Abstract
The prevalence of Helicobacter pylori remains high in the older population. Specific age-related peculiarities may impact the outcomes of H. pylori treatment. The aim of the study was to evaluate the diagnostics and effectiveness of H. pylori eradication between the younger and older European populations. “European Registry on H. pylori Management (Hp-EuReg)” data from 2013 to 2022 were analyzed. Patients were divided into older (≥ 60 years) and younger (18–59 years) groups. Modified intention-to-treat (mITT) and per-protocol (PP) analysis was performed. 49,461 patients included of which 14,467 (29%) were older-aged. Concomitant medications and penicillin allergy were more frequent among the older patients. Differences between younger and older populations were observed in treatment duration in first-line treatment and in proton pump inhibitors (PPIs) doses in second-line treatment. The overall incidence of adverse events was lower in the older adults group. The overall first-line treatment mITT effectiveness was 88% in younger and 90% in the older patients (p < 0.05). The overall second-line mITT treatment effectiveness was 84% in both groups. The effectiveness of the most frequent first- and second-line triple therapies was suboptimal (< 90%) in both groups. Optimal efficacy (≥ 90%) was achieved by using bismuth and non-bismuth-based quadruple therapies. In conclusion, the approach to the diagnostics and treatment of H. pylori infection did not generally differ between younger and older patients. Main differences were reported in the concurrent medications, allergy to penicillin and adverse events both in first- and second-line treatment. Optimal effectiveness rates were mostly achieved by using bismuth and non-bismuth-based quadruple therapies. No clinically relevant differences in the effectiveness between the age groups were observed.
Subject terms: Gastrointestinal diseases, Infectious diseases, Geriatrics, Therapeutics, Diseases, Gastroenterology, Health care, Signs and symptoms, Medical research, Epidemiology, Antibiotics, Infectious-disease epidemiology, Microbiology, Infectious-disease diagnostics
Introduction
Helicobacter pylori (H. pylori) is the main cause of chronic gastritis as well as one of the main etiopathogenetic factors in the development of peptic ulcer disease. It is the only bacterium that is classified as Class I (definite) carcinogen by World Health Organization (WHO) and is the main risk factor in the etiopathogenesis of gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma1–4.
Despite the fact that the prevalence of H. pylori is declining, especially in the younger population5,6, it is estimated that around 50% of the world’s populations is still infected with this bacterium7. Based on a wide systematic review and meta-analysis, the prevalence of H. pylori in Western Europe is 34%8 and is significantly higher in Eastern and Southern European countries9.
The prevalence of H. pylori is related to the cohort effect, meaning that it is higher among the cohorts with earlier date of birth, i.e., the older the cohort, the higher the prevalence of H. pylori. The vast majority of people acquire this bacterium in their early childhood (up to 10 years of age) and the possibility of a later infection is rather low10. After the treatment of H. pylori the reinfection rate is also low11; however it might be higher in the areas with low socioeconomic status and high prevalence of H. pylori.
Epidemiological studies have reported that the world’s population is shifting towards older adults (> 60 years old) and the number of persons aged over 60 years is expected to double in the upcoming 10 years12,13. It has been reported that the prevalence of H. pylori in older population is significantly higher compared to younger people14, resulting in higher incidence of gastric cancer, peptic ulcer disease and other H. pylori-associated conditions in this population15. Even though there is a clear lack of epidemiological research in older patients, the available studies have reported the prevalence of H. pylori ranging from 40% up to 75% in these subjects14,15. Older adults are also associated with other age-related peculiarities, such as a higher number of comorbidities and concurrent medications, lower treatment compliance, impaired renal function, changes in drug metabolism16,17 as well as other physiological changes18.
Both the currently updated Maastricht VI/Florence, as well as previous Maastricht V/Florence, Consensus Reports4,19 on the management of H. pylori infection have decided not to separate the recommended treatment regimens in different age groups as it has been reported that these regimens are equally effective both in older and younger patients20. In fact, the limited available studies have reported that the standard triple therapy achieved close to optimal or, in seldom cases, optimal effectiveness and most of the quadruple therapies and sequential therapies have been reported to achieve optimal (> 90%) eradication rates in older-aged groups19–21. It has been described that H. pylori eradication prescriptions are safe and effective in older adults, although the experience is quite confined21,22.
There is a scarcity of data not only regarding the H. pylori diagnostics and treatment in older patients but also comparing the results with younger subjects. The latter could evaluate more in depth both population’s characteristics and potentially help in the therapeutical tailoring in the different age groups. In fact, after performing a literature search, we could not retrieve any European studies in recent years that have compared the diagnostics and treatment of H. pylori between older and younger populations.
On the other hand, it is known that H. pylori resistance rates to antibiotics are increasing worldwide. An extensive analysis reported that primary clarithromycin resistance in European countries has doubled in the last 20 years23. It is likely that older adults might have patterns of higher antimicrobial resistance due to the lifelong exposure to various antibiotics24,25. However, a population based study in China revealed that the failure rates of clarithromycin containing triple therapy were especially high in younger populations, whereas the higher cure rates were observed in the older subjects26.
The aim of this study was therefore to evaluate and compare the diagnostic methods and treatment prescriptions for H. pylori infection as well as the effectiveness of the most frequent first- and second-line H. pylori eradication regimens between the older and younger adults in Europe.
Methods
European registry on H. pylori management (Hp-EuReg)
The Hp-EuReg is an international, multicentre, prospective, non-interventional registry that has been recording information on the management of H. pylori infection since the year 2013. The Hp-EuReg protocol27 establishes national coordinators in the selected 29 countries, where gastroenterologists have been recruited at over 300 study centres to provide input to the registry.
Participants and study groups
This study data was obtained from the centres of all the participating countries. The participants were patients that were included in the Hp-EuReg since the year 2013 up to the January of 2022. All of the patients were adults, who were diagnosed with H. pylori infection.
The study participants were divided into two groups based on the age: younger (18–59 years old) and older (≥ 60 years old). The age cut-off value between the groups was selected based on the “older adults” definition by WHO and some sources from the United Nations (UN)28,29.
Data collection
Data were collected through an electronic Case Report Form (e-CRF), collecting the patient’s demographic information, any previous eradication attempts, and the treatments employed, as well as the outcomes of the treatment, recording details such as the compliance, the cure rate, the follow-up, etc. and the adverse events (AEs). This information was registered at the REDCap database30 managed and hosted by the "Asociación Española de Gastroenterología" (AEG; http://www.aegastro.es), a non-profit Scientific and Medical Society that focuses on Gastroenterology research.
Data management
Data were quality reviewed by evaluating whether the study selection criteria had been met, whether information was correctly registered and ultimately to ensure the study was conducted according to the highest scientific and ethical standards, in accordance with the latest revision of the ethical guidelines firstly announced in 1975 Declaration of Helsinki. Data discordances were resolved by querying the investigators and through group emailing.
Variables categorization and definitions
PPI doses were categorized according to the potency of acid inhibition, as low-dose (4.5–27 mg of omeprazole equivalents given twice a day), standard-dose (32–40 mg of omeprazole equivalents given twice a day), or high-dose (54–128 mg of omeprazole equivalents given twice a day)31,32. Likewise, the duration of treatment was categorized as 7, 10, or 14 days to ease the interpretation. Sub-analyses were performed according to the treatment duration and PPI doses comparing both age groups (younger and older subjects).
The following categories were used for the most frequently prescribed first-line treatments: Triple-CA/M (clarithromycin, amoxicillin/metronidazole), Seq CAT-CAM (clarithromycin, amoxicillin, tinidazole—clarithromycin, amoxicillin, metronidazole), Quad-CAT-CAM, Quad-Pylera® (three-in-one single-capsule containing metronidazole, tetracycline and bismuth) + Quad Bi (bismuth), Quad-CAB (clarithromycin, amoxicillin, bismuth) and other. Likewise, following categories were used for the most frequent second-line treatments: Quad Pylera® + Quad Bi, Quad-LAB (levofloxacin, amoxicillin, bismuth), Triple-AL (amoxicillin, levofloxacin), Conco-Seq (concomitant-sequential), and other.
In order to calculate the effectiveness of different H. pylori eradication regimens, per-protocol (PP), intention to treat (ITT) and modified intention-to-treat (mITT) analyses were used. The ITT analysis includes all cases registered in Hp-EuReg, allowing at least a 6-month follow-up, and lost to follow-up cases were considered treatment failures. The PP analysis includes all cases that have completed follow-up (i.e., had undertaken a valid confirmatory test after the eradication treatment) and had taken at least 90% of the treatment drugs, as defined in the protocol. The mITT was designed to reach the closest results to those obtained in clinical practice, and therefore included all cases that had completed the follow-up, regardless of the compliance to treatment. All the patients, that were empirically treated (that is, without prescribing a susceptibility-guided antibiotic treatment) were included in the effectiveness analysis.
AEs and compliance were evaluated through patient questioning with both open-ended questions and a predefined questionnaire, by face-to-face interview. Compliance was defined, through physician questioning, as having taken at least 90% of the prescribed drugs.
AEs were classified depending on the intensity of symptoms evaluated by the corresponding physician: mild (not interfering with daily routine), moderate (affecting daily routine), intense/severe (not allowing normal daily routine), and serious (causing death, hospitalization, disability, congenital anomaly, and/or requiring intervention to prevent permanent damage).
Statistical analyses
Continuous variables were summarized as the mean and standard deviation, while qualitative variables were presented as the absolute relative frequencies, displayed as percentages (%) together with their 95% CIs, where applicable. The selected level of statistical significance was p < 0.05 (two-tailed).
All treatments were accounted in the descriptive and univariate analyses; for the purpose of the multivariate analysis, logistic regression (LR) was performed first controlling by the most frequently prescribed first-line treatments and then by the most frequently second-line treatments.
The LR used a backward modelling strategy and compared models using the log-likelihood ratio. The mITT population was the dependent variable used to evaluate the association between the treatments' eradication rate and the following independent variables: age [ref. 18–59 years old group], sex [ref. female], indication [ref. dyspepsia], compliance [ref. No, < 90% drug intake], PPI dose [ref. low dose], treatment length [ref. 7 days] and prescribed eradication regimens (for the first line-treatment [ref. Triple-CA/M]; for the second-line treatment [ref. Quad Pylera® + Quad Bi] as defined earlier]. In the multivariate analysis, the effect was evaluated by calculating odds ratios (ORs) and the corresponding 95% confidence intervals (95% CIs).
Ethics approval statement
The study was approved by the Ethics Committee of La Princesa University Hospital (Madrid, Spain). It was registered at ClinicalTrials.gov (NCT02328131).
Results
Baseline characteristics
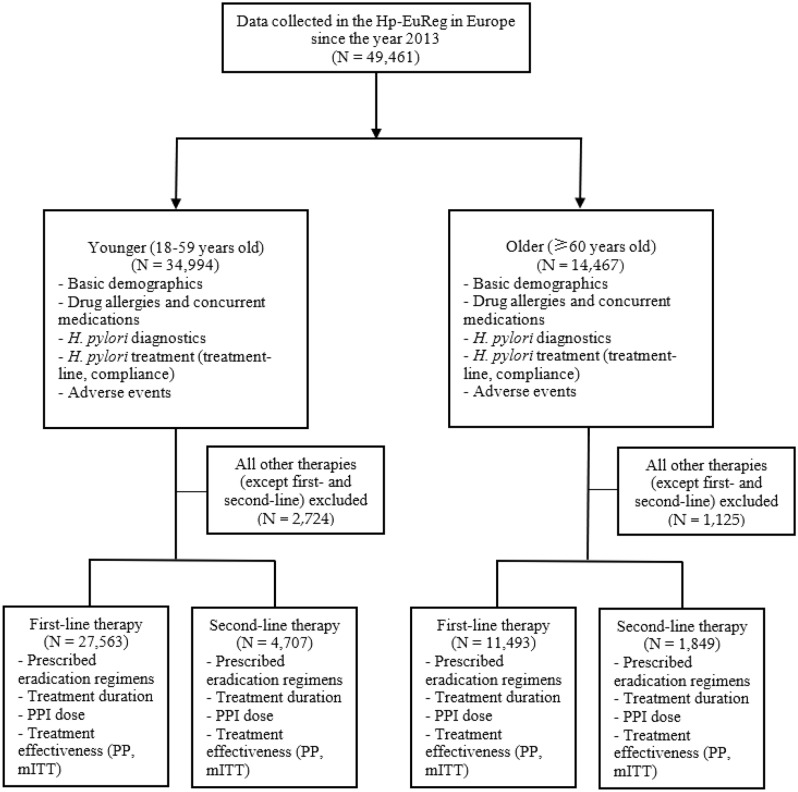
Figure 1 depicts the flow-chart of the study. The main results of the differences in the demographics, diagnostics, and treatment of H. pylori infection between the older and younger age groups is presented in Table 1.
Figure 1.
Study flow chart. PP per-protocol, mITT modified Intention-to-treat.
Table 1.
Demographics, diagnostics, and treatment prescriptions in H. pylori management between older and younger European populations.
| Older (≥ 60 years) | Younger (18–59 years) | p-value | |
|---|---|---|---|
| Total number of patients, N (%) | 14,467 (29%) | 34,994 (71%) | |
| Gender, N (%) | |||
| Male | 5365 (37%) | 14,244 (41%) | < 0.05 |
| Female | 9093 (63%) | 20,730 (59%) | < 0.05 |
| Average age (mean ± standard deviation) | 68.1 ± 6.3 | 42.8 ± 10.7 | < 0.05 |
| Main treatment indications, N (%) | |||
| Uninvestigated dyspepsia | 5111 (36%) | 13,248 (38%) | < 0.05 |
| Peptic ulcer disease | 2893 (20%) | 5094 (15%) | < 0.05 |
| Main symptoms, N (%) | |||
| Dyspepsia | 10,571 (73%) | 27,070 (77%) | < 0.05 |
| Heartburn | 3831 (26.3%) | 9183 (26.5%) | > 0.05 |
| Concurrent medications, N (%) | 7364 (55%) | 8834 (27%) | < 0.05 |
| Drug allergies, N (%) | 796 (6%) | 1306 (4%) | < 0.05 |
| Penicillin | 650 (4.5%) | 1050 (3%) | < 0.05 |
| Macrolides | 43 (0.3%) | 92 (0.3%) | > 0.05 |
| Fluoroquinolones | 27 (0.2%) | 50 (0.1%) | > 0.05 |
| Main diagnostic methods pre-treatment, N (%) | |||
| Histology | 6840 (47%) | 13,191 (38%) | < 0.05 |
| Rapid urease test | 5463 (38%) | 13,138 (38%) | > 0.05 |
| Urea breath test | 3550 (25%) | 10,702 (31%) | < 0.05 |
| Main diagnostic tests post-treatment, N (%) | |||
| Urea breath test | 9544 (66%) | 21,755 (62%) | < 0.05 |
| Stool antigen test | 1968 (13.6%) | 5401 (15.4%) | < 0.05 |
| Histology | 715 (5%) | 1529 (4%) | < 0.05 |
| Treatment compliance, N (%) | 12,938 (97%) | 31,270 (97%) | > 0.05 |
| First-line prescriptions, N (%) | |||
| Triple-therapy | 4851 (42%) | 12,492 (45%) | < 0.05 |
| Quadruple-therapy | 5182 (45%) | 12,641 (46%) | < 0.05 |
| Second-line prescriptions, N (%) | |||
| Triple-therapy | 788 (43%) | 2134 (45%) | < 0.05 |
| Quadruple-therapy | 956 (52%) | 2393 (51%) | < 0.05 |
| PPI potency in first-line treatment, N (%) | |||
| Low | 5275 (46%) | 12,312 (45%) | > 0.05 |
| Standard | 2726 (24%) | 6831 (25%) | > 0.05 |
| High | 3516 (31%) | 8539 (31%) | > 0.05 |
| PPI potency in second-line treatment, N (%) | |||
| Low | 750 (40%) | 1691 (36%) | < 0.05 |
| Standard | 423 (23%) | 1059 (23%) | > 0.05 |
| High | 705 (38%) | 1983 (42%) | < 0.05 |
| First-line treatment duration, N (%) | |||
| 7 days | 1583 (14%) | 3170 (12%) | < 0.05 |
| 10 days | 5700 (50%) | 13,351 (49%) | < 0.05 |
| 14 days | 4185 (37%) | 11,019 (40%) | < 0.05 |
| Second-line treatment duration, N (%) | |||
| 7 days | 75 (4%) | 197 (4%) | > 0.05 |
| 10 days | 1023 (56%) | 2618 (56%) | > 0.05 |
| 14 days | 744 (40%) | 1881 (40%) | > 0.05 |
| Adverse events | |||
| Overall, N (%) | 3062 (23%) | 8144 (25%) | < 0.05 |
| Treatment cessation due to AEs, % | 1.5% | 1.2% | > 0.05 |
PPI—proton pump inhibitor.
Significant p-values are in bold.
Since the year 2013, data from 49,461 cases were included in the Hp-EuReg and used for current analysis. As already mentioned, the patients were divided into two groups based on their age: there were 14,467 (29%) older (aged 60 years or older) and 34,994 (71%) younger (18–59 years old) patients. There were 63% female in the older group and 59% female in the younger group (p < 0.05 between the age groups).
The older adults group reported a significantly higher intake of concurrent medications (the main evaluated drug groups were PPIs, acetylsalicylic acid, non-steroidal anti-inflammatory drugs and statins) as compared to the younger subjects (55% vs. 27% respectively, p < 0.05). Older-aged group was characterized by statistically significantly higher rate of allergy to penicillin (5% in the older compared to 3% in the younger group, p < 0.05), whereas there were no differences in other evaluated drug groups, such as macrolides (0.3% in both groups) and fluoroquinolones (0.2% vs. 0.1% respectively, p > 0.05).
Before H. pylori eradication dyspeptic symptoms were more frequently reported in older subjects as compared to the younger ones (77% vs. 73% respectively, p < 0.05), whereas there were no differences in the prevalence of heartburn (both groups 26%, p > 0.05). The main indications for the treatment of H. pylori infection in the older and younger age groups were dyspeptic syndrome (36% vs. 38% respectively) and peptic ulcer disease (20% vs. 15% respectively, p < 0.05). Further causes (for instance, gastroesophageal reflux disease, erosive gastritis etc.) accounted for 32% of the indications in older and 29% in younger patients groups (p < 0.05).
The main H. pylori diagnostic tests used in older and younger subjects before the treatment were histological examination (47% and 38% respectively, p < 0.05), rapid urease test (RUT) (both groups 38%, p > 0.05), urea breath test (UBT) (25% and 31% respectively, p < 0.05), microbiological culture (11% and 10% respectively, p < 0.05) and serology (7% vs. 8% respectively, p < 0.05). The main diagnostic tests post-treatment in both groups were UBT (66% and 62% respectively, p < 0.05), stool antigen test (14% and 15% respectively, p < 0.05), histological examination (5% and 4% respectively, p < 0.05) and RUT (3% in both groups, p > 0.05).
Prescriptions, treatment duration and PPIs
In both age groups 81% of the cases were treatment-naive. In the first-line treatment, quadruple therapy was prescribed in 45% of the older and 46% of the younger subjects (p < 0.05) and triple therapy in 42% and 45% respectively (p < 0.05). In the second-line treatment quadruple therapy was prescribed in 52% of the older and 51% of the younger age cases (p < 0.05) and triple therapy in 43% and 45% respectively (p < 0.05).
The overall duration of first-line treatment in older and younger adults was 7 days in 14% and 12% of the cases respectively; 10 days in 50% and 49% respectively; and 14 days in 36% and 40% respectively (p < 0.05 in all groups). The overall duration of the second-line treatment was as follows: 7 days in 4%, 10 days in 56% and 14 days in 40% of the cases in both age groups (p > 0.05).
In the first-line treatment low PPI doses were most frequently prescribed in both groups and no significant differences in prescribed PPI doses were found between the groups. In the second-line treatment low doses of PPIs were most frequently prescribed in older adults (40% vs 36% respectively, p < 0.05) and high doses of PPIs most frequently in the younger adults (38% vs 42% respectively, p < 0.05).
Treatment effectiveness
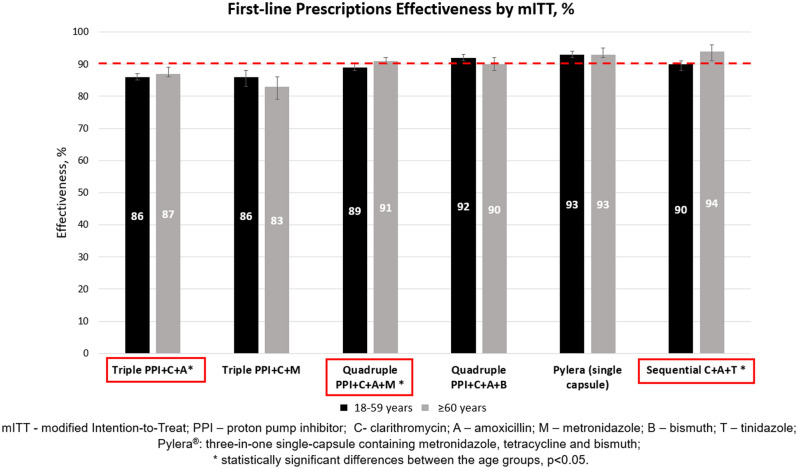
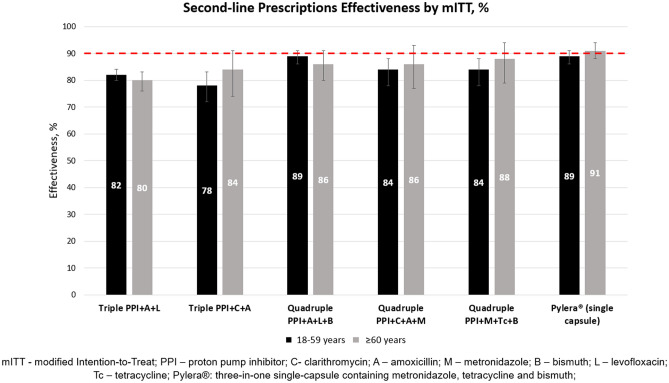
The detailed analysis of the most widely used first- and second-line prescriptions in the older and younger age groups and their effectiveness (ITT, mITT and PP) comparison is presented in Table 2. The graphic comparison of the mITT effectiveness of the six most frequently prescribed first- and second-line treatments between the age groups is illustrated in Figs. 2 and 3, respectively.
Table 2.
First- and second-line H. pylori treatment effectiveness in the younger and older European populations.
| Treatment | Younger adults (18–59 years) | Older adults (≥ 60 years) | ||||||
|---|---|---|---|---|---|---|---|---|
| Use, N | PP % (95% CI) | mITT % (95% CI) | ITT % (95% CI) | Use, N | PP % (95% CI) | mITT % (95% CI) | ITT % (95% CI) | |
| Effectiveness of first-line treatment prescriptions between the younger and older populations | ||||||||
| Triple PPI + C + A | 10,540 | 87 (86–87) | 86 (85–87)* | 67 (66–68)* | 4065 | 88 (87–89) | 87 (86–89)* | 71 (69–72)* |
| Triple PPI + C + M | 1150 | 86 (84–88) | 86 (83–88) | 68 (65–71) | 491 | 83 (79–86) | 83 (79–86) | 71 (66–75) |
| Triple PPI + A + L | 425 | 83 (79–87) | 83 (78–86) | 76 (72–80) | 162 | 83 (75–88) | 83 (76–89) | 76 (69–82) |
| Quadruple PPI + C + A + M | 4199 | 90 (89–91)* | 89 (88–90)* | 86 (85–87)* | 1940 | 91 (90–93)* | 91 (90–92)* | 89 (87–90)* |
| Quadruple PPI + C + A + B | 3489 | 92 (91–93) | 92 (91–93) | 76 (75–77) | 1086 | 91 (89–93) | 90 (88–92) | 76 (73–79) |
| Quadruple PPI + C + A + T | 309 | 98 (95–99) | 96 (93–98) | 92 (88–95) | 127 | 96 (89–99) | 92 (85–96) | 86 (78–92) |
| Pylera® (single capsule)1 | 3233 | 94 (93–95) | 93 (92–94) | 89 (87–90) | 1519 | 94 (93–95) | 93 (92–95) | 89 (87–91) |
| Sequential C + A + T | 1291 | 90 (88–92)* | 90 (88–91)* | 80 (78–82)* | 623 | 94 (92–96)* | 94 (91–96)* | 85 (81–87)* |
| Sequential C + A + M | 458 | 84 (80–87) | 82 (78–86) | 76 (72–80) | 222 | 84 (78–89) | 82 (76–87) | 75 (69–81) |
| Effectiveness of second-line treatment prescriptions between the younger and older populations | ||||||||
| Triple PPI + A + L | 1422 | 83 (80–85) | 82 (80–84) | 74 (71–76) | 524 | 80 (76–84) | 80 (76–83) | 73 (69–77) |
| Triple PPI + C + A | 323 | 78 (72–83) | 78 (72–83) | 60 (54–65) | 102 | 84 (74–91) | 84 (74–91) | 68 (58–77) |
| Triple PPI + A + R | 105 | 80 (70–87) | 79 (69–87) | 68 (58–76) | 49 | 82 (67–92) | 82 (67–92) | 74 (59–85) |
| Triple PPI + A + Mx | 105 | 92 (85–97) | 92 (85–97) | 90 (82–95) | 35 | 87 (70–96) | 87 (70–96) | 77 (60–90) |
| Quadruple PPI + A + L + B | 584 | 89 (86–92) | 89 (86–91) | 73 (69–76) | 225 | 86 (80–91) | 86 (80–91) | 75 (69–81) |
| Quadruple PPI + C + A + M | 234 | 84 (79–89) | 84 (78–88) | 80 (74–85) | 91 | 86 (77–92) | 86 (77–93) | 83 (73–90) |
| Quadruple PPI + M + Tc + B | 224 | 86 (80–91) | 84 (78–88) | 75 (69–81) | 101 | 87 (78–93) | 88 (79–94) | 77 (68–85) |
| Quadruple PPI + C + A + B | 229 | 91 (85–96)* | 91 (85–95)* | 52 (45–59) | 66 | 79 (64–89)* | 80 (66–90)* | 60 (47–72) |
| Pylera® (single capsule)1 | 843 | 89 (87–91) | 89 (86–91) | 83 (80–86)* | 384 | 92 (89–95) | 91 (88–94) | 88 (84–91)* |
PP—per protocol, mITT—modified Intention-To-Treat, ITT—Intention-To-Treat, 95% CI—95% confidence interval, PPI—proton pump inhibitor, C—clarithromycin, A—amoxicillin, M—metronidazole, B—bismuth, T—tinidazole, L—levofloxacin, Tc—tetracycline, Mx—moxifloxacin, R—rifabutin.
1Pylera®: three-in-one single-capsule containing metronidazole, tetracycline and bismuth.
*Statistically significant differences between the age groups, p < 0.05.
Figure 2.
Comparison of the mITT effectiveness of 6 most frequent first-line prescriptions between the younger and older adults.
Figure 3.
Comparison of the mITT effectiveness of six most frequent second-line prescriptions between the younger and older adults.
The overall first-line treatment effectiveness was 89% (95% CI 89–90%) by PP and 88% (95% CI 88–89%) by mITT in the younger and 89% (95% CI 88–89%) by PP and 90% (95% CI 89–91%) by mITT in the older groups. The effectiveness of most frequently prescribed first-line regimen, which was standard triple therapy (PPI + C + A), was 88% by PP and 87% by mITT in the older group, whereas it was 87% by PP and 86% by mITT in the younger group (p < 0.05 only comparing the mITT effectiveness), meaning it reached suboptimal (< 90%) efficacy rates in either group. The remaining triple therapies failed to reach optimal eradication rates as well. Optimal (≥ 90%) eradication rates in the first-line treatment were achieved by using quadruple therapies (quadruple PPI + C + A + B, quadruple PPI + C + A + T and single-capsule bismuth quadruple therapy) and the most frequently used sequential therapy (PPI + C + A + T) in both age groups. Statistically significant differences in the eradication effectiveness between the age groups were reported when standard triple therapy (PPI + C + A), quadruple PPI + C + A + M or sequential PPI + C + A + T therapies were used.
The overall second-line treatment effectiveness was 84% both by PP and by mITT in the younger and older subjects. The efficacy of the most popular second-line prescription PPI + A + L was 80% by PP and by mITT in the older adults group, whereas it was 83% by PP and 82% by mITT in the younger adults group (p > 0.05 by both analyses), meaning that it also achieved only suboptimal eradication rates. Optimal eradication rates in the younger group were achieved by using triple PPI + A + Mx (moxifloxacin) or quadruple PPI + C + A + B therapies and in the older group only single-capsule bismuth quadruple therapy managed to reach optimal treatment effectiveness. Statistically significant difference between the age groups in the second-line treatment effectiveness was only reported when quadruple PPI + C + A + B therapy was used.
Multivariate analysis
The detailed multivariate analysis of the first- and second-line treatments is presented in Table 3. After performing the multivariate analysis it has been calculated that compliance with treatment, quadruple bismuth-based prescriptions (including single-capsule bismuth quadruple therapy), longer treatment durations (14 days vs. 7 days; 10 days vs. 7 days), higher acid inhibition (high PPIs doses vs. low; medium PPIs doses vs. low), sequential CAT/CAM, quadruple CAT/CAM and quadruple CAB prescriptions as well as belonging to older adults group were the main factors associated with higher mITT cure rate in the first-line treatment.
Table 3.
Independent variables associated with higher mITT rates in the first- and second-line treatments.
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| First-line treatment | |||
| Treatment compliance | 7.34 | 6.12–8.80 | < 0.005 |
| Quadruple bismuth-based prescriptions | 2.36 | 2.06–2.70 | < 0.005 |
| 14 days vs. 7 days treatment duration | 1.73 | 1.54–1.95 | < 0.005 |
| High PPIs doses vs. low PPIs doses | 1.64 | 1.48–1.80 | < 0.005 |
| Sequential CAT/CAM prescriptions | 1.52 | 1.30–1.77 | < 0.005 |
| Medium PPIs doses vs. low PPIs doses | 1.42 | 1.27–1.57 | < 0.005 |
| Quadruple CAT/CAM prescriptions | 1.29 | 1.16–1.43 | < 0.005 |
| 10 days vs. 7 days treatment duration | 1.17 | 1.05–1.31 | < 0.005 |
| Quadruple CAB prescription | 1.17 | 1.02–1.35 | < 0.05 |
| Older adults group | 1.13 | 1.05–1.12 | < 0.005 |
| Second-line treatment | |||
| 14 days vs. 7 days treatment duration | 4.45 | 3.21–6.17 | < 0.005 |
| 10 days vs. 7 days treatment duration | 3.76 | 2.80–5.04 | < 0.005 |
| Treatment compliance | 3.56 | 2.38–5.23 | < 0.005 |
| High PPIs doses vs. low PPIs doses | 2.17 | 1.80–2.61 | < 0.005 |
| Medium PPIs doses vs. low PPIs doses | 1.52 | 1.25–1.83 | < 0.005 |
OR—odds ratio, 95% CI—95% confidence interval, PPIs—proton pump inhibitors, CAT—clarithromycin, amoxicillin, tinidazole, CAM—clarithromycin, amoxicillin, metronidazole, CAB—clarithromycin, amoxicillin, bismuth salts, mITT—modified intention-to-treat.
In the second-line treatment the main factors associated with higher mITT rates were longer treatment durations (14 days vs. 7 days; 10 days treatment vs. 7 days), good treatment compliance and higher PPIs doses (high PPIs doses vs. low; medium PPIs doses vs. low). Compared to quadruple bismuth-based prescriptions (including single-capsule bismuth quadruple therapy), Triple-AL and Conco-Seq prescriptions were associated with significantly lower mITT rates.
Compliance and safety
The treatment compliance was 97% in both groups. The overall AEs rate was lower in older subjects compared to the younger adults (23%; 95% CI 24–26% vs. 25%; 95% CI 22–24% respectively, p < 0.05), however severe AEs were more frequent in older patients. The most frequent AEs in older and younger subjects were dysgeusia (7% in both groups), nausea (7% and 8% respectively), diarrhea (6% and 8% respectively) and vomiting (2% and 3% respectively). Most of the AEs were mild to moderate in intensity and lasted 7–14 days. The most frequent severe AEs among the older and younger age groups were asthenia (23% and 11% respectively, p < 0.05), anorexia (16% and 10% respectively, p < 0,05) and abdominal pain (8% and 7% respectively, p > 0.05). Among the older adults 1.5% had to stop taking medications due to AEs, whereas it was 1.2% in the younger group (p > 0.05).
Discussion
In this study we have evaluated the differences of H. pylori diagnostics and treatment between the older and younger European populations. We would like to point out that such analysis is one of only very few available both evaluating an older-aged population and comparing it to younger subjects. Additionally, this is one of only few studies providing data on such a large number of patients from all Europe and enabling more accurate data and more reliable statistical results.
Our analysis confirmed the expected epidemiological hypothesis, regarding the baseline characteristics—compared to the younger patients; more older subjects were taking concurrent medications and reported a higher rate of allergy to penicillin. Even though a higher number of concurrent medications and higher rate of drug allergies is usually associated with worse treatment compliance33,34, this was not confirmed in our study and had no influence on the efficacy of the eradication therapy.
The diagnostics of H. pylori also complied with the current guidelines4,19 and the study confirmed that the invasive diagnostic methods (histology, RUT) were more frequent for the initial diagnosis of H. pylori prior to the treatment and, as anticipated, some of these methods were also more common in the older population. For the confirmation of eradication UBT was the preferred choice, followed by SAT in both age groups.
Most of the performed studies35–37 have revealed that quadruple therapies were superior to triple therapies regarding the effectiveness and, in fact, our study has demonstrated that quadruple therapies were more frequently prescribed for the first- and second-line treatment, in both age groups. The most frequent treatment duration was 10–14 days in both age groups, in line with the current guidelines. Seven days duration treatment is no longer recommended4,37,38 and even though there were some cases with 7 days treatment duration in both age groups, these cases were registered in the early years of the registry, as reported in previous Hp-EuReg research and, also in accordance with the Maastricht V/Florence consensus report, updated in the year 2016, where 7 days treatment duration was no longer recommended.
We could have expected that the doses of PPI might have been lower in the older population due to the higher chance of possible AEs (e.g., diarrhea, Clostridioides difficile infection); however we did not find any significant differences between the age groups regarding the PPI dose in the first line therapy and the differences in the second-line were not clinically relevant. The multivariate analysis revealed that high doses of PPIs were associated with better mITT rate in the first- and second-line therapies; however, low doses of PPIs were prescribed most frequently in the first-line treatment in both age groups. We could speculate that some of the prescribing gastroenterologists were not acquainted with the guidelines or were cautious of the possible AEs, especially in older patients. On the other hand, we should also point out that there was a clear shift from low doses of PPIs to high doses of PPIs throughout the duration of the Hp-EuReg. Low doses of PPIs were predominant in the beginning of the registry up until the year 2017; however, since the year 2017, after the release of updated Maastricht V/Florence consensus report, the rate of higher PPIs doses started increasing and is now predominantly represented by high-dose PPIs—almost half of the Hp-EuReg cases39.
Concerning H. pylori eradication regimens, we can state that the use of the main first and second-line prescriptions in the older and younger European populations met the recommendations of Maastricht V/Florence consensus report. However, the most frequently prescribed first-line therapy in both age groups, in spite of many countries with > 15% clarithromycin resistance rate, was standard triple therapy (PPI + C + A). Levofloxacin containing triple therapy was the most frequently used rescue regimen in both age groups, as previously recommended.
One of the main goals of this research was to evaluate the effectiveness of main first- and second-line H. pylori eradication regimens in the older and younger age groups. We have found that the overall first-line treatment effectiveness was very close to optimal (≥ 90%) eradication rates in younger subjects (89% by PP and 88% by mITT); whereas it was optimal by PP (90%) and very close to optimal by mITT (89%) in the older ones. The effectiveness of the most popular first-line prescription – standard triple therapy (PPI + C + A) was suboptimal in both age groups. Other triple therapies (PPI + C + M, PPI + A + L) did show even worse effectiveness. Optimal eradication rates were achieved only by using bismuth and non-bismuth-based quadruple therapies (PPI + C + A + B, PPI + C + A + T and single-capsule bismuth quadruple therapy (Pylera®)) and the most popular sequential therapy (PPI + C + A + T) in both age groups. The optimal effectiveness of these treatment regimens was also confirmed in other published studies40–42. Statistically significant differences in the effectiveness between the age groups were reported when standard triple therapy (PPI + C + A), quadruple PPI + C + A + M or sequential PPI + C + A + T therapies were used, while there were no differences in the remaining analysed prescriptions. In this respect, it is worthwhile mentioning that even though there were statistically significant differences between the age groups in various parameters, including the effectiveness of different prescriptions, these differences might be clinically non-significant and should be interpreted with caution, as most of them ranged between 1–2% and could be due merely to the very large sample size. Therefore, in most of the cases, we considered these differences to be clinically irrelevant, even though statistically significant.
The overall effectiveness of second-line treatment was suboptimal (84%) both by PP and mITT in both age groups. The effectiveness of the main second-line regimen (PPI + A + L) was suboptimal in both age groups. In fact, the only regimen that achieved optimal eradication rate in the older age group was the single-capsule bismuth quadruple therapy (Pylera®). In the younger group optimal eradication rates were achieved by using triple PPI + A + Mx and quadruple PPI + C + A + B prescriptions. In fact, the only statistically significant difference in second-line treatment effectiveness between the older and younger adults was obtained with the previously mentioned bismuth quadruple PPI + C + A + B prescription (80% vs. 91% by mITT respectively).
The multivariate analysis revealed the expected results – non-bismuth and, especially, bismuth-based quadruple therapies (the most frequent being PPI + C + A + B, single capsule Pylera® and PPI + C + A + M) were associated with better mITT cure rates in the first-line treatment as our effectiveness analysis revealed that most of these bismuth-containing regimens achieved optimal or close-to-optimal eradication rates. This was also confirmed in other studies4,35,36,41,42 and the current guidelines are shifting towards bismuth-based quadruple therapies as the main H. pylori treatment regimen4 recommendation given clarithromycin resistance rates are increasing worldwide23,43,44.
Another possible issue, which albeit was not confirmed in our study, was the possibility of worse treatment compliance in older subjects. Even though the older-aged populations were associated with a higher number of concurrent medications, the treatment compliance was very satisfactory in both age groups, reaching 97%.
Interestingly, the older adults experienced statistically significantly less AEs compared to the younger group; however, we should consider whether this difference is really clinically relevant and may be due, as previously stated, to the very large number of subjects in both age groups. Nonetheless, both age groups presented a similar safety profile (77% of the older and 75% of the younger adults without any AEs), whereas severe AEs were slightly more frequent in the older-aged subjects.
We can compare our study results to only a few other available similar studies. A Japanese study in the year 2019 also compared the diagnostics, efficacy and safety of H. pylori eradication between the age groups (younger (≤ 65 years), old (65–74 years), and super-old (≥ 75 years)). The study reported similar indications (chronic gastritis, PUD) for the eradication; however, the AEs rate in the old (9%) and super-old (12%) groups was significantly lower as compared to those in our study. Compared to our analysis, this study also reported a better effectiveness of the main standard triple therapy (PPI + A + C), which achieved optimal overall eradication rate (92%). When comparing the age groups, super-old patients had a significantly less frequent indication of chronic gastritis but more frequent indications of PUD compared to other groups. In this same Japanese study, the H. pylori eradication rates for older patients were not reported lower when compared to the younger patients. No remarkable differences were seen among the groups for the efficacy of prescribed regimens, no significant differences were observed in comparisons of AE rates among the groups21. Another small Chinese study also analysed the efficacy of H. pylori eradication between the age groups by using bismuth-based quadruple therapies for 14 days and did not yield significant differences either in the ITT and PP analysis between the age groups. This study also reported excellent eradication rates (> 92%) in both age groups22.
One of the main weaknesses of our study is the possible heterogeneity of the data. The Hp-EuReg currently includes 32 European countries and various regions might have different approaches to the management of H. pylori infection, which could be affected by diverse factors, such as the availability of local antimicrobial resistance rates, financial capabilities, availability of diagnostic methods, local antibiotics market as well as the knowledge and objectivity of the gastroenterologists. Our study has included a very large European cohort; providing accurate Pan-European data. Such is the case of previously published available studies from Hp-EuReg from different European countries39, where, despite the aforementioned concerns, it has to be acknowledged that multicentre collaboration gathering information on the daily routine of the gastroenterological practice is one of the best ways to secure a critical mass of knowledge encompassing the inclusion of those even difficult-to-treat cases as well as offering power to the statistical analyses.
Conclusions
In general, the approach to the diagnostics and treatment of H. pylori infection did not differ between the older and younger European populations. The main differences were reported in the concurrent medications, allergy to penicillin, AEs and the type of prescribed regimen (triple vs. quadruple) both in first- and second-line treatment, which were generally well-tolerated in both age groups. The effectiveness of the most frequent first- and second-line triple therapies in the younger and older populations was suboptimal (< 90%) and optimal effectiveness rates (> 90%) were mostly achieved by using bismuth and non-bismuth-based quadruple therapies (quadruple PPI + C + A + M, quadruple PPI + C + A + B, quadruple PPI + C + A + T, single capsule bismuth quadruple Pylera®, sequential C + A + T for the first line first-line treatment; quadruple PPI + C + A + B (only in the younger adults), single capsule bismuth Pylera® for the second-line treatment). Although statistically significant, no clinically relevant differences in the effectiveness between older and younger patients was observed in the most frequently prescribed first- and second-line prescriptions.
Supplementary Information
Acknowledgements
The details regarding the Hp-EuReg investigators are provided in the Supplementary Information file 1.
Author contributions
Guarantor of the article: P.J. P.J. analysed and synthesized the data, wrote the manuscript draft, and approved the final submitted manuscript. O.P.N., Hp-EuReg Scientific Director, performed the data extraction, the monitoring and the quality check, performed the statistical analyses, assisted with data interpretation and synthesis, and approved the final submitted manuscript. I.M.S., G.F., D.V., A.P.A., B.T., M.C.F., M.P.C., A.K.H., I.V., A.J.L., A.L., S.J.M.D., E.A.A., L.R., L.V., N.B.J., M.D., L.B., U.M., M.L., F.L., G.B., D.S.B., A.G., O.G., T.R., R.M.P., P.S.P., S.M.S., A.T., D.B., G.M.B., Š.Š., H.S., T.M.B., V.M., W.M., M.V., L.B., M.D., L.G.C., A.C.C., R.B., E.M., R.A.A., G.F., J.M.H.: collected data, critically reviewed the manuscript’s drafts, and approved the final submitted manuscript. J.K., National coordinator of Lithuania, recruited patients, assisted with data interpretation, critically reviewed the manuscript’s drafts, and approved the final submitted manuscript. C.O., F.M., L.M. and O.P.N. are Members of the Hp-EuReg Scientific Committee; they assisted with data interpretation, critically reviewed the manuscript’s drafts, and approved the final submitted manuscript. L.J. recruited patients, designed the protocol and planned the study, assisted with data interpretation, critically reviewed the manuscript’s drafts, and approved the final submitted manuscript. J.P.G., Principal investigator, directed the project, obtained funding, designed the protocol and planned the study, recruited patients, analysed and interpreted the data, critically reviewed the manuscript drafts, and approved the final submitted manuscript.
Funding
This project was promoted and funded by the European Helicobacter and Microbiota Study Group (EHMSG) and received support from the Spanish Association of Gastroenterology (AEG) and the Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd). The Hp-EuReg was co-funded by the European Union programme HORIZON (grant agreement number 101095359) and supported by the UK Research and Innovation (grant agreement number 10058099). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the Health and Digitial Executive Agency (HaDEA). Neither the European Union nor the granting authority can be held responsible for them. The Hp-EuReg was co-funded by the European Union programme EU4Health (grant agreement number 101101252). This study was funded by Diasorin; however, clinical data were not accessible and the company was not involved in any stage of the Hp-EuReg study (design, data collection, statistical analysis, or manuscript writing). We want to thank Diasorin for their support.
Data availability
Data transparency statement: Raw data were generated at AEG-REDCap. Derived data supporting the findings of this study are available from the Hp-EuReg Scientific Director and the PI of the project (OPN and JPG) upon request. Data sharing statement: The data that support the findings of this study are not publicly available given that containing information could compromise the privacy of research participants. However, previous published data on the Hp-EuReg study, or de-identified raw data referring to current study, as well as further information on the methods used to explore the data could be shared, with no particular time constraint. Individual participant data will not be shared.
Competing interests
Prof. J. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen. Dr. Nyssen has received research funding from Mayoly and Allergan. The remaining authors have declared no conflict of interest.
Footnotes
The original online version of this Article was revised: In the original version of this Article, ‘Hp-EuReg investigators’ was omitted from the author list. The details regarding the Hp-EuReg investigators were provided in the Supplementary Information.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Javier P. Gisbert and Laimas Jonaitis.
A list of authors and their affiliations appears at the end of the paper.
Change history
11/1/2023
A Correction to this paper has been published: 10.1038/s41598-023-45888-5
Contributor Information
Olga P. Nyssen, Email: opn.aegredcap@aegastro.es
Hp-EuReg investigators:
Renāte Būmane, Emin Mammadov, Rustam A. Abdulkhakov, Galina Fadeenko, and Jose M. Huguet
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-43287-4.
References
- 1.Toh JWT, Wilson RB. Pathways of gastric carcinogenesis, Helicobacter pylori virulence and interactions with antioxidant systems, vitamin C and phytochemicals. Int. J. Mol. Sci. 2020;21(17):6451. doi: 10.3390/ijms21176451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusters JG, van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006;19(3):449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guevara B, Cogdill AG. Helicobacter pylori: A review of current diagnostic and management strategies. Dig. Dis. Sci. 2020;65(7):1917–1931. doi: 10.1007/s10620-020-06193-7. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou J-M, Schulz C, et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut. 2022 doi: 10.1136/gutjnl-2022-327745. [DOI] [PubMed] [Google Scholar]
- 5.Jonaityte IR, Ciupkeviciene E, Jonaitis P, Kupcinskas J, Petkeviciene J, Jonaitis L. Changes in the seroprevalence of Helicobacter pylori among the Lithuanian medical students over the last 25 years and its relation to dyspeptic symptoms. Medicina. 2021;57:254. doi: 10.3390/medicina57030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowland M. The continuing decline in the prevalence of Helicobacter pylori infection. Lancet Child Adolesc. Health. 2022;6(3):139–140. doi: 10.1016/S2352-4642(22)00026-8. [DOI] [PubMed] [Google Scholar]
- 7.Park JS, Jun JS, Seo J-H, Youn H-S, Rhee K-H. Changing prevalence of Helicobacter pylori infection in children and adolescents. Clin. Exp. Pediatr. 2021;64(1):21–25. doi: 10.3345/cep.2019.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Roberts SE, Morrison-Rees S, Samuel DG, Thorne K, Akbari A, Williams JG. Review article: The prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol. Ther. 2016;43(3):334–345. doi: 10.1111/apt.13474. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard TG, Czinn SJ. Helicobacter pylori acquisition and transmission: Where does it all begin? Gastroenterology. 2001;121:483–485. doi: 10.1053/gast.2001.26769. [DOI] [PubMed] [Google Scholar]
- 11.Gisbert JP. The recurrence of Helicobacter pylori infection: Incidence and variables influencing it. A critical review. Am. J. Gastroenterol. 2005;100(9):2083–2099. doi: 10.1111/j.1572-0241.2005.50043.x. [DOI] [PubMed] [Google Scholar]
- 12.Kanasi E, Ayilavarapu S, Jones J. The aging population: Demographics and the biology of aging. Periodontology 2000. 2016;72(1):13–18. doi: 10.1111/prd.12126. [DOI] [PubMed] [Google Scholar]
- 13.De Luca DE, Bonacci S, Giraldi G. Aging populations: The health and quality of life of the elderly. Clin. Ter. 2011;162(1):e13–e18. [PubMed] [Google Scholar]
- 14.Huang Q, Jia X, Chu Y, Zhang X, Ye H. Helicobacter pylori infection in geriatric patients: Current situation and treatment regimens. Front. Med. 2021;8:713908. doi: 10.3389/fmed.2021.713908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilotto A, Franceschi M. Helicobacter pylori infection in older people. World J. Gastroenterol. 2014;20(21):6364–6373. doi: 10.3748/wjg.v20.i21.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shruthi R, Jyothi R, Pundarikaksha HP, Nagesh GN, Tushar TJ. A study of medication compliance in geriatric patients with chronic illnesses at a tertiary care hospital. J. Clin. Diagn. Res. 2016;10(12):FC40–FC43. doi: 10.7860/JCDR/2016/21908.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 2009;41(2):67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- 18.van Beek JHGM, Kirkwood TBL, Bassingthwaighte JB. Understanding the physiology of the ageing individual: Computational modelling of changes in metabolism and endurance. Interface Focus. 2016;6(2):20150079. doi: 10.1098/rsfs.2015.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 20.Liou J-M, Lin J-T, Lee Y-C, Wu C-Y, Wu M-S. Helicobacter pylori infection in the elderly. Int. J. Gerontol. 2008;2(4):145–153. doi: 10.1016/S1873-9598(09)70002-X. [DOI] [Google Scholar]
- 21.Kobayashi S, Joshita S, Yamamoto C, Yanagisawa T, Miyazawa T, Miyazawa M, et al. Efficacy and safety of eradication therapy for elderly patients with Helicobacter pylori infection. Medicine. 2019;98(30):e16619. doi: 10.1097/MD.0000000000016619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao C, Fan Y-H. Effect and safety of Helicobacter pylori eradication treatment based on molecular pathologic antibiotic resistance in Chinese elderly people. Infect. Drug Resist. 2022;15:3277–3286. doi: 10.2147/IDR.S371113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Megraud F, Bruyndonckx R, Coenen S, Wittkop L, Huang T-D, Hoebeke M, et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021;70(10):1815–1822. doi: 10.1136/gutjnl-2021-324032. [DOI] [PubMed] [Google Scholar]
- 24.Denkinger CM, Grant AD, Denkinger M, Gautam S, D’Agata EMC. Increased multi-drug resistance among the elderly on admission to the hospital—a 12-year surveillance study. Arch. Gerontol. Geriatr. 2013;56(1):227–230. doi: 10.1016/j.archger.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Hujer AM, Bethel CR, Hujer KM, Bonomo RA. Antibiotic resistance in the institutionalized elderly. Clin. Lab. Med. 2004;24(2):343–361. doi: 10.1016/j.cll.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Jiang F, Guo C-G, Cheung KS, Li B, Law SYK, Leung WK. Age of eradication and failure rates of clarithromycin-containing triple therapy for Helicobacter pylori: A 15-year population-based study. Helicobacter. 2022;27(3):e12893. doi: 10.1111/hel.12893. [DOI] [PubMed] [Google Scholar]
- 27.McNicholl AG, O’Morain CA, Megraud F, Gisbert JP. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg) Helicobacter. 2019;24(5):e12630. doi: 10.1111/hel.12630. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Ageing and Health by World Health Organization. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (Accessed 15 Aug 2022).
- 29.UN. Older persons by United Nations. https://emergency.unhcr.org/entry/43935/older-persons. An older person is defined, or age-related health conditions (Accessed 15 Aug 2022).
- 30.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham DY, Lu H, Dore MP. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter. 2019;24(1):e12554. doi: 10.1111/hel.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur. J. Clin. Pharmacol. 2009;65(1):19–31. doi: 10.1007/s00228-008-0576-5. [DOI] [PubMed] [Google Scholar]
- 33.Jin J, Sklar GE, Min Sen OhV, Chuen LS. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther. Clin. Risk Manag. 2008;4(1):269–286. doi: 10.2147/tcrm.s1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimmy B, Jose J. Patient medication adherence: Measures in daily practice. Oman Med. J. 2011;26(3):155–159. doi: 10.5001/omj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNicholl AG, Bordin DS, Lucendo A, Fadeenko G, Fernandez MC, Voynovan I, et al. Combination of bismuth and standard triple therapy eradicates Helicobacter pylori infection in more than 90% of patients. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020;18(1):89–98. doi: 10.1016/j.cgh.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 36.Nyssen OP, Perez-Aisa A, Castro-Fernandez M, Pellicano R, Huguet JM, Rodrigo L, et al. European Registry on Helicobacter pylori management: Single-capsule bismuth quadruple therapy is effective in real-world clinical practice. United Eur. Gastroenterol. J. 2021;9(1):38–46. doi: 10.1177/2050640620972615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyssen OP, Bordin D, Tepes B, Pérez-Aisa Á, Vaira D, Caldas M, et al. European Registry on Helicobacter pylori Management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut. 2021;70(1):LP40–LP54. doi: 10.1136/gutjnl-2020-321372. [DOI] [PubMed] [Google Scholar]
- 38.Nyssen OP, Vaira D, Tepes B, Kupcinskas L, Bordin D, Pérez-Aisa Á, et al. Room for improvement in the treatment of Helicobacter pylori infection: Lessons from the European Registry on H. pylori Management (Hp-EuReg) J. Clin. Gastroenterol. 2022;56(2):e98–108. doi: 10.1097/MCG.0000000000001482. [DOI] [PubMed] [Google Scholar]
- 39.Nyssen OP, Moreira L, García-Morales N, Cano-Català A, Puig I, Mégraud F, et al. European Registry on Helicobacter pylori Management (Hp-EuReg): Most relevant results for clinical practice. Front. Gastroenterol. 2022 doi: 10.3389/fgstr.2022.965982. [DOI] [Google Scholar]
- 40.Zullo A, Fiorini G, Scaccianoce G, Portincasa P, De Francesco V, Vassallo R, et al. Sequential therapy for first-line Helicobacter pylori eradication: 10- or 14-day regimen? J. Gastrointestin. Liver Dis. 2019;28(1):11–14. doi: 10.15403/jgld.2014.1121.281.hpy. [DOI] [PubMed] [Google Scholar]
- 41.Tursi A, Franceschi M, Allegretta L, Savarino E, De Bastiani R, Elisei W, et al. Effectiveness and safety of Pylera® in patients infected by Helicobacter pylori: A multicenter, retrospective, real life study. Dig. Dis. 2018;36(4):264–268. doi: 10.1159/000487391. [DOI] [PubMed] [Google Scholar]
- 42.Sun Q, Liang X, Zheng Q, Liu W, Xiao S, Gu W, et al. High efficacy of 14-day triple therapy-based, bismuth-containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter. 2010;15(3):233–238. doi: 10.1111/j.1523-5378.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 43.Sukri A, Lopes BS, Hanafiah A. The emergence of multidrug-resistant Helicobacter pylori in Southeast Asia: A systematic review on the trends and intervention strategies using antimicrobial peptides. Antibiotiotics. 2021;10(9):1061. doi: 10.3390/antibiotics10091061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hulten KG, Lamberth LB, Kalfus IN, Graham DY. National and regional US antibiotic resistance to Helicobacter pylori: Lessons from a clinical trial. Gastroenterology. 2021;161(1):342–344.e1. doi: 10.1053/j.gastro.2021.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data transparency statement: Raw data were generated at AEG-REDCap. Derived data supporting the findings of this study are available from the Hp-EuReg Scientific Director and the PI of the project (OPN and JPG) upon request. Data sharing statement: The data that support the findings of this study are not publicly available given that containing information could compromise the privacy of research participants. However, previous published data on the Hp-EuReg study, or de-identified raw data referring to current study, as well as further information on the methods used to explore the data could be shared, with no particular time constraint. Individual participant data will not be shared.