Abstract
The activity of malate synthase (MS) (EC 4.1.3.2) appears and increases during cotton (Gossypium hirsutum L.) seed maturation, persists through desiccation and imbibition, then increases again following germination. The research reported herein is a comparative study of the synthesis and acquisition of MS into glyoxysomes as they occur in maturing and germinated seeds. Rate-zonal centrifugation of cotyledon extracts revealed that the 5 Svedberg unit (S) cytosolic form of MS was the only form present at 42 days postanthesis (DPA) when activity was first detectable. At later stages (48 DPA, 0 day, 26 hours, and 48 hours), both the 5S and glyoxysomal 20S forms were present, with the 20S form becoming much more prevalent. Western blot analyses revealed that no other form(s) of MS were present in the phosphate-buffered gradients, and that 5S and 20S forms had the same subunit molecular weight in maturing and germinated seeds. Comparisons of radiospecific activity of MS immunoprecipitates following in vivo labeling with [35S]methionine for varying time intervals provided strong evidence for a 5S-precursor to 20S-product relationship during both seed maturation and seedling growth. Comparisons of MS labeled in vivo and in vitro in wheat germ and rabbit reticulocyte lysates programmed with poly(A)+RNA (from maturing and germinated seeds) revealed no detectable differences in subunit molecular weights. These results reinforced our other data indicating that MS was synthesized in the cytosol and acquired by glyoxysomes in both maturing and germinated cotton seeds without involvement of an intervening aggregate pool in the endoplasmic reticulum, or via processing of a cleavable precursor molecule. MS was translated from poly(A)+RNA extracted from 28 DPA cotton seeds. This was nearly 2 weeks before MS activity or protein was detected in vivo. This finding invites further study on the regulation of RNA transcripts during maturation.
Full text
PDF

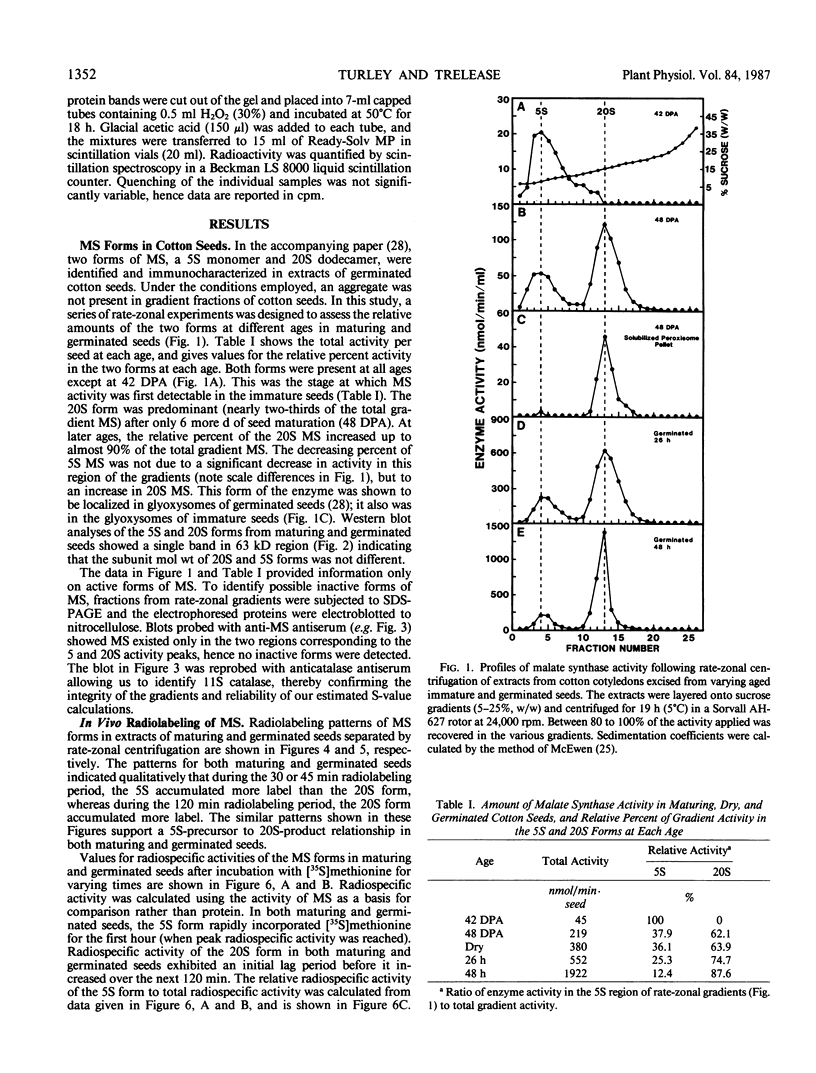
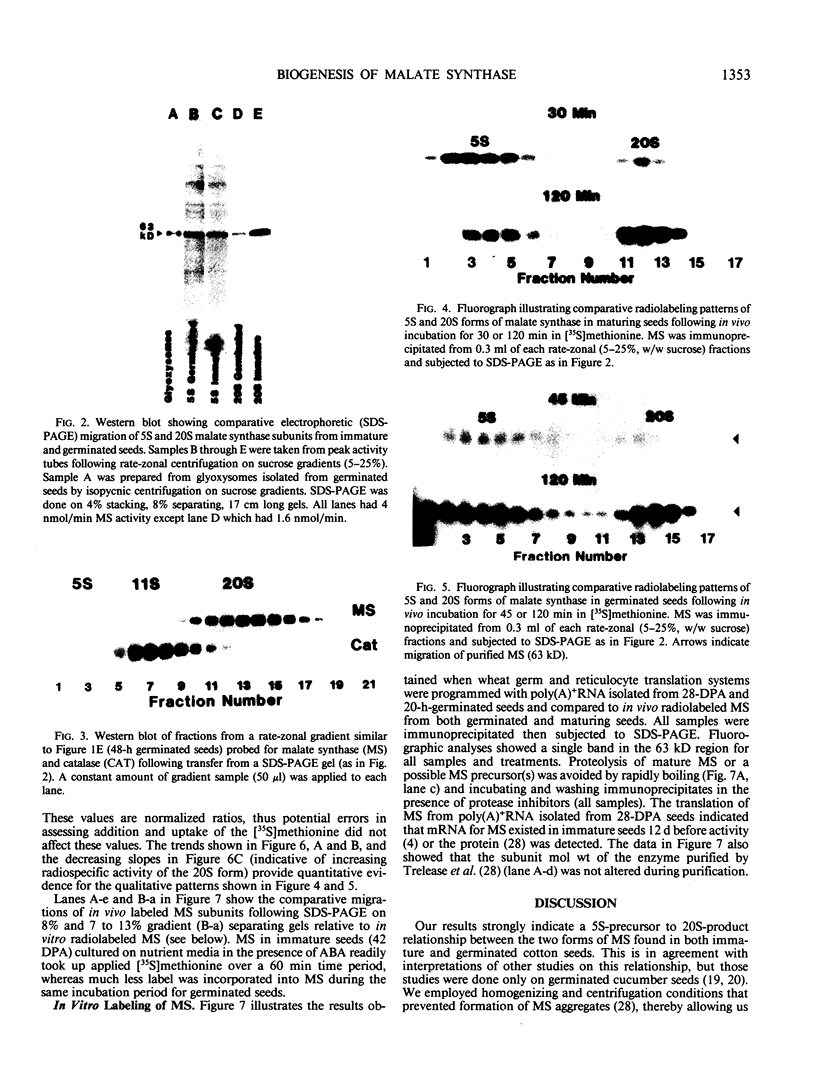
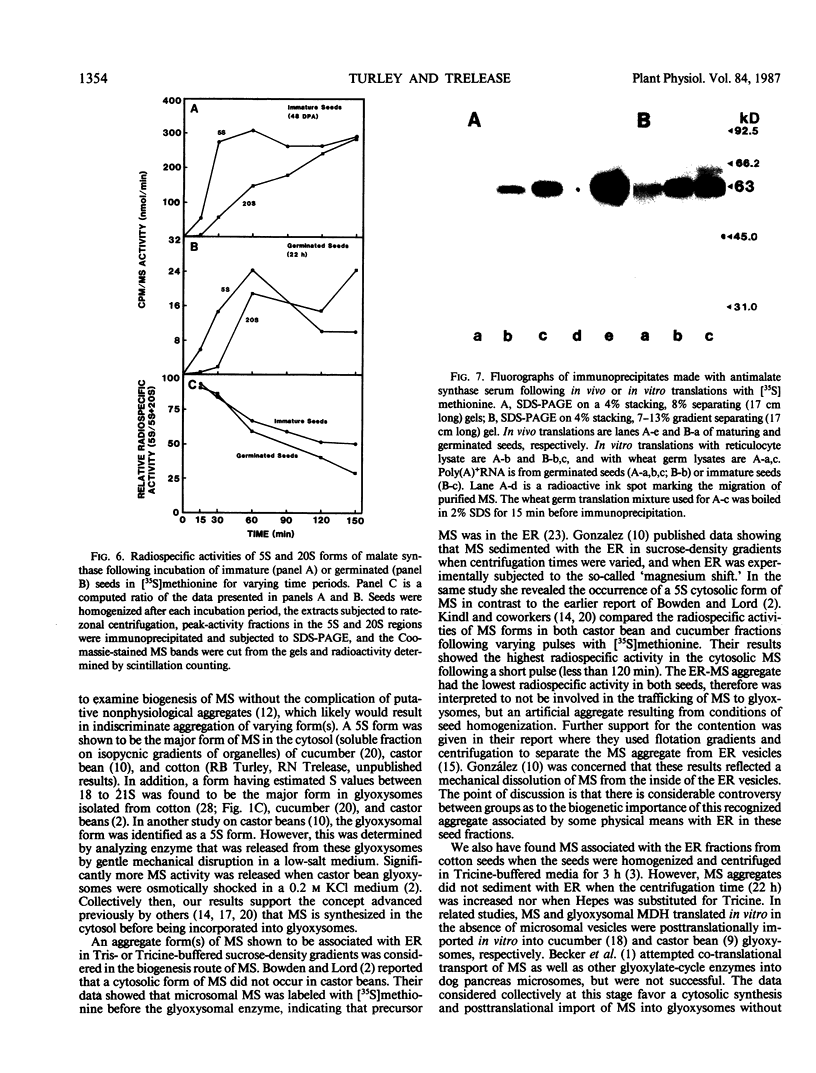


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden L., Lord J. M. Purification and comparative properties of microsomal and glyoxysomal malate synthase from castor bean endosperm. Plant Physiol. 1978 Feb;61(2):259–265. doi: 10.1104/pp.61.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choinski J. S., Trelease R. N. Control of Enzyme Activities in Cotton Cotyledons during Maturation and Germination: II. Glyoxysomal Enzyme Development in Embryos. Plant Physiol. 1978 Jul;62(1):141–145. doi: 10.1104/pp.62.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert J., Köller W., Kindl H. Occurrence and biosynthesis of glyoxysomal enzymes in ripening cucumber seeds. Hoppe Seylers Z Physiol Chem. 1980 Oct;361(10):1557–1565. doi: 10.1515/bchm2.1980.361.2.1557. [DOI] [PubMed] [Google Scholar]
- González E. Aggregated Forms of Malate and Citrate Synthase are Localized in Endoplasmic Reticulum of Endosperm of Germinating Castor Bean. Plant Physiol. 1982 Jan;69(1):83–87. doi: 10.1104/pp.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindl H., Köller W., Frevert J. Cytosolic precursor pools during glyoxysome biosynthesis. Hoppe Seylers Z Physiol Chem. 1980;361(3):465–467. [PubMed] [Google Scholar]
- Kindl H. The biosynthesis of microbodies (peroxisomes, glyoxysomes). Int Rev Cytol. 1982;80:193–229. doi: 10.1016/s0074-7696(08)60370-8. [DOI] [PubMed] [Google Scholar]
- Kruse C., Kindl H. Malate synthase: aggregation, deaggregation, and binding of phospholipids. Arch Biochem Biophys. 1983 Jun;223(2):618–628. doi: 10.1016/0003-9861(83)90626-4. [DOI] [PubMed] [Google Scholar]
- Kruse C., Kindl H. Oligomerization of malate synthase during glyoxysome biosynthesis. Arch Biochem Biophys. 1983 Jun;223(2):629–638. doi: 10.1016/0003-9861(83)90627-6. [DOI] [PubMed] [Google Scholar]
- Kunce C. M., Trelease R. N. Heterogeneity of catalase in maturing and germinated cotton seeds. Plant Physiol. 1986 Aug;81(4):1134–1139. doi: 10.1104/pp.81.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köller W., Kindl H. 19S cytosolic malate synthase. A small pool characterized by rapid turnover. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1437–1444. doi: 10.1515/bchm2.1980.361.2.1437. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Bowden L. Evidence that glyoxysomal malate synthase is segregated by the endoplasmic reticulum. Plant Physiol. 1978 Feb;61(2):266–270. doi: 10.1104/pp.61.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Riezman H., Weir E. M., Leaver C. J., Titus D. E., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 3. IN VITRO TRANSLATION AND CHARACTERIZATION OF FOUR GLYOXYSOMAL ENZYMES. Plant Physiol. 1980 Jan;65(1):40–46. doi: 10.1104/pp.65.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Leaver C. J. Glyoxysomal Malate Synthase of Cucumber: Molecular Cloning of a cDNA and Regulation of Enzyme Synthesis during Germination. Plant Physiol. 1986 Jul;81(3):762–767. doi: 10.1104/pp.81.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Hermerath C. A., Turley R. B., Kunce C. M. Cottonseed malate synthase : purification and immunochemical characterization. Plant Physiol. 1987 Aug;84(4):1343–1349. doi: 10.1104/pp.84.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir E. M., Riezman H., Grienenberger J. M., Becker W. M., Leaver C. J. Regulation of glyoxysomal enzymes during germination of cucumber. Temporal changes in translatable mRNAs for isocitrate lyase and malate synthase. Eur J Biochem. 1980 Dec;112(3):469–477. [PubMed] [Google Scholar]








