier lymph nodes were completely retrieved. Combined resection was defined as resection of the other organ involved in direct tumor invasion or concurrent cancer14,15. On the other hand, we defined limited surgery as surgery with lesser fields of recommended lymphadenectomy according to gastric cancer treatment guidelines, omitting splenectomy.
Statistical analysis
The Chi-squared test and Fisher's exact probability test were performed for categorical variables, and Student's t-test and Mann–Whitney U test for unpaired continuous variables to compare clinicopathological characteristics between comparison groups. Survival curves were estimated using the Kaplan–Meier method, and statistical differences were examined with the log-rank test. Data were stratified for multivariate analysis using backward stepwise Cox regression methods. A P-value < 0.05 was considered significant.
Approval of the research protocol
This study was approved by the institutional review board of the Kyoto Prefectural University of Medicine.
Informed consent
Patients’ data were collected with written informed consent, approved by the Kyoto Prefectural University of Medicine.
Ethics approval
This study was institutionally approved by Kyoto Prefectural University of Medicine.
Results
Clinicopathological features of gastric cancer in male and female patients
Comorbidities were present in 67% of all patients. Frequent comorbidities were hypertension (38%), heart disease (21%), diabetes (14%), and respiratory disease (9%) (Supplementary Table S1). Male patients tended to have more comorbidities than females (P = 0.077). Specifically, heart disease (P = 0.106), cerebral disease (P = 0.161), and renal disease (P = 0.161) tended to be higher in male patients. Table 1 shows that male patients had significantly higher incidences of undifferentiated and upper gastric cancers than female patients (P = 0.003, P = 0.001). The incidence of postoperative complications was also higher in male patients than in female patients (P = 0.045). The most common postoperative complications were anastomotic leakage (P = 0.506), pneumonia (P = 0.161), pancreatic fistula (P = 0.158), and intra-abdominal abscess (P = 1.000) (Supplementary Table S2).
Clinical effect of sex differences and clinicopathological factors on postoperative complications
First, we compared clinicopathological factors between patients with and without postoperative complications. Multivariate analysis using logistic regression revealed that being male [P = 0.040, OR (Odds ratio) 2.15 (95% CI 1.03–4.46)] and having open gastrectomy [P = 0.021, OR 2.73 (95% CI 1.16–6.39)] were independent risk factors for postoperative complications (Table 2).
Table 2.
Univariate and multivariate analyses to detect possible risk factors for postoperative complications.
| ≥ Grade II | < Grade II | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| n = 45 | n = 250 | P-value | OR | 95% CI | P-value | |||
| Gender | 0.045 | 2.15 | 1.03–4.46 | 0.040 | ||||
| Male | 34 | 76% | 147 | 59% | ||||
| Female | 11 | 24% | 103 | 41% | ||||
| Age (years) | 0.799 | |||||||
| ≥ 85 | 4 | 9% | 30 | 12% | ||||
| < 85 | 41 | 91% | 220 | 88% | ||||
| BMI (kg/m2) | 0.229 | |||||||
| ≥ 25 | 12 | 27% | 47 | 19% | ||||
| < 25 | 33 | 73% | 203 | 81% | ||||
| Histological type | 1 | |||||||
| Undifferentiated | 17 | 38% | 94 | 38% | ||||
| Differentiated | 28 | 62% | 156 | 62% | ||||
| Lymphatic invasion | 0.259 | |||||||
| Positive | 18 | 40% | 124 | 50% | ||||
| Negative | 27 | 60% | 126 | 50% | ||||
| Venous invasion | 0.193 | |||||||
| Positive | 20 | 44% | 140 | 56% | ||||
| Negative | 25 | 56% | 110 | 44% | ||||
| Tumor location | 0.1 | |||||||
| U | 17 | 38% | 63 | 25% | ||||
| M and L | 28 | 62% | 187 | 75% | ||||
| Pathological N status | 0.06 | |||||||
| N3 | 8 | 18% | 21 | 8% | ||||
| N0–2 | 37 | 82% | 229 | 92% | ||||
| Pathological T status | 0.145 | |||||||
| T4 | 9 | 20% | 29 | 12% | ||||
| T1–3 | 36 | 80% | 221 | 88% | ||||
| Tumor size (mm) | 0.483 | |||||||
| ≥ 60 | 15 | 33% | 71 | 28% | ||||
| < 60 | 30 | 67% | 179 | 72% | ||||
| Surgical approach | 0.015 | 2.73 | 1.16–6.39 | 0.021 | ||||
| Open | 38 | 84% | 166 | 66% | ||||
| Laparoscopic | 7 | 16% | 84 | 34% | ||||
| Surgical procedure | 0.048 | |||||||
| Total | 23 | 49% | 69 | 28% | ||||
| Distal, proximal | 22 | 51% | 181 | 72% | ||||
| Comorbidities | 1.000 | |||||||
| Positive | 35 | 78% | 162 | 65% | ||||
| Negative | 10 | 22% | 88 | 35% | ||||
| Extent of lymph node | 0.121 | |||||||
| Limited | 18 | 40% | 99 | 40% | ||||
| Standard | 27 | 60% | 151 | 60% | ||||
Prognostic factors of elderly male gastric cancer patients
Next, we investigated the prognostic factors of elderly male gastric cancer patients. In our cohort of 181 elderly male patients with gastric cancer, 36 patients died due to metastasis or recurrence of gastric cancer, 8 patients died from other types of cancer, and 44 patients died from other diseases (Supplementary Table S3). Among these patients, only one patient undergone neoadjuvant chemotherapy (S-1 oral administration). As for adjuvant chemotherapy, only a few patients did receive this treatment: 19 patients were administered S-1 orally, 6 patients received UFT orally, 2 patients were given 5-FU, and 2 other patients received other treatments. Univariate analysis using a log-rank test revealed that elderly male patients had poorer prognoses than female patients after gastrectomy (P = 0.003) (Fig. 1). Multivariate analysis using Cox’s proportional hazards model revealed that being male [P = 0.008, HR (Hazard ratio) 1.81 (95% CI 1.17–2.80)], elderly [P < 0.001, HR 2.79 (95% CI 1.70–4.58)], pN3 [P < 0.001, HR 3.03 (95% CI 1.86–4.95)], pT4 [P = 0.002, HR 2.11 (95% CI 1.32–3.38)], having open gastrectomy [P = 0.004, HR 2.01 (95% CI 1.25–3.23)] and postoperative complications [P < 0.001, HR 2.46 (95% CI 1.60–3.77)] were independent poor prognostic factors (Table 3). Regarding male patients, total gastrectomy was an independent poor prognostic factor [P = 0.036, OR 2.25 (95% CI 1.06–4.78)], determined using logistic regression (Supplementary Table S4).
Figure 1.
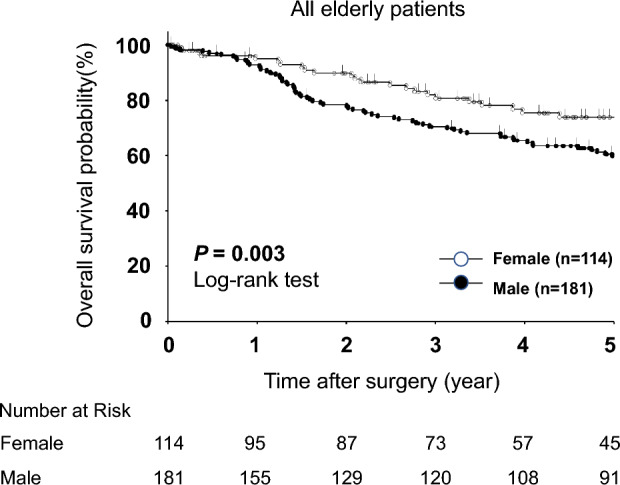
Comparison of overall survival curves between elderly male and female patients. Elderly male patients had poorer prognoses after gastrectomy than female patients (P = 0.003).
Table 3.
Univariate and multivariate analyses of overall survival after surgery in elderly patients.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | ||
| Gender | Male vs. female | 0.003 | 1.81 | 1.17–2.80 | 0.008 |
| Age (years) | ≥ 85 vs. < 85 | 0.002 | 2.79 | 1.70–4.58 | < 0.001 |
| BMI (kg/m2) | ≥ 25 vs. < 25 | 0.765 | |||
| Histological type | Undifferentiated vs. differentiated | 0.469 | |||
| Lymphatic invasion | Positive vs. negative | < 0.001 | |||
| Venous invasion | Positive vs. negative | 0.001 | |||
| Tumor location | U vs. M and L | 0.328 | |||
| Pathological N status | N3 vs. N0–2 | < 0.001 | 3.03 | 1.86–4.95 | < 0.001 |
| Pathological T status | T4 vs. T1–3 | < 0.001 | 2.11 | 1.32–3.38 | 0.002 |
| Tumor size (mm) | ≥ 60 vs. < 60 | 0.004 | |||
| Surgical approach | Open vs. laparoscopic | < 0.001 | 2.01 | 1.25–3.23 | 0.004 |
| Surgical procedure | TG vs. DG, PG | 0.008 | |||
| Complications | ≥ Grade II vs. < Grade II | < 0.001 | 2.46 | 1.60–3.77 | < 0.001 |
| Comorbidities | Present vs. absent | 0.870 | |||
| Extent of lymph node | Limited vs. standard | 0.186 | |||
Prognostic analysis of male gastric cancer patients considering postoperative complications and type of surgery
Finally, we analyzed the prognostic impact of postoperative complications following limited surgery in male patients (standard surgery (T1: n = 50, T2–4: n = 60), limited surgery (T1: n = 36, T2–4: n = 35)). Patients who underwent standard surgery had better prognoses than patients who underwent limited surgery (3-year overall survival: 78% vs. 72%, P = 0.186). However, regarding the efficacy of limited surgery to avoid postoperative complications in male patients, patients who underwent limited surgery without postoperative complications tended to have a better prognosis than patients receiving standard surgery who developed postoperative complications (3-year overall survival: 78% vs. 55%, P = 0.156) (Fig. 2).
Figure 2.
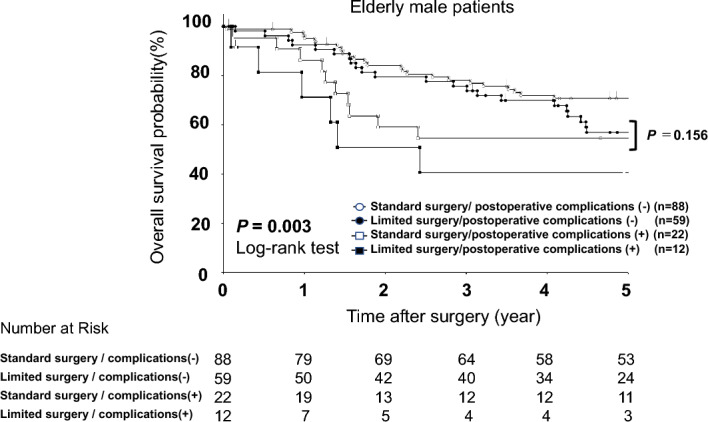
Comparison of overall survival curves with elderly male patients according to the extent of lymphadenectomy and the presence of postoperative complications. Patients who underwent standard surgery had better prognoses than patients who underwent limited surgery (3-year overall survival: 78% vs. 72%, P = 0.186). However, patients who underwent limited surgery without developing postoperative complications tended to have a better prognosis than patients receiving standard surgery with postoperative complications (3-year overall survival: 78% vs. 55%, P = 0.156).
Discussion
Gastric cancer is among the most common causes of cancer-related death worldwide16. Recent advances in diagnostic techniques, minimally invasive surgical methods, and perioperative management have led to the early detection of gastric cancer and declines in mortality and morbidity17–19. However, little is known about differences in clinical features and short and long-term outcomes between male and female elderly gastric cancer patients. In this study, we clearly demonstrated that changes associated with advancing age that differ between males and females affect clinical features, postoperative complications, and long-term outcomes in gastric cancer. Namely, being male was an independent risk factor for postoperative complications and an independent poor prognostic factor in elderly gastric cancer patients. Receiving limited surgery without developing postoperative complications contributed to a better prognosis than standard surgery with postoperative complications in male gastric cancer patients. Our results may provide evidence that changes associated with advancing age that differ between elderly male and female gastric cancer patients affect clinical features, postoperative complications, and long-term outcomes and that sex-based treatment strategies might be needed to improve outcomes.
Several studies have examined the relationship between prognosis and sex in various types of cancer, including gastric cancer. Using a large database20 in Korea, Huafu et al. reported that male gastric cancer patients had worse prognoses than female patients. On the other hand, Kim et al. reported that males had better prognoses than females in young gastric cancer patients because younger males had a lower incidence of signet ring cell carcinoma21,22. In colorectal cancer, Yang et al. conducted a meta-analysis to reveal that males had worse overall survival and cancer-specific survival than females23. Previous studies and several meta-analyses in various solid tumors including gastric cancer suggested that males are tended to have postoperative complications more frequently than females as shown in our results24,25. Whereas Sah et al. indicated that females were more prone to have postoperative complications following gastric cancer surgery26,27. The authors report that females were more prone to serious complications, possibly due to the influence of sex hormones on the overall prognosis. Azzurra et al. suggested the theory that female hormones could maintain the immune tolerance and prevent the excessive inflammatory responses28. Thereby, female hormones might potentially contribute to postoperative recovering and reducing the risk of postoperative complications. We also suggest that the hormonal differences between male and female might affect the aging phenomenon and postoperative complications. These issues are currently under evaluation by investigating the various hormone levels, and we will report details in near future.
Another striking finding in this study was that limited surgery without postoperative complications might contribute to a better prognosis than standard surgery with postoperative complications in elderly male patients. Indeed, 3-year overall survival following limited surgery without postoperative complications was higher than for standard surgery with postoperative complications (78% vs. 55% (P = 0.156)) in elderly male patients. Regarding the extent of lymphadenectomy, various studies have shown the efficacy of D2 gastrectomy to be controversial compared to D1 gastrectomy in elderly gastric cancer patients29–32. However, two recent studies report that standard D2 surgery contributes to a better prognosis compared to limited surgery, even in elderly gastric cancer patients33,34. In real-world data from nationwide general hospitals, patients had more high-risk comorbidities than those in high-volume centers. Therefore, a safer operation strategy might be performing limited surgery to avoid postoperative complications. A recent pivotal study identified that, postoperatively, elderly gastric cancer patients are more likely to die from other diseases rather than from gastric cancer35. Moreover, postoperative complications affect the incidence of death from other diseases, especially respiratory disease36. Therefore, performing limited surgery for high-risk and/or elderly male patients could be a sex-based treatment strategy for elderly gastric cancer patients.
The European Society for Medical Oncology (ESMO) recommends a geriatric assessment to evaluate functional age for treatment in elderly gastric cancer patients as it is a better predictor of treatment response than chronological age37. In the ESMO guideline, limited surgery is already recommended for high-risk patients evaluated using the geriatric assessment. This guideline is very useful considering the aging society globally. On the other hand, a recent Japanese nationwide study proved that the introduction of minimally invasive surgery reduced postoperative complications in high-risk patients, including elderly patients38. Indeed, robotic surgery can reduce postoperative complications39,40. Therefore, minimally invasive surgery with standard lymphadenectomy may reduce postoperative complications and be an alternative strategy in elderly patients41. This issue is also under evaluation at our institute and will be reported in the near future.
A limitation of our study was that the results were retrospectively demonstrated in a small cohort. The long accrual period of this retrospective analysis at a single institute may have incorporated variations in treatment strategies. Therefore, a prospective observational study using several large cohorts or a nationwide clinical database study may be needed to validate our finding that being male is a poor prognostic factor and proposal for a sex-based surgical strategy. Nevertheless, elderly male patients had higher frequencies of comorbidities and postoperative complications. They should be specifically targeted in an effort to improve prognosis by considering comorbidities and performing limited surgery to avoid complications. Also, minimally invasive surgery may be the future strategy for avoiding postoperative complications and improving prognosis. The findings of our research are awaiting confirmation through a prospective trial.
Supplementary Information
Author contributions
H.A. and S.K. designed this study, and H.A., S.K., H.K., K.N., T.O., H.K., A.S., T.K., H.F. and E.O. performed the research and analyzed data, and H.A. and S.K. wrote the paper.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the personal information protection law in Japan but are available after the permission from the institutional review board and the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original PDF version of this Article contained a repeated error where sentences were duplicated. Full information regarding the correction can be found in the correction published with this article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hiroshi Arakawa and Shuhei Komatsu.
Change history
1/23/2024
A Correction to this paper has been published: 10.1038/s41598-024-51810-4
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44465-0.
References
- 1.Kanasi E, Ayilavarapu S, Jones J. The aging population: Demographics and the biology of aging. Periodontol. 2016;2000(72):13–18. doi: 10.1111/prd.12126. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y, Han BG, KoGES Group Cohort profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017;46:1350. doi: 10.1093/ije/dyx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagger C, et al. A comparison of health expectancies over two decades in England: Results of the Cognitive Function and Ageing Study I and II. Lancet. 2016;387:779–786. doi: 10.1016/S0140-6736(15)00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborators GBDSC. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020;5:42–54. doi: 10.1016/S2468-1253(19)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards SJG, Frizelle FA, Geddes JA, Eglinton TW, Hampton MB. Frailty in surgical patients. Int. J. Colorectal Dis. 2018;33:1657–1666. doi: 10.1007/s00384-018-3163-y. [DOI] [PubMed] [Google Scholar]
- 6.Kurita N, et al. Risk model for distal gastrectomy when treating gastric cancer on the basis of data from 33,917 Japanese patients collected using a nationwide web-based data entry system. Ann. Surg. 2015;262:295–303. doi: 10.1097/SLA.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe M, et al. Total gastrectomy risk model: Data from 20,011 Japanese patients in a nationwide internet-based database. Ann. Surg. 2014;260:1034–1039. doi: 10.1097/SLA.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 8.Sierzega M, Kolodziejczyk P, Kulig J, Polish Gastric Cancer Study Group Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br. J. Surg. 2010;97:1035–1042. doi: 10.1002/bjs.7038. [DOI] [PubMed] [Google Scholar]
- 9.Yoo HM, et al. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J. Surg. Oncol. 2011;104:734–740. doi: 10.1002/jso.22045. [DOI] [PubMed] [Google Scholar]
- 10.Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J. Gastroenterol. 2013;19:4060–4065. doi: 10.3748/wjg.v19.i25.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann. Surg. Oncol. 2013;20:1575–1583. doi: 10.1245/s10434-012-2720-9. [DOI] [PubMed] [Google Scholar]
- 12.Ministry of Health Labour and Welfare of Japan. https://www.mhlw.go.jp/english/ (2022).
- 13.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma (October 2017 [The 15th Edition]). https://www.jgca.jp/rule.html (2017). [DOI] [PubMed]
- 14.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 16.Ferlay J, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, et al. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: A meta-analysis. Gastric Cancer. 2016;19:543–552. doi: 10.1007/s10120-015-0500-5. [DOI] [PubMed] [Google Scholar]
- 18.Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu S, Otsuji E. Essential updates 2017/2018: Recent topics in the treatment and research of gastric cancer in Japan. Ann. Gastroenterol. Surg. 2019;3:581–591. doi: 10.1002/ags3.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, et al. Gender differences in gastric cancer survival: 99,922 cases based on the SEER database. J. Gastrointest. Surg. 2020;24:1747–1757. doi: 10.1007/s11605-019-04304-y. [DOI] [PubMed] [Google Scholar]
- 21.Kim HW, et al. Sex disparity in gastric cancer: Female sex is a poor prognostic factor for advanced gastric cancer. Ann. Surg. Oncol. 2016;23:4344–4351. doi: 10.1245/s10434-016-5448-0. [DOI] [PubMed] [Google Scholar]
- 22.Fajkovic H, et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J. Urol. 2011;29:457–463. doi: 10.1007/s00345-011-0709-9. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, et al. Gender differences in colorectal cancer survival: A meta-analysis. Int. J. Cancer. 2017;141:1942–1949. doi: 10.1002/ijc.30827. [DOI] [PubMed] [Google Scholar]
- 24.van Kooten RT, et al. Patient-related prognostic factors for anastomotic leakage, major complications, and short-term mortality following esophagectomy for cancer: A systematic review and meta-analyses. Ann. Surg. Oncol. 2022;29:1358–1373. doi: 10.1245/s10434-021-10734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun R, et al. Gender-related differences in patients with colon cancer resection. Eur. Surg. 2018;50:50–57. doi: 10.1007/s10353-018-0513-5. [DOI] [Google Scholar]
- 26.Sah BK, et al. Does testosterone prevent early postoperative complications after gastrointestinal surgery? World J. Gastroenterol. 2009;15:5604–5609. doi: 10.3748/wjg.15.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sah BK, et al. Post-operative complications of gastric cancer surgery: Female gender at high risk. Eur. J. Cancer Care. 2009;18:202–208. doi: 10.1111/j.1365-2354.2008.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irelli A, Sirufo MM, D'Ugo C, Ginaldi L, De Martinis M. Sex and gender influences on cancer immunotherapy response. Biomedicines. 2020;8:232. doi: 10.3390/biomedicines8070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuschieri A, et al. Patient survival after D1 and D2 resections for gastric cancer: Long-term results of the MRC randomized surgical trial. Br. J. Cancer. 1999;9:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degiuli M, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br. J. Surg. 2014;101:23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 31.Bonenkamp JJ, Ermans JH, Asako MS, Elde CJH. Extended lymph-node dissection for gastric cancer. N. Engl. J. Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 32.Hartgrink HH, et al. Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch gastric cancer group trial. J. Clin. Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Kiyokawa T, et al. Feasibility of gastrectomy with standard lymphadenectomy for patients over 85 years old with gastric cancer. Ann. Surg. Oncol. 2015;22:3962–3969. doi: 10.1245/s10434-015-4489-0. [DOI] [PubMed] [Google Scholar]
- 34.Ko CS, et al. Comparison of standard D2 and limited lymph node dissection in elderly patients with advanced gastric cancer. Ann. Surg. Oncol. 2022;29:5076–5082. doi: 10.1245/s10434-022-11480-w. [DOI] [PubMed] [Google Scholar]
- 35.Nunobe S, et al. Surgical outcomes of elderly patients with Stage I gastric cancer from the nationwide registry of the Japanese Gastric Cancer Association. Gastric Cancer. 2020;23:328–338. doi: 10.1007/s10120-019-01000-3. [DOI] [PubMed] [Google Scholar]
- 36.Kamiya H, et al. Postoperative complications and open gastrectomy affect non-cancer-related death and shorten life expectancy in elderly patients with gastric cancer. Am. J. Cancer Res. 2021;7:5038–5044. [PMC free article] [PubMed] [Google Scholar]
- 37.Bellera CA, et al. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012;23:2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 38.Inokuchi M, et al. Feasibility of laparoscopic gastrectomy for patients with poor physical status: A retrospective cohort study based on a nationwide registry database in Japan. Gastric Cancer. 2019;23:310–318. doi: 10.1007/s10120-019-00993-1. [DOI] [PubMed] [Google Scholar]
- 39.Uyama I, et al. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: A multi-institutional prospective single-arm study. Gastric Cancer. 2019;22:377–385. doi: 10.1007/s10120-018-00906-8. [DOI] [PubMed] [Google Scholar]
- 40.Ojima T, et al. Short-term outcomes of robotic gastrectomy vs laparoscopic gastrectomy for patients with gastric cancer. JAMA Surg. 2021;156:954–963. doi: 10.1001/jamasurg.2021.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joharatnam-Hogan N, Shiu KK, Khan K. Challenges in the treatment of gastric cancer in the older patient. Cancer Treat. Rev. 2020;85:101980. doi: 10.1016/j.ctrv.2020.101980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to the personal information protection law in Japan but are available after the permission from the institutional review board and the corresponding author on reasonable request.


