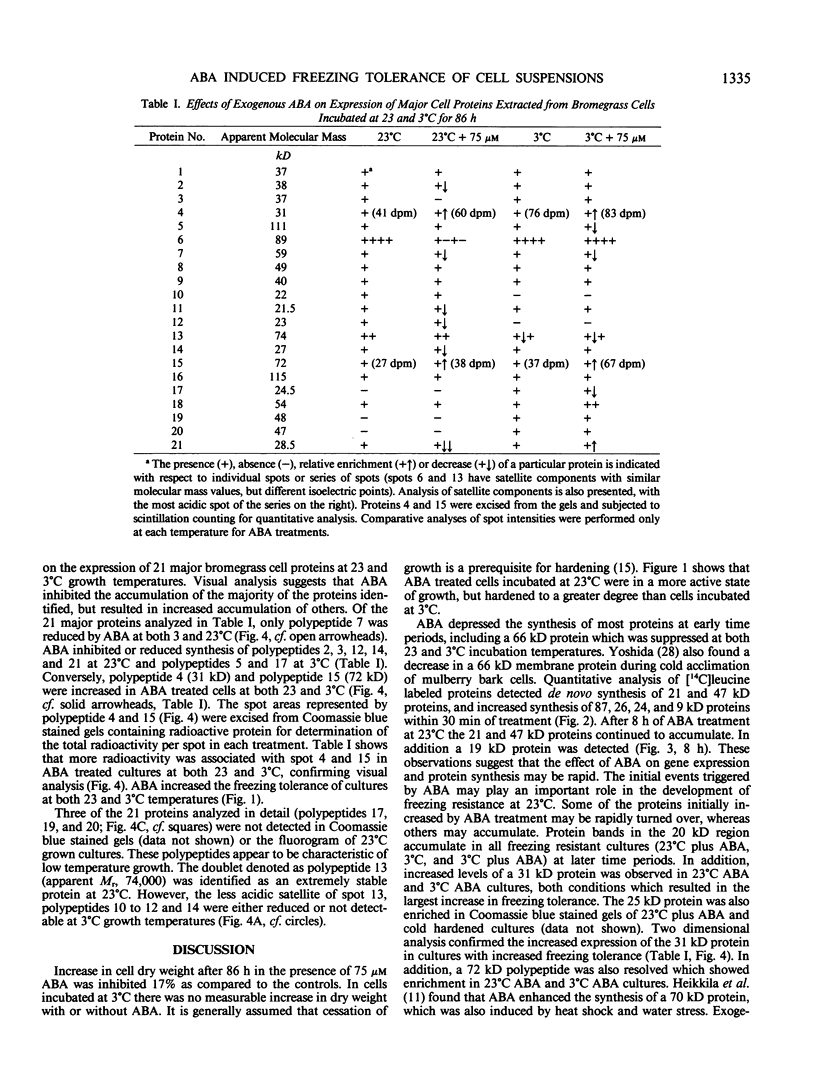
Abstract
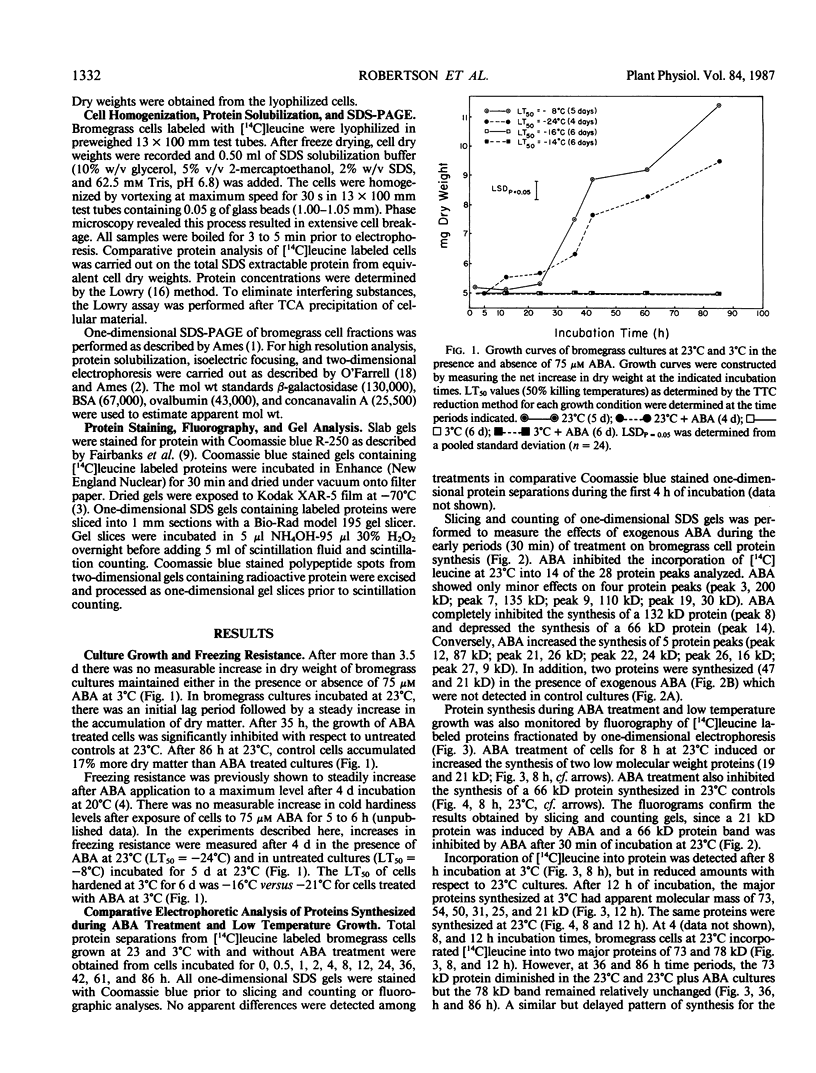
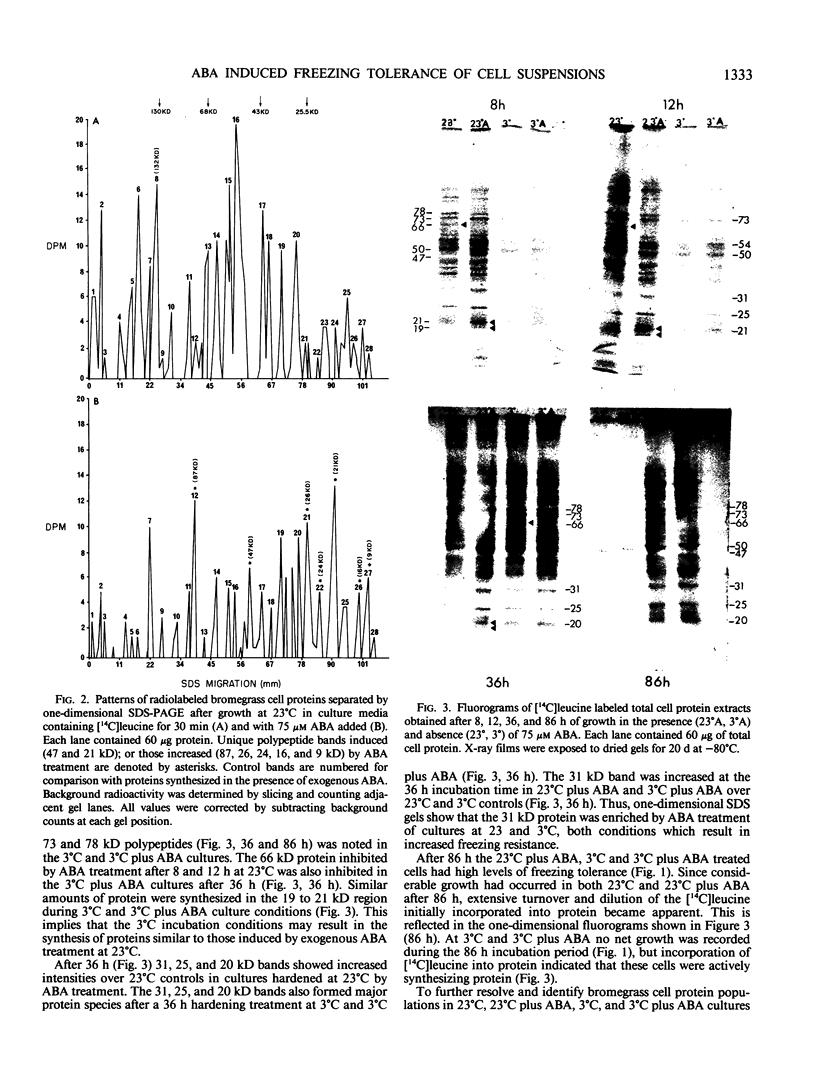
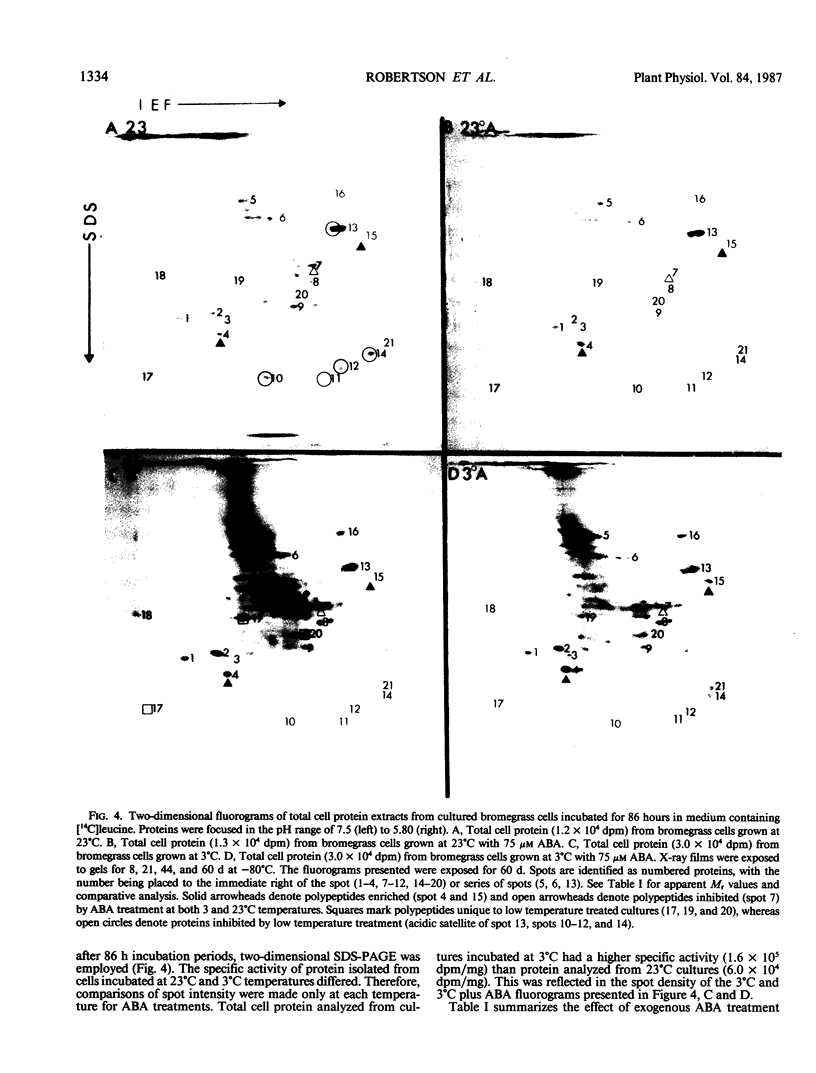
Bromus inermis Leyss cell cultures treated with 75 micromolar abscisic acid (ABA) at both 23 and 3°C developed more freezing resistance than cells cultured at 3°C. Protein synthesis in cells induced to become freezing tolerant by ABA and low temperature was monitored by [14C]leucine incorporation. Protein synthesis continued at 3°C, but net cell growth was stopped. Most of the major proteins detected at 23°C were synthesized at 3°C. However, some proteins were synthesized only at low temperatures, whereas others were inhibited. ABA showed similar effects on protein synthesis at both 23 and 3°C. Comparative electrophoretic analysis of [14C]leucine labeled protein detected the synthesis of 19, 21 and 47 kilodalton proteins in less than 8 hours after exposure to exogenous ABA. Proteins in the 20 kilodalton range were also synthesized at 3°C. In addition, a 31 kilodalton protein band showed increased expression in freezing resistant ABA treated cultures after 36 hours growth at both 3 and 23°C. Quantitative analysis of [14C]leucine labeled polypeptides in two-dimensional gels confirmed the increased expression of the 31 kilodalton protein. Two-dimensional analysis also resolved a 72 kilodalton protein enriched in ABA treated cultures and identified three proteins (24.5, 47, and 48 kilodaltons) induced by low temperature growth.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chen H. H., Li P. H., Brenner M. L. Involvement of abscisic Acid in potato cold acclimation. Plant Physiol. 1983 Feb;71(2):362–365. doi: 10.1104/pp.71.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. H., Gusta L. V. Abscisic Acid-induced freezing resistance in cultured plant cells. Plant Physiol. 1983 Sep;73(1):71–75. doi: 10.1104/pp.73.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier Y. Changes in the Electrophoretic Patterns of the Soluble Proteins of Winter Wheat and Rye following Cold Acclimation and Desiccation Stress. Plant Physiol. 1983 Feb;71(2):400–403. doi: 10.1104/pp.71.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie J., Campbell W. F. Response of Tomato Plants to Stressful Temperatures : INCREASE IN ABSCISIC ACID CONCENTRATIONS. Plant Physiol. 1981 Jan;67(1):26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gerloff E. D., Stahmann M. A., Smith D. Soluble proteins in alfalfa roots as related to cold hardiness. Plant Physiol. 1967 Jul;42(7):895–899. doi: 10.1104/pp.42.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J. J., Papp J. E., Schultz G. A., Bewley J. D. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic Acid, and wounding. Plant Physiol. 1984 Sep;76(1):270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith C. N., McKersie B. D. The Effect of Abscisic Acid on the Freezing Tolerance of Callus Cultures of Lotus corniculatus L. Plant Physiol. 1986 Mar;80(3):766–770. doi: 10.1104/pp.80.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meza-Basso L., Alberdi M., Raynal M., Ferrero-Cadinanos M. L., Delseny M. Changes in Protein Synthesis in Rapeseed (Brassica napus) Seedlings during a Low Temperature Treatment. Plant Physiol. 1986 Nov;82(3):733–738. doi: 10.1104/pp.82.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Triplett B. A., Quatrano R. S. Timing, localization, and control of wheat germ agglutinin synthesis in developing wheat embryos. Dev Biol. 1982 Jun;91(2):491–496. doi: 10.1016/0012-1606(82)90057-4. [DOI] [PubMed] [Google Scholar]
- Weiser C. J. Cold Resistance and Injury in Woody Plants: Knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science. 1970 Sep 25;169(3952):1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Chemical and Biophysical Changes in the Plasma Membrane during Cold Acclimation of Mulberry Bark Cells (Morus bombycis Koidz. cv Goroji). Plant Physiol. 1984 Sep;76(1):257–265. doi: 10.1104/pp.76.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]