Abstract
Recent research has focused on nanoparticles. Aedes albopictus is a potential vector that transmits fatal diseases. Recently, Phyto-reduced silver nanoparticles (AgNPs) were shown to be mosquito larvicides. This study aimed to synthesize silver nanoparticles using Diospyros montana leaf extract, characterize them, and test their efficacy as larvicide and pupicide against Ae. albopictus mosquitoes, determine their duration of effectiveness as a larvicide, identify plant compounds that help to synthesize nanoparticles, and assess their effects on non-target organisms. Quercetin, luteolin, kaempferol, gallocatechin gallate, epigallocatechin gallate, and capsaicin are among the novel reducing and capping agents found in D. montana leaf through LCMS analysis. The color shift and distinctive peak in UV–Vis spectroscopy made it simple to see how biogenic AgNPs were produced by converting Ag+ ions into Ag0. Substantial negative value (− 19.10 mv) of zeta potential demonstrated the long-term stability of AgNPs. A moderate range (8.72 − 50.75 nm) of particle size distribution pattern was obtained using the DLS technique. SEM and TEM images depicted the quasi-spherical (or polyhedral) and spherical shape of the nanoparticles, having approximately 16.75 nm average size. Synthesized AgNPs had a low LC90 value (< 10 ppm) for all larval instars and pupae of Ae. albopictus and had negligible mal effect on non-target organisms. Regression equations showed dose-dependent mortality by the positive correlation between mortality rate and AgNPs concentration, and each time the regression coefficient (R2) value was larger than zero. This study shows that D. montana leaf extract is an environment-friendly and sustainable source of an effective reducing and capping agent to synthesize highly stable, ecologically acceptable silver nanoparticles and their application as mosquitocide.
Subject terms: Zoology, Nanoscience and technology
Introduction
The study of nanoparticles is the focus of recent scientific research on chemical biology. Nanoparticles are particles of atomic and molecular scale, ranging from 1 to 100 nm in size. They possess various intriguing physical, biological, catalytic, and optical properties that have piqued the interest of modern advanced research thinking. Different physical, chemical, and biological methods have produced various types of metal nanoparticles. Silver nanoparticles (AgNPs) are widely synthesized due to their extensive applications and area of use perspective. Production of silver nanoparticles using various chemicals, namely hydrazine hydrate, citrate, sodium borohydride, DMF, ethylene glycol, etc., as reducing agents may lead to the absorption of hazardous substances on the surfaces of nanoparticles, generating toxicity issues1. Also, these methods are relatively more expensive2. Synthesis of nanoparticles employing a green root, i.e., using a plant-based material as both a reducing agent and capping agent, is comparatively less toxic and cost-effective3. Different plant primary and secondary metabolites, such as carbohydrates, proteins, terpenoids, and alkaloids, have been reported as reducing agents of AgNO3 for the green synthesis of metallic nanoparticles. Terpenoids, Alkaloids, phenolic acid, and polyphenol compounds like flavonoids are the major bioactive phytochemicals that assist in such processes due to their substantial reducing and stabilizing properties4. Silver nanoparticles exhibit size and shape-dependent unique properties which are of interest for applications in different fields such as medical imaging5, filters, drug delivery, sensor technology6, optical devices7, biological labeling8, catalysis9, molecular recognition and diagnosis10. Recently, Ag nanoparticles have also been applied to harmful biological organisms as antibacterial11, antiviral12, antifungal13, anti-inflammatory14, anticancer15 and mosquitocidal16 agents.
Aedes is a threatening genus of mosquito vectors that spread several death-dealing viral diseases, including dengue, yellow fever, zika virus, chikungunya, etc.17, and found in all tropical, subtropical, and temperate regions throughout the world. An estimated 390 million dengue virus infections occur yearly, of which 96 million manifests clinically18. According to WHO (2019), 47 countries (34 in Africa and 13 in Central and South America) are either endemic for yellow fever. As of 2023, 34 African countries and 13 Central and South American countries are endemic for yellow fever or have regions that are endemic for yellow fever19. WHO20 also announced that 86 countries and territories had reported evidence of Zika virus infection transmitted by mosquito vectors. According to the European Centre for Disease Prevention and Control, in 2022, and as of 24 August, 229,029 cases and 41 deaths from chikungunya have been reported21. Such alarming increases in vector-borne diseases demand control of the vector population because that is the principal and most effective method to combat the spreading of these diseases22. However, challenges for mosquito vector control are the choice and availability of effective, economical, and secured insecticides. Most of the insecticides available in the market are synthetic chemicals, which are of high cost. Their frequent unscientific applications have many unexpected implications, including the production of resistant strains of mosquitoes, the elimination of non-target organisms, and the establishment of ecological imbalance in the environment23. Because of these disadvantages, there is a definite requirement for alternative control methods which may replace these synthetic insecticides. Green synthesized nanoparticles using plant extract may be a suitable alternative for vector control strategies24. Recently, the use of phyto-reduced silver nanoparticles as mosquito larvicides has been reported25.
Diospyros montana Roxb. The Bombay ebony is a small deciduous, dicotyledonous tree under the family Ebenaceae. This plant is a naturally occurring deciduous forest tree widely distributed throughout India. It is used in traditional Indian medicines, including Ayurveda and Unani. Parts of this plant are used in treating several diseases, particularly in folk medicine and also for treating anti-inflammatory, anticancer, etc.26. Crude extract from the leaves of this plant also showed mosquito larvicidal properties27.
The present study was aimed at synthesizing silver nanoparticles using leaf extract of D. montana as a reducing as well as stabilizing agent, characterization of the synthesized nanoparticles, evaluation of its efficacy as larvicidal and pupicidal agent against Aedes albopictus mosquitoes, Examination of the length of its efficacy as mosquito larvicide, identification of plant compounds those assist the synthesis process of metal nanoparticles and assessment of its influence on different non-target organisms.
Materials and methods
Chemicals
The experiments used silver nitrate (AgNO3) acquired from Merck, double distilled water, and normal saline.
Collection and identification of plants
Fresh and mature leaves of D. montana, a native plant to India with cosmopolitan distribution, were collected from Ramnabagan Wildlife Sanctuary (23°16′ N, 87°54′), adjacent to the Golapbag campus of the University of Burdwan (Fig. 1). As an endemic species, this plant is available in good numbers on our university campus. The use of D. montana does not violate the local regulations of India. During the sample collection from a specific plant, permission was taken from the authority of the sanctuary. Prof. Ambarish Mukherjee, Department of Botany, The University of Burdwan, identified the plant. A herbarium sheet bearing reference No (GCRKM/2017/S011) of the plant was preserved in the Department of Zoology, The University of Burdwan.
Figure 1.
Collected leaves of Diospyros montana during the study.
Complies with international, national and/or institutional guidelines
Experimental research and field studies on plants, including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation. Studies of the plant (Diospyros montana) were carried out in accordance with relevant institutional, national, and international guidelines and legislation.
Collections of mosquito larvae and culture
Larvae of Ae. albopictus were collected from rainwater-dampened tires along the side road and small water-filled waste containers. Larvae were maintained in the culture trays filled one-third with chlorine-free tap water. Artificial food was prepared by mixing dog biscuits and yeast extract in a 1:3 ratio and supplied. The amount of food was 0.5 mg/larva. A temperature of 27 ± 2 °C, a humidity of 85%, and a 14:10 light–dark hour condition were maintained to ensure their metamorphosis28. After emergence, adult mosquitoes were allowed to imbibe blood meal from the pigeon. Adult Aedes albopictus mosquitoes were identified according to the key of Chandra29. Adults were allowed to lay eggs in the water-filled small container in the mosquito cage. Immatures hatched from eggs were used for mosquito larvicidal and pupicidal bioassays.
Preparation of aqueous phytoextract and its phytochemicals analysis
Leaves were cleaned under the running cold tap water to remove unwanted dust and litter. Washed leaves were allowed to be air-dried for 30 min. Ten grams of fresh leaves were taken and cut into small pieces with scissors. Small amounts of plant leaves were mixed with 200 ml of double distilled water in a glass beaker to prepare aqueous phytoextract. The mixture was warmed in a hot water bath for 10 min at 50 °C and then allowed for rigorous shaking in a magnetic stirrer for an hour. A yellowish-green extract was filtered from the mixture by a Whatman filter paper no 1 and used to synthesize silver nanoparticles. The extract was also subjected to qualitative phytochemical analysis for the identification of primary and secondary metabolites using standard methods of Harborne (1984), Stahl (1989), Trease and Evans (1989), and Sofowara (1993) with slight alterations as follows30–32. To detect plant compounds responsible for the reduction of AgNO3 and stabilization of nanoparticles, Liquid chromatography-mass spectrometry (LCMS) analysis of the leaf extract was performed.
Synthesis, separation, and collection of silver nanoparticles
25-mmol AgNO3 stock solution was prepared by mixing AgNO3 crystals and 100 ml of double distilled water. Twenty-five milliliters of phytoextract of D. montana was mixed with 20 ml of silver nitrate stock solution drop by drop in a conical flask. The conical flask was wrapped with aluminum foil. The volume was adjusted by adding deionized water to 250 ml so that the final concentration of Ag + ion becomes 2 × 10–3 M and plant extract concentration remains 10% (v/v) of the stock solution. The mixture was then stirred with a magnetic stirrer at a temperature of 50 °C for 5 min, and then the flask was transferred to a thermostat water bath and warmed at 60 °C for 2 h. The transformation of the color of the solution into reddish brown from yellowish green was the first indication of the reduction of Ag+ to Ag0 form. The resultant reddish-brown colored solution was allowed to cool overnight in an air-tight, light-protected container. In a REMI Research Centrifuge instrument, the final nano-colloidal solution was centrifuged twice to eliminate un-interacted biological materials at 10,000 rpm for 15 min. The final pellet was collected and transferred to a vacuum desiccator for drying. Synthesized nanoparticles were stored properly for characterization and bioassays.
Different analytical techniques used for the characterization of synthesized silver nanoparticles
Synthesized nanoparticles from D. montana were characterized by various scientific analytical methodologies. To detect the absorption maxima, the optical density of the reaction mixture was first scanned at a fixed wavelength range between 200 and 700 nm at regular intervals using a Multiskan GO spectrophotometer (version 1.00.40) with 1-nm resolution. Then, a dried pellet of nano-materials obtained after centrifugation was coated on XRD grid. The spectra were scanned by X-ray diffractometer (Rigaku, Smartlab) with a Cu Kα (I = 1.54A) radiation source. The scanning range was 10°–80°. An aliquot from the final pellet was used for field emission scanning electron microscopy (FE-SEM) integrated with energy dispersive X-ray spectroscopy (EDS). For structural characteristics of synthesized nanoparticles, images from a transmission electron microscope (TEM) were obtained using the instrument of JEOL; JEM 1400 plus operated at 200 kV. A sample for TEM analysis was prepared by dropping the aqueous AgNPs on carbon-coated copper grids (300 mesh sizes) and then drying them in a vacuum at 25 °C overnight. Clear nano solution, prepared by repeated centrifugation of the reaction mixture, was used for Malvern's dynamic light scattering (DLS) and zeta potential analyzer. Dried powder of nano-materials was used for FTIR measurements to obtain an idea about the chemical framework around the synthesized nanoparticles by an FT-IR Spectrometer (Perkin Elmer Lx10-8873). The scanning range was 450–4000 cm−1 at a resolution of 4 cm−1.
Mosquitocidal bioassays
The efficacy of synthesized nanoparticles was tested on immatures (larvae and pupae) of Ae. albopictus according to the standard protocol specified by WHO33. Twenty-five larvae of each instar (I-IV) and pupae were taken in separate glass beakers filled with 100 ml of double distilled water. Six concentrations of nano solution (0.3125, 0.6250, 1.2500, 2.5000, 5.0000, and 10.0000 ppm) were used to examine larvicidal and pupicidal efficacies. 0.5 mg larval food was provided in each glass beaker. Three trials for each concentration against each larval instar and pupae were performed in an experimental setup. The experimental setup was replicated on three different days (i.e., n = 9). A control using only distilled water was maintained for each concentration during experimentation. Mortality of immature was recorded after 24, 48, and 72 h of exposure. Control mortality was corrected by Abbott's formula34.
Examination of the length of larvicidal efficacy of synthesized nanoparticles up to three and half years
An experiment was done for up to three and a half years to determine the stability of synthesized silver nanoparticles at an interval of three months. Twenty-five 4th instar Ae. albopictus larvae were kept in a 250 ml glass container filled with 100 ml of distilled water. A five ppm concentration of synthesized AgNPs was used for that long-term bioassay. The experiment was triplicated in three separate containers for more accuracy. After that experiment, DLS analysis and Zeta potential measurement were done to test the stability of synthesized silver nanoparticles.
Test on non-target organisms
Synthesized nanoparticles using D. montana were tested for any toxicity and abnormalities against some non-target organisms following the same protocol. Five concentrations (0.6250, 1.2500, 2.5000, 5.0000, and 10.0000 ppm) were used for the experiment against non-target species. Toxorhynchites splendens larvae (mosquito predator), Chironomus circumdatus larvae (chironomid), and Aplocheilus panchax, a mosquito larvivorous fish, were used as non-target organisms as they share common habitats with mosquito larvae (Fig. 2).
Figure 2.
Pictures of three non-target organisms (A) Chironomus circumdatus, (B) Toxorhynchites splendens, and (C) Aplocheilus panchux.
Data analysis
Larval and pupal mortality were subjected to probit analysis35. The LC90 values, regression equations (Y = mortality; X = concentrations), and regression coefficient values were computed using the software "STAT PLUS 2007 (Trial version)" and MS EXCEL 2003. The Abbott׳s formula was used to adjust the control mortality percentage.
Results
Determination of plant compounds
Phytochemical analysis
Qualitative phytochemical analysis of the aqueous plant extract revealed the presence of alkaloids, flavonoids, terpenoids, steroids, saponins, coumarin, glycosides, etc. (Table 1). These secondary metabolites can reduce metal salts.
Table 1.
Preliminary qualitative phytochemical screening of leaf extract of Diospyros montana.
| Phytochemicals | Results |
|---|---|
| Alkaloid | + |
| Flavonoids | + |
| Tannins | – |
| Terpenoids | + |
| Steroids | + |
| Glycosides | + |
| Saponins | + |
| Coumarin | + |
‘+’ = present, ‘–’ = absent.
Determination of reducing and stabilizing performances of Plant Compounds
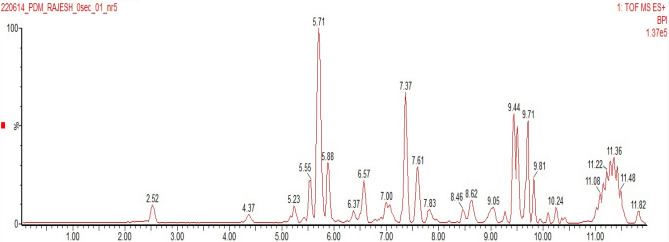
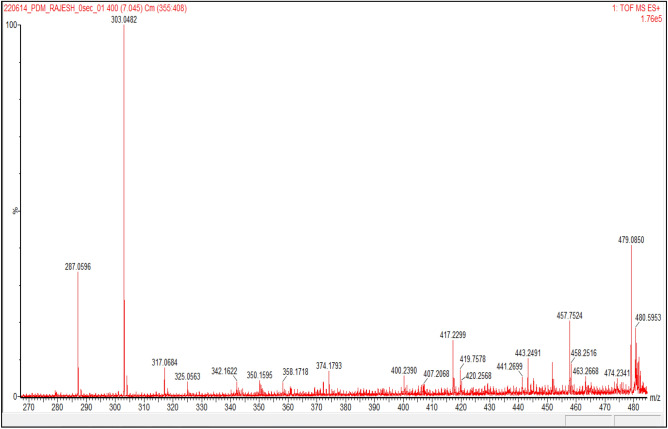
To determine the phytochemical compounds of D. montana responsible for reduction and stabilization during the synthesis of silver nanoparticles, Liquid chromatography-mass spectrometry (LC–MS) analysis of the leaf extract was done. Figure 3 represents the LC–MS chromatogram of the leaf extract of D. montana. LCMS spectrum revealed flavonoid compounds, including quercetin (303.05 Da.), luteolin (287.05 Da.), kaempferol (287.11 Da.), and other polyphenol compounds, including Gallocatechin gallate (458.25 Da.) and Epigallocatechin gallate (458.25 Da.) and also alkaloid compound like capsaicin (306.15 Da.) were present in the leaf extract of D. montana (Fig. 4).
Figure 3.
LC–MS chromatogram of leaf extract of Diospyros montana.
Figure 4.
LC–MS spectrum of leaf extract of Diospyros montana.
Characterization of synthesized silver nanoparticles
UV–VIS spectroscopy study
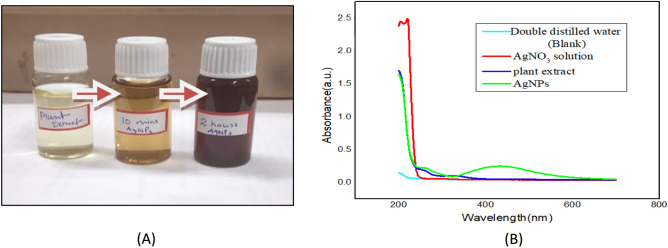
Changes in color from yellowish green to reddish brown occurred due to the excitation of surface plasmon vibrations in metal nanoparticles. Here, the characteristic surface plasmon absorption spectral band is shown at (max) 430 nm (Fig. 5B). The absorbance of the AgNPs solution showed (color change) after 2 h in Fig. 5A. 20 ml of aqueous 10–3 M AgNO3 solution was exposed to sunlight for the blank analysis and subjected to UV–vis spectroscopy study. It was observed that there was no color change in the aqueous AgNO3 solution since nanoparticles were not synthesized.
Figure 5.
(A) The solution of diluted silver nanoparticles exhibits color change. (B) Distinctive surface plasmon absorption spectrum of silver nanoparticles (AgNPs) synthesized using leaf extract of Diospyros montana, with a maximum wavelength of 430 nm.
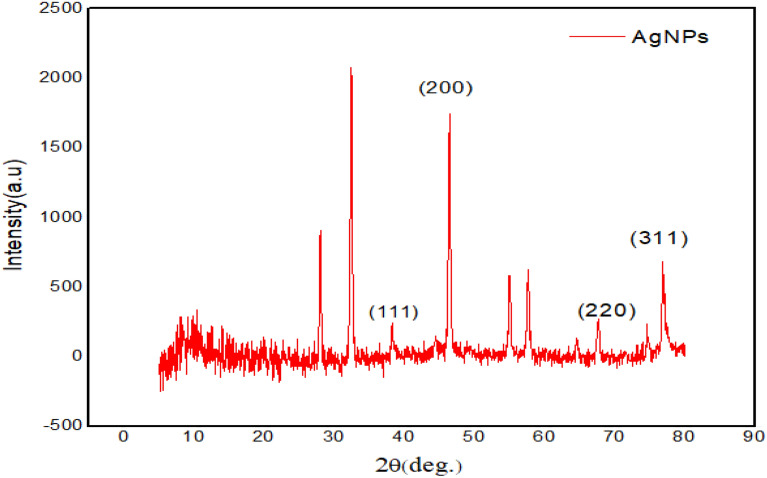
X-ray diffraction analysis
Any crystal that receives an X-ray reflection produces a variety of diffraction patterns, which are representations of the physicochemical properties of the crystal formations. Here, diffraction peaks were found at corresponding 2θ vales of 38.4°, 44.45°, 64.7°, and 77.15° those were attributed to the four different facets known for zero-valent face-centered cubic silver, which represented the (111), (200), (220), and (311) crystal planes respectively (Fig. 6). Additionally, a few unassigned peaks at 32.5°, 55°, and 57.67° were seen close to the typical peaks. These distinct Bragg peaks could result from the capping agent's stabilizing effects on the nanoparticles. Analysis was made by comparing the diffracted beams with the reference database in the Joint Committee on Powder Diffraction Standards (JCPDS) library.
Figure 6.
X-ray Diffraction pattern of the silver nanoparticles synthesized using leaf extract of Diospyros montana.
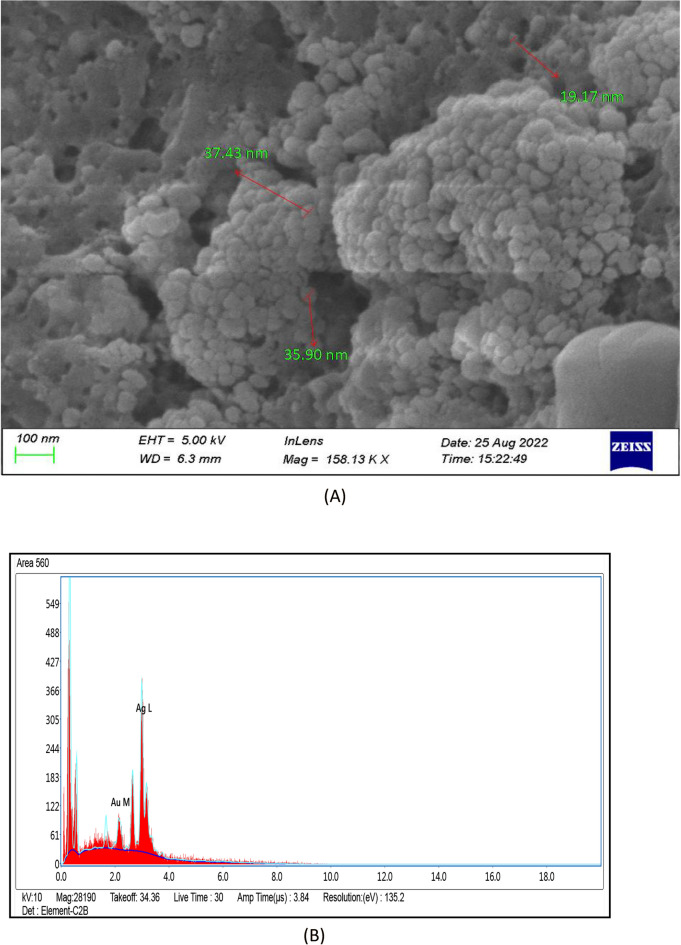
FE-SEM and EDX Analysis
The produced nanoparticles in the SEM micrograph had spherical and semi-spherical structural features (Fig. 7A). Energy-dispersive X-ray spectroscopy (EDX) combined with FE-SEM could be used to examine silver powder morphology and also perform chemical composition analysis. The outcome showed that pure AgNPs were present in the reaction product. EDX spectrum exhibited an optical absorption peak at about 3 keV. The significant signal in the silver region of the EDX spectrum provided evidence that silver particles were formed (Fig. 7B). 89.43% yield of silver element was determined through EDX analysis.
Figure 7.
(A) Field emission scanning electron microscopy images of silver nanoparticles (AgNPs) synthesized by Diospyros montana (B) EDX spectrum of synthesized silver nanoparticles (AgNPs) using leaf extract of Diospyros montana.
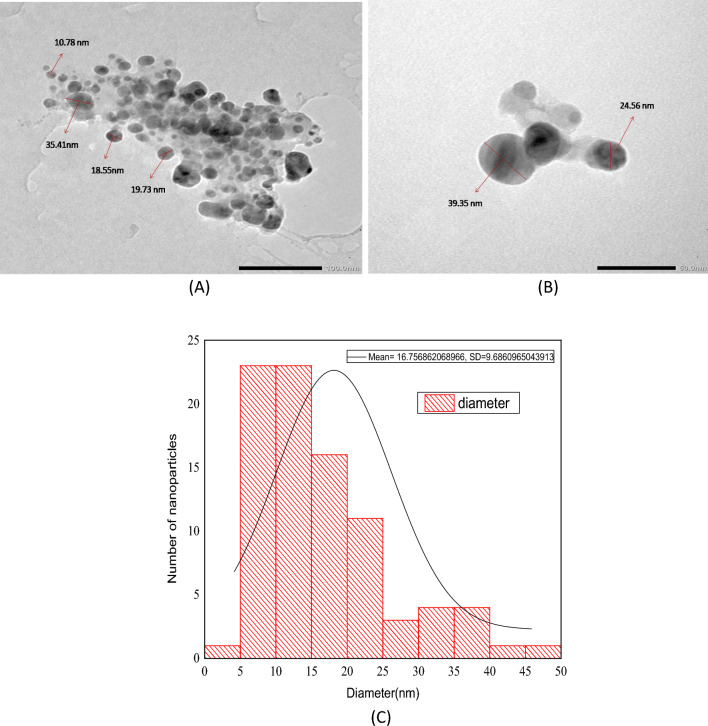
TEM analysis
Transmission electron microscopy (TEM) is a valuable, widely used, and essential technology to get precise measurements of particle size, size distribution, and shape of nanoparticles. TEM image of synthesized nanoparticles presented in Fig. 8A 100 nm and B 50 nm scale imparted that the majority of newly generated nanocrystals had a quasi-spherical (or polyhedral) shape. Some were irregular in shape. The average size of synthesized AgNPs determined through the TEM image was 16.75 nm (Fig. 8C).
Figure 8.
(A) Representative Transmission Electron Microscopy photograph of silver nanoparticles (AgNPs) synthesized using leaf extract of Diospyros montana. (B) Closer view of the polyhedral shape of silver nanocrystals. (C) Size distribution histogram of silver nanoparticles synthesized using leaf extract of Diospyros montana.
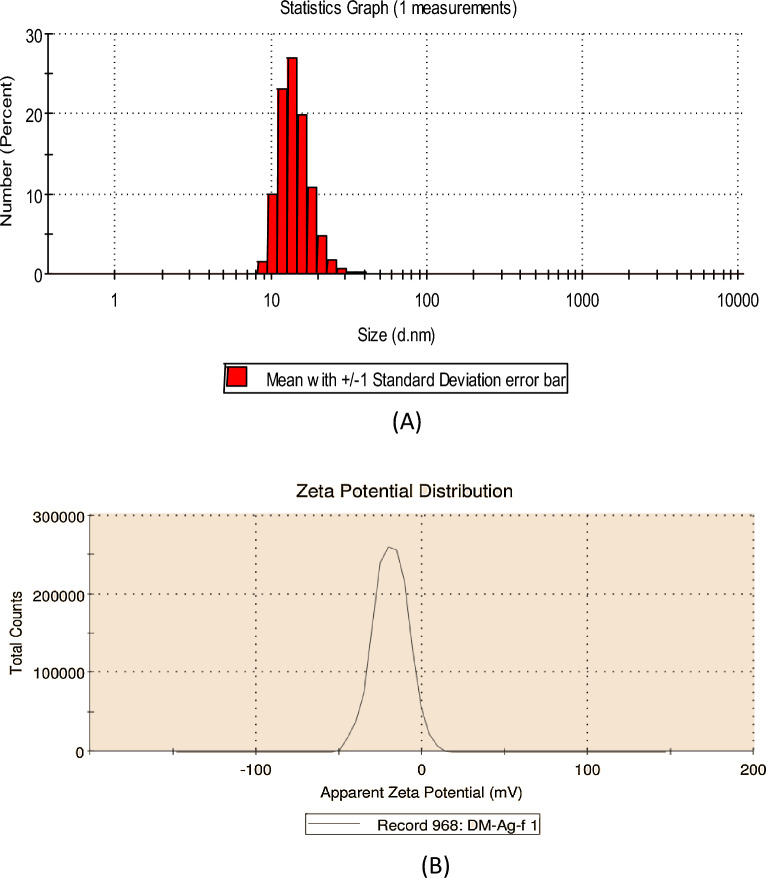
Dynamic light scattering (DLS) and zeta potential analysis
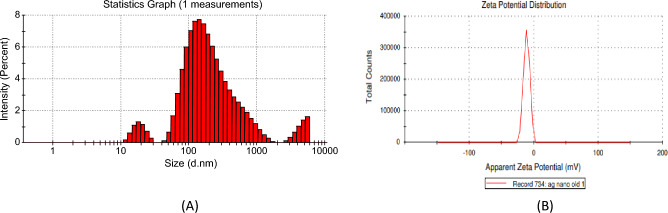
DLS makes it possible to calculate the hydrodynamic size of particles by analyzing the variation of the scattered light intensity as a function of time. The size obtained from DLS was larger than TEM, which might be due to the influence of Brownian motion. Size distribution through DLS analysis of nanoparticles synthesized using D. montana is presented in Fig. 9A. Particle size ranges from 8.72 to 50.75 nm. Supplementary Table 1 illustrates the size distribution of synthesized AgNPs through percent values. The Zeta potential of the nano solution was also measured. The Zeta potential of the nano solution was highly negative with a value of minus nineteen, approximately (− 19.10) mV. The zeta potential value is presented in Fig. 9B.
Figure 9.
(A) Particle size histogram of silver nanoparticles (AgNPs) synthesized using leaf extract of Diospyros montana. (B) Zeta potential chromatogram of silver nanoparticles (AgNPs) synthesized using leaf extract of Diospyros montana.
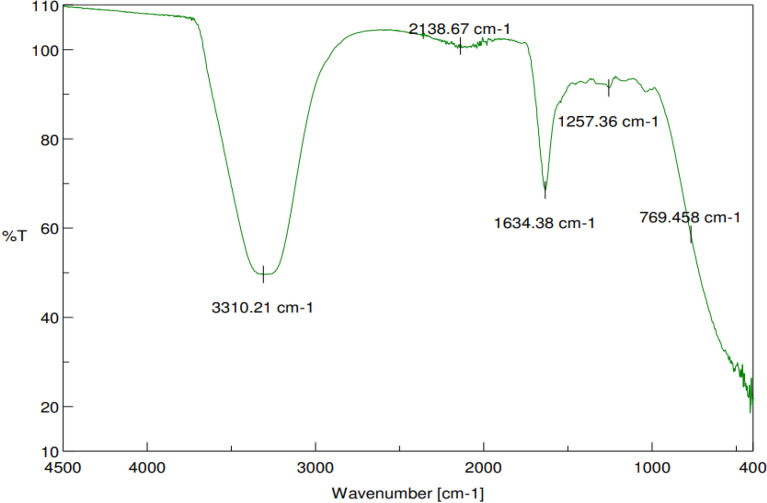
FT-IR analysis
FTIR spectrum of biosynthesized silver nanoparticles (Fig. 10) revealed distinct peaks at 3310.21 cm−1, 2138.67 cm−1, 1634.38 cm−1, 1257.36 cm−1, and 769.45 cm−1. Each of these bands represents different functional groups, which are interpreted in Table 2.
Figure 10.
FTIR spectrum of silver nanoparticles synthesized by Diospyros montana leaf extract.
Table 2.
FTIR absorption spectra bands of synthesized silver nanoparticles and their probable functional groups.
| Absorption spectra peaks | Range (cm−1) | Probable functional groups |
|---|---|---|
| 3310.21 cm−1 | 3270–3320 | C=O and N–H stretching of amide |
| 3200–3400 | O–H stretching of alcohol | |
| 2138.67 cm−1 | 2100–2140 | C≡C stretching of alkynes |
| 1634.38 cm−1 | 1630–1680 | C=O stretching of ketones and amide |
| 1620–1680 | C–C stretching of alkenes | |
| 1630–1680 | C=O stretching of amide | |
| 1257.36 cm−1 | 1180–1260 | C=O stretching of alcohol |
| 1200–1305 | N–H stretching of amide | |
| 769.45 cm−1 | 1000–1100 | N–H stretching of amine |
Mosquitocidal bioassay study
The percent mortality of mosquito immatures subjected to aqueous solutions containing pure AgNPs pellets at various concentrations (0.3125, 0.6250, 1.2500, 2.5000, 5.0000, and 10.0000 ppm) for 72 h is shown in Table 3. Cent percent mortality has been noted at a 10 ppm concentration of nano-material after 48 h of exposure against all larval instars. In pupicidal bioassay, 100% mortality was found at 10 ppm after 72 h of exposure. The mortality of mosquito immatures (larvae and pupae) increased with increasing concentration of nano solution and time exposure. The LC90 values, regression equations, and R2 values of synthesized Ag NPs against immures of Ae. albopictus are depicted in Table 4. Table 5 shows the outcomes of three-way factorial ANOVA of mortality with three fixed variables: larval instar, extract concentration, and exposure hour.
Table 3.
Larvicidal and pupicidal activity of Diospyros montana induced green synthesized silver nanoparticles (AgNPs) on the larvae and pupae of Aedes albopictus.
| Larval instars | Concentrations (ppm) | Mortality percentage (mean ± SE) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| FIRST | 0.3125 | 60.44 ± 5.51 | 72.44 ± 4.29 | 86.22 ± 2.50 |
| 0.6250 | 74.22 ± 5.54 | 86.22 ± 4.11 | 94.22 ± 2.32 | |
| 1.2500 | 76.88 ± 5.48 | 87.55 ± 3.42 | 100.00 ± 0.00 | |
| 2.5000 | 89.77 ± 3.77 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 5.0000 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 10.0000 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| SECOND | 0.3125 | 52.00 ± 5.81 | 64.88 ± 4.79 | 69.33 ± 4.32 |
| 0.6250 | 62.66 ± 5.65 | 77.77 ± 4.42 | 90.66 ± 2.66 | |
| 1.2500 | 67.55 ± 3.01 | 83.55 ± 3.42 | 94.22 ± 2.01 | |
| 2.5000 | 81.77 ± 6.39 | 89.33 ± 5.03 | 95.11 ± 2.81 | |
| 5.0000 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 10.0000 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| THIRD | 0.3125 | 18.22 ± 2.22 | 34.22 ± 2.41 | 49.77 ± 2.83 |
| 0.6250 | 45.77 ± 3.34 | 65.33 ± 3.05 | 79.11 ± 3.03 | |
| 1.2500 | 54.66 ± 4.66 | 73.77 ± 2.32 | 87.55 ± 2.35 | |
| 2.5000 | 62.66 ± 5.49 | 75.55 ± 4.29 | 88.00 ± 2.58 | |
| 5.0000 | 86.22 ± 2.50 | 92.44 ± 2.53 | 96.44 ± 1.04 | |
| 10.0000 | 95.11 ± 1.29 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| FOURTH | 0.3125 | 12.88 ± 1.85 | 19.55 ± 1.81 | 25.33 ± 1.15 |
| 0.6250 | 34.66 ± 3.12 | 44.88 ± 3.31 | 53.77 ± 2.75 | |
| 1.2500 | 42.22 ± 2.75 | 56.44 ± 3.42 | 71.55 ± 2.86 | |
| 2.5000 | 58.22 ± 5.81 | 68.88 ± 4.65 | 82.66 ± 2.21 | |
| 5.0000 | 78.22 ± 5.85 | 89.33 ± 3.33 | 96.44 ± 1.55 | |
| 10.0000 | 95.55 ± 1.23 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| PUPA | 0.3125 | 7.11 ± 0.88 | 15.55 ± 1.23 | 22.22 ± 1.17 |
| 0.6250 | 21.77 ± 2.50 | 30.66 ± 2.58 | 38.22 ± 2.41 | |
| 1.2500 | 45.77 ± 2.22 | 58.66 ± 2.40 | 71.11 ± 2.08 | |
| 2.5000 | 67.55 ± 2.15 | 84.88 ± 1.60 | 92.44 ± 1.55 | |
| 5.0000 | 85.77 ± 1.77 | 97.33 ± 0.94 | 99.11 ± 0.58 | |
| 10.0000 | 94.66 ± 1.15 | 98.66 ± 0.66 | 100.00 ± 0.00 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
S.E Standard error, ppm parts per millions.
Table 4.
Assessment of LC90 values of synthesized silver nanoparticles on Aedes albopictus larvae through log-probit and regression analyses.
| Larval instars | Period of exposure (hour) | LC90 | SE | LCL | UCL | Regression equation | R2 value |
|---|---|---|---|---|---|---|---|
| FIRST | 24 | 1.987 | 0.621 | 1.156 | 6.386 | y = 3.506x + 72.04 | 0.678 |
| 48 | 0.900 | 0.242 | 0.526 | 2.315 | y = 2.091x + 84.17 | 0.483 | |
| 72 | 0.3997 | 0.053 | 0.301 | 0.506 | y = 0.818x + 94.05 | 0.288 | |
| SECOND | 24 | 2.9910 | 1.446 | 1.537 | 20.507 | y = 4.684x + 61.95 | 0.753 |
| 48 | 1.6873 | 0.249 | 1.314 | 2.340 | y = 2.984x + 76.12 | 0.663 | |
| 72 | 0.8721 | 0.116 | 0.686 | 1.157 | y = 1.912x + 85.27 | 0.382 | |
| THIRD | 24 | 7.5227 | 1.237 | 5.697 | 10.826 | y = 6.572x + 38.87 | 0.760 |
| 48 | 3.8676 | 1.390 | 2.135 | 15.093 | y = 5.047x + 56.99 | 0.654 | |
| 72 | 1.9473 | 0.276 | 1.532 | 2.669 | y = 3.393x + 72.34 | 0.484 | |
| FOURTH | 24 | 9.2699 | 1.473 | 7.0716 | 13.154 | y = 7.494x + 29.03 | 0.851 |
| 48 | 5.1974 | 1.220 | 3.259 | 11.867 | y = 6.982x + 40.26 | 0.769 | |
| 72 | 3.0254 | 0.357 | 2.467 | 3.919 | y = 5.963x + 52.05 | 0.610 | |
| PUPA | 24 | 6.6435 | 0.808 | 5.376 | 8.652 | y = 8.132x + 27.08 | 0.738 |
| 48 | 3.5041 | 0.363 | 2.923 | 4.387 | y = 7.526x + 39.59 | 0.624 | |
| 72 | 2.3244 | 0.226 | 1.962 | 2.873 | y = 6.667x + 48.63 | 0.551 |
LC90 Lethal concentration (to kill 90% of exposed population), SE standard error, LCL Lower control limit, UCL Upper control limit.
Table 5.
Three-way ANOVA analysis of mortality of Aedes albopictus using green synthesized silver nanoparticles using Diospyros montana leaf extract.
| Source of variation | Sum of square | df | Mean square | F value | p-level | F crit. value | Omega square |
|---|---|---|---|---|---|---|---|
| Instars (I) | 103,409.97 | 4 | 25,852.49 | 332.87 | 0 | 4.66 | 0.17 |
| Concentrations (C) | 328,429.43 | 5 | 65,685.88 | 845.76 | 0 | 4.15 | 0.54 |
| Hours (H) | 39,569.81 | 2 | 19,784.90 | 254.74 | 0 | 4.15 | 0.06 |
| I × C | 59,793.58 | 20 | 2989.67 | 38.494 | 0 | 2.30 | 0.09 |
| I × H | 1676.00 | 8 | 209.50 | 2.69 | 0.006 | 3.31 | 0.00 |
| C × H | 9987.04 | 10 | 998.70 | 12.85 | 0 | 3.00 | 0.01 |
| I × C × H | 3400.61 | 40 | 85.01 | 1.09 | 0.32 (N.S) | 1.09 | 0.00 |
| Within groups | 55,918.22 | 720 | 77.66 | ||||
| Total | 602,184.69 | 809 | 744.35 | ||||
| Omega squared for combined effect | 0.89 |
Significant level p < 0.05.
df Degree of freedom, N.S Not significant.
Examination of the duration of larvicidal efficacy of synthesized nanoparticles
The duration of larvicidal efficacy examined up to three and half years is presented in Fig. 11. Again, remarkable stability in larvicidal effectiveness was recorded with a prolonged decreasing trend. In November 2018, the mortality was 93.33%. In November 2019, mortality decreased by 16–77.33%. In November 2020, mortality decreased by 30.67–62.66%. In November 2021, mortality decreased by 62.66–30.66%. Lastly, up to February 2022, mortality was reduced by 69.33%. So, Aedes albopictus larvae mortality gradually decreased to 24% in three and a half years. DLS analysis of synthesized silver nanoparticles after that long duration showed that the particles were relatively larger because of their aggregation. The Zeta potential value of synthesized AgNPs increased to minus eleven millivolts, approximately (− 11.3 mV) (Fig. 12).
Figure 11.
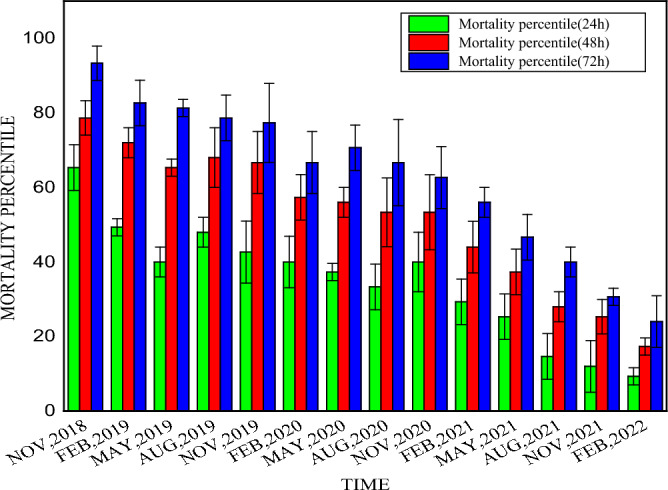
Mortality percentiles in long-term efficacy (stability) test of synthesized silver nanoparticles (AgNPs).
Figure 12.
(A) DLS analysis and (B) Zeta potential measurement of synthesized silver nanoparticles after three and half year of synthesis.
Effect on non-target organisms
The effect of synthesized AgNPs on three non-target organisms, Toxorhynchites splendens larvae, Chironomus circumdatus larvae, and Aplocheilus panchux were presented in Table 6. LC90 values of synthesized silver nanoparticles against Toxorhynchites splendens larvae and Chironomus circumdatus larvae are presented in Table 7. When treated on Aplocheilus panchux fish, synthesized silver nanoparticles showed the least mortalities at the highest concentration. But in lower concentrations, Aplocheilus panchux didn't show any mortality.
Table 6.
Mortality of non-target organisms on different concentrations of synthesized silver nanoparticles (AgNPs).
| Non–target organisms | Concentrations (ppm) | Mortality percentage (mean ± SE) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| Toxorhynchites splendens larvae | 0.6250 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 1.2500 | 2.66 ± 1.33 | 6.66 ± 1.33 | 8.00 ± 0.00 | |
| 2.5000 | 9.33 ± 1.33 | 10.66 ± 1.33 | 14.66 ± 1.33 | |
| 5.0000 | 10.66 ± 1.33 | 16.00 ± 2.30 | 18.66 ± 1.33 | |
| 10.0000 | 18.66 ± 3.52 | 24.00 ± 2.30 | 26..66 ± 1.33 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Chironomus circumdatus larvae | 0.6250 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 1.2500 | 4.00 ± 2.30 | 8.00 ± 2.30 | 10.66 ± 1.33 | |
| 2.5000 | 8.00 ± 0.00 | 12.00 ± 2.30 | 16.00 ± 2.30 | |
| 5.0000 | 16.00 ± 2.30 | 21.33 ± 1.33 | 22.66 ± 2.66 | |
| 10.0000 | 20.00 ± 2.30 | 25.33 ± 1.33 | 29.33 ± 1.33 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Aplocheilus panchax | 0.6250 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 1.2500 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 2.5000 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 5.0000 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| 10.0000 | 6.66 ± 1.33 | 10.66 ± 2.66 | 13.33 ± 1.33 | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
S.E Standard error, ppm Parts per millions.
Table 7.
Assessment of LC90 values of synthesized silver nanoparticles on Toxorhynchites splendens and Chironomus circumdatus larvae through log-probit analyses.
| Non–target organisms | Period of exposure (hour) | LC90 (ppm) | SE | LCL | UCL |
|---|---|---|---|---|---|
| Toxorhynchites splendens larvae | 24 | 569.87 | 819.04 | 141.23 | 13,298.48 |
| 48 | 418.61 | 454.59 | 123.80 | 4948.64 | |
| 72 | 184.42 | 276.91 | 48.74 | 5288.25 | |
| Chironomus circumdatus larvae | 24 | 451.97 | 532.27 | 127.43 | 6462.79 |
| 48 | 417.55 | 439.75 | 124.88 | 4572.88 | |
| 72 | 349.58 | 330.74 | 112.57 | 3063.33 |
LC90 Lethal concentration (to kill 90% of exposed population), SE Standard error, LCL Lower control limit, UCL Upper control limit, ppm parts per millions.
Discussion
The current study demonstrated for the first time how to make biogenic AgNPs and how effective they are at killing mosquitoes by using Diospyros montana leaf extract as a reducing and capping agent.
Quercetin, luteolin, kaempferol, gallocatechin gallate, epigallocatechin gallate, and capsaicin are among the novel reducing and capping agents found in D. montana leaf through LCMS analysis. These substances have the capacity to end-cap the AgNPs, greatly improving their ability to kill mosquito larvae. Lee and Park36 reported the role of quercetin as a reducing agent for the synthesis of silver and gold nanoparticles. According to Osonga et al.37, luteolin tetraphosphate (LTP) was the reducing and capping agent for AgNPs synthesis using water as the solvent. In 2018 Deng et al.38 reported kaempferol as a reducing and stabilizing compound for silver nanoparticle synthesis. Hussain and Khan39 reported Gallocatechin gallate and Epigallocatechin gallate as reducing agents for AgNPs synthesis. Amruthraj et al.40 described that an alkaloid compound, capsaicin, was a bio-reductant to reduce silver nitrate to form silver nanoparticles. In light of this, the plant metabolites identified in the current investigation utilizing LC–MS may function as reducing and capping agents in the formation of silver nanoparticles.
The production of biogenic AgNPs by reducing Ag+ ions into Ag0 is easily observed by visual observation (color change) and characteristic peaks in UV–Vis spectroscopy. In AgNPs, the conduction band and valence band lie very close to each other, in which electrons move freely. These free electrons give rise to a surface plasmon resonance (SPR) absorption band, arising due to the collective oscillation of electrons of AgNPs in resonance with the light wave41. The XRD data also suggests crystallization of the bio-organic phase on the surface of the silver nanoparticles, or vice versa. Generally, the broadening of peaks in the XRD patterns of solids is assigned to particle size effects42. The presence of broader peaks in the observed data can be attributed to the impact of the experimental conditions on the process of nucleation and growth of crystal nuclei. Additionally, these broader peaks suggest the existence of smaller particle sizes43. The size distribution pattern acquired through DLS method shows a moderate particle size range from 8.72 to 50.75 nm, of which the majority are found between 10 and 21 nm. A high negative value (− 19.10 mV) of zeta potential indicates the long-term stability of synthesized AgNPs and better colloidal properties due to electrostatic repulsion and high dispersity. SEM and TEM images depicted that the plurality of newly generated nanocrystals had a quasi-spherical (or polyhedral) and spherical shape. Typical optical absorption peak at about 3 keV due to surface plasmon resonance in EDX analysis confirmed the formation of metallic silver nanocrystals44. FTIR analysis verifies functional molecules covalently grafted onto silver nanoparticles or interactions between an enzyme and a substrate during catalysis45. The prominent peak at 3310.21 cm−1 revealed the O–H stretching in the produced sample, which might be caused by the presence of hydroxyl-containing substances like carboxylic acid, phenols, or alcohol. The carbonyl (C=O) group stretching was thought to be responsible for the peak at 1634.38 cm−146. These results might point to proteins that could reduce and stabilize the silver nanoparticles. The apparent bands at 1257.36 cm−1 might reveal the stretching of the (C–O–C) group. The silver nanoparticles might be stabilized by Ag–O, Ag–N, or Ag-C bonding, which might be visible in the observable bands.
Dengue fever is a significant public health threat that affects the entire world and is carried by Aedes mosquitoes. It is indigenous to tropical and subtropical countries, especially in urban and suburban areas47. Earlier studies by different authors suggested that plant extracts, essential oils, etc., produced from plants and bacteria, especially distinct strains of B. thuringiensis, are potential alternative resources for mosquito control48. D. montana-derived green silver nanoparticles exhibit a promising mosquitocidal property. In earlier studies, Adhikari et al.49 revealed larvicidal properties of Swietenia mahagoni-derived silver nanoparticles against Anopheles stephensi, Culex quinquefasciatus, and Culex vishnui group with LC90 values of 55.34, 202.05, and 113.07 ppm, respectively after 72 h exposure. Suganya et al.50 showed the LC90 value of synthesized nanoparticles by using Leucas aspera against Aedes aegypti, which was 21.5685 mg L−1 after 24 h post-exposure. D. montana-derived nanoparticles are significantly more efficient regarding LC90 value for all instars larvae and pupae of the dengue vector, Asian tiger mosquito (Ae. albopictus). The established regression equations clearly show a dose-dependent mortality, which is demonstrated by the positive correlation between the rate of mortality (Y) and the concentration of the AgNPs (X) and by the fact that each time the regression coefficient (R2) value is greater than zero. Outcomes of three-way ANOVA analysis revealed that different concentrations, instars, and times of exposure singly or in complex interaction significantly influenced the mortality of larvae and pupae because, in most cases, F values were greater than critical F values and p values were less than 0.05. Only the influence of one complex interaction (I × C × H) was not significant. The exact mechanisms of larvicidal and pupicidal effects of AgNPs are unknown. When applied to non-target organisms, synthesized silver nanoparticles showed LC90 values ranging from 184.42 to 569.87 ppm, but in the case of Ae. albopictus LC90 values were less than 10 ppm, indicating their target specificity. It is conceivable that the AgNPs penetrate the larval membrane and cause the sensitive larvae to perish. Previously, it was claimed that the passage of AgNPs through the membrane of a treated larva induced an interaction with cell molecules that led to the death of larvae51. Additionally, the enzymes were deactivated and produced peroxide once the AgNPs reached the midgut epithelial membrane, which caused cell death52. It is worth mentioning that nanoparticles synthesized during the present study are extremely stable and have long-lasting mosquitocidal effects that continue for years with a very slow diminishing efficacy.
Conclusion
The results of the current study demonstrate that aqueous leaf extract of D. montana is a sustainable and environmentally acceptable source that can be employed as an efficient reducing and stabilizing agent for the production of exceptionally stable silver nanoparticles (AgNPs). The procedure used in this work to fabricate green synthesized AgNPs is safe, non-toxic, and environmentally acceptable. The biosynthesized AgNPs exhibit significant mosquitocidal properties against the Dengue vector Ae. albopictus at low concentrations. Biogenic AgNPs are not as hazardous to organisms that are not their intended targets, indicating the target-specificity of synthesized AgNPs. There may be an essential usage in various domains, particularly in vector control programs.
Supplementary Information
Acknowledgements
The authors appreciatively acknowledged Late. Dr. Ambarish Mukherjee, Former Professor, Department of Botany, The University of Burdwan, for the authentication of the plant species. The authors thank the Department of Zoology, The University of Burdwan, for the instrumentation facilities and the Central Facilities Unit, Bose Institute, Kolkata, for providing us with the LC-MS instrumentation facility.
Author contributions
R.K.M. conducted the experiments, collected the data, prepared the first draft of the manuscript, data acquisition, and manuscript editing, statistical analysis. G.C. designed the experiments, analyzed the data, and critically revised the manuscript. Both authors reviewed the manuscript. All authors approved the final manuscript for publication.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44442-7.
References
- 1.Mallick K, Witcomb MJ, Scurrell MS. Polymer stabilized silver nanoparticles: A photochemical synthesis route. J. Mater. Sci. 2004;39:4459–4463. [Google Scholar]
- 2.Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, Muniyandi J, Hariharan N, Eom SH. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces. 2009;74:328–335. doi: 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 3.Rawani A, Ghosh A, Chandra G. Mosquito larvicidal and antimicrobial activity of synthesized nano-crystalline silver particles using leaves and green berry extract of Solanum nigrum L. (Solanaceae: Solanales) Acta Trop. 2013;128(3):613. doi: 10.1016/j.actatropica.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Marslin G, Siram K, Maqbool Q, Selvakesavan RK, Kruszka D, Kachlicki P, Franklin G. Secondary metabolites in the green synthesis of metallic nanoparticles. Mater. 2018;11:940. doi: 10.3390/ma11060940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J. Nucl. Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 6.Ren X, Meng X, Chen D, Tang F, Jiao J. Using silver nanoparticles to enhance current response of biosensor. Biosensor. Bioelectro. 2005;21:433–437. doi: 10.1016/j.bios.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 7.Kamat PV. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. 2002;106:7729–7744. [Google Scholar]
- 8.Nicewarner-Peña SR, Freeman RG, Reiss BD, He L, Peña DJ, Walton ID, Cromer R, Keating CD, Natan MJ. Submicrometer metallic barcodes. Science. 2001;294:137–141. doi: 10.1126/science.294.5540.137. [DOI] [PubMed] [Google Scholar]
- 9.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 2008;10:507–517. [Google Scholar]
- 10.Agasti SS, Rana S, Park MH, Kim CK, You CC, Rotello VM. Nanoparticles for detection and diagnosis. Adv Drug Deliv Rev. 2010;62:316–328. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanda A, Saravanan M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine. 2009;5:452–456. doi: 10.1016/j.nano.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Orlowski P, Tomaszewska E, Gniadek M, Baska P, Nowakowska J, Sokolowska J, Nowak Z, Donten M, Celichowski G, Grobelny J, et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE. 2014;9:e104113. doi: 10.1371/journal.pone.0104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteiro D, et al. Antifungal activity of silver nanoparticles in combination with nystatin and chlorhexidine digluconate against Candida albicans and Candida glabrata biofilms. Mycoses. 2013;56:672–680. doi: 10.1111/myc.12093. [DOI] [PubMed] [Google Scholar]
- 14.David L, Moldovan B, Vulcu A, Olenic L, Perde-Schrepler M, Fischer-Fodor E, Florea A, Crisan M, Chiorean I, Clichici S, et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces. 2014;122:767–777. doi: 10.1016/j.colsurfb.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 15.O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photothermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Mallick HK, Haldar B, Chandra G. Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall) Parasitol. Res. 2013;112:1451–1459. doi: 10.1007/s00436-013-3288-4. [DOI] [PubMed] [Google Scholar]
- 17.Duane JG. prevention and control of Aedes aegypti borne diseases: Lesson learned from past successes and failures. Asian Pac. J. Mol. Biol. Biotechnol. 2011;19(3):111–114. [Google Scholar]
- 18.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–505. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . Yellow fever. Geneva: World Health Organization; 2023. [Google Scholar]
- 20.WHO . Zika virus disease. Geneva: World Health Organization; 2016. [Google Scholar]
- 21.E.C.D.P.C. Chikungunya. European Centre for Disease Prevention and Control; 2022. [Google Scholar]
- 22.Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, Lindsay SW. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14(1):e0007831. doi: 10.1371/journal.pntd.0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anyaele OO, Amusan AAS. Toxicity of hexanoic extracts of Dennettia tripetala (G. Baxer) on larvae of Aedes aegypti (L) Afr. J. Biomed. Res. 2003;6:49–53. [Google Scholar]
- 24.Albrecht MA, Evan CW, Raston CL. Green chemistry and the health implications of nanoparticles. Green Chem. 2006;8:417–432. [Google Scholar]
- 25.Patil CD, Borase HP, Patil SV, Salunkhe RB, Salunke BK. Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and non-target fish Poecillia reticulata. Parasitol. Res. 2012;111(2):555–562. doi: 10.1007/s00436-012-2867-0. [DOI] [PubMed] [Google Scholar]
- 26.Venugopal S, Alagesan V. Brief review of the genus Diospyros Montana Roxb: Phytopharmacological properties. Ext. Rev. 2022;2(1):11–19. [Google Scholar]
- 27.Malla RK, Mandal P, Chandra G. Evaluation of larvicidal efficacy of Diospyros montana leaf extract on Aedes albopictus. Int. J. Mosq. Res. 2023;10(2):15–22. [Google Scholar]
- 28.Kamaraj C, Bagavan A, Rahuman AA, Zahir AA, Elango G, Pandiyan G. Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera Culicidae) Parasitol. Res. 2009;104:1163–1171. doi: 10.1007/s00436-008-1306-8. [DOI] [PubMed] [Google Scholar]
- 29.Chandra G. Mosquito. Sribhumi Publication Company; 2000. pp. 1–102. [Google Scholar]
- 30.Harborne JB. Phytochemical methods. Chapman and Hall Ltd; 1984. pp. 49–188. [Google Scholar]
- 31.Trease GE, Evans WC. Pharmacognosy Brailliar Tiridel can. 13. Macmillian Publishers; 1989. [Google Scholar]
- 32.Sofowara A. Medicinal plants and traditional medicine in Africa. Spectrum Books Ltd; 1993. p. 289. [Google Scholar]
- 33.WHO-World Health Organization . Report of the WHO informal consultation on the evaluation on the testing of insecticides. CTD/WHO PES/IC/96.1. Geneva: WHO; 1996. p. 69. [Google Scholar]
- 34.Abbott WS. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–266. [Google Scholar]
- 35.Finney DJ. Probit analysis. 3. Cambridge University Press; 1971. [Google Scholar]
- 36.Lee YJ, Park Y. Green synthetic Nanoarchitectonics of gold and silver nanoparticles prepared using quercetin and their cytotoxicity and catalytic applications. J. Nanosci. Nanotechnol. 2020;20(5):2781–2790. doi: 10.1166/jnn.2020.17453. [DOI] [PubMed] [Google Scholar]
- 37.Osonga FJ, Akgul A, Yazgan I, Akgul A, Ontman R, Kariuki VM, Eshun GB, Sadik OA. Flavonoid-derived anisotropic silver nanoparticles inhibit growth and change the expression of virulence genes in Escherichia coli SM10. RSC Adv. 2018;8(9):4649–4661. doi: 10.1039/c7ra13480k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng SP, Zhang JY, Ma ZW, Wen S, Tan S, Cai JY. Facile synthesis of long-term stable silver nanoparticles by kaempferol and their enhanced antibacterial activity against Escherichia coli and Staphylococcus aureus. J. Inorg. Organomet. Polym. Mater. 2018;31:2766–2778. [Google Scholar]
- 39.Hussain S, Khan Z. Epigallocatechin-3-gallate-capped Ag nanoparticles: Preparation and characterization. Bioprocess Biosyst Eng. 2014;37:1221–1231. doi: 10.1007/s00449-013-1094-0. [DOI] [PubMed] [Google Scholar]
- 40.Amruthraj NJ, Raj JP, Lebel A. Capsaicin-capped silver nanoparticles: Its kinetics, characterization and biocompatibility assay. Appl Nanosci. 2015;5:403–409. [Google Scholar]
- 41.Mulvaney P. Surface plasmon spectroscopy of Nanosized metal particles. Langmuir. 1996;12:788–800. [Google Scholar]
- 42.Jenkins, R., Snyder, R. L. Introduction to X-ray powder diffractometry, 138 (1996).
- 43.Becheri A, Dürr M, Lo Nostro P, Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J. Nanoparticle Res. 2008;10:679–689. [Google Scholar]
- 44.Magudapatty P, Gangopadhyayrans P, Panigrahi B, Nair KGM, Dhara S. Electrical transport studies of Ag nanoparticles embedded in glass matrix. Phys. B. 2001;299:142–146. [Google Scholar]
- 45.Baudot C, Tan CM, Kong JC. FTIR spectroscopy as a tool for nano-material characterization. Infrar. Phys. Technol. 2010;53:434–438. [Google Scholar]
- 46.Suganya A, Murugan K, Kovendan K, Mahesh Kumar P, Hwang JS. Green synthesis of silver nanoparticles using Murraya koenigii leaf extract against Anopheles stephensi and Aedes aegypti. Parasitol. Res. 2013;112:1385–1397. doi: 10.1007/s00436-012-3269-z. [DOI] [PubMed] [Google Scholar]
- 47.Guzman MG, Halstead SB, Artsob H, et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010;8:7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad ZZ, Che DN, Ahmed AI. Efficacy of Bacillus thuringiensis treatment on Aedes population using different applications at high-rise buildings. Trop. Med. Infect. Dis. 2020;5(2):67. doi: 10.3390/tropicalmed5020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adhikari U, Bhattacharya K, Mitra P, Chandra G. Larvicidal efficacy of silver nanoparticles synthesized biologically using Swietenia mahagoni (L.) Jacq. leaf extract against Anopheles stephensi, Culex quinquefasciatus and Cx. vishnui group. Indian J. Exp. Biol. 2018;56:14–19. [Google Scholar]
- 50.Suganya G, Karthi S, Shivakumar MS. Larvicidal potential of silver nanoparticles synthesized from Leucas aspera leaf extracts against dengue vector Aedes aegypti. Parasitolo. Res. 2014;113:875–880. doi: 10.1007/s00436-013-3718-3. [DOI] [PubMed] [Google Scholar]
- 51.Sundaravadivelan C, Nalini Padmanabhan M, Sivaprasath P, Kishmu L. Biosynthesized silver nanoparticles from Pedilanthus tithymaloides leaf extract with anti-developmental activity against larval instars of Aedes aegypti L. (Diptera; Culicidae) Parasitol Res. 2013;112(1):303–311. doi: 10.1007/s00436-012-3138-9. [DOI] [PubMed] [Google Scholar]
- 52.Raffi M, Hussain F, Bhatti TM, Akhter JI, Hameed A, Hasan MM. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008;24:192–196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).