Abstract
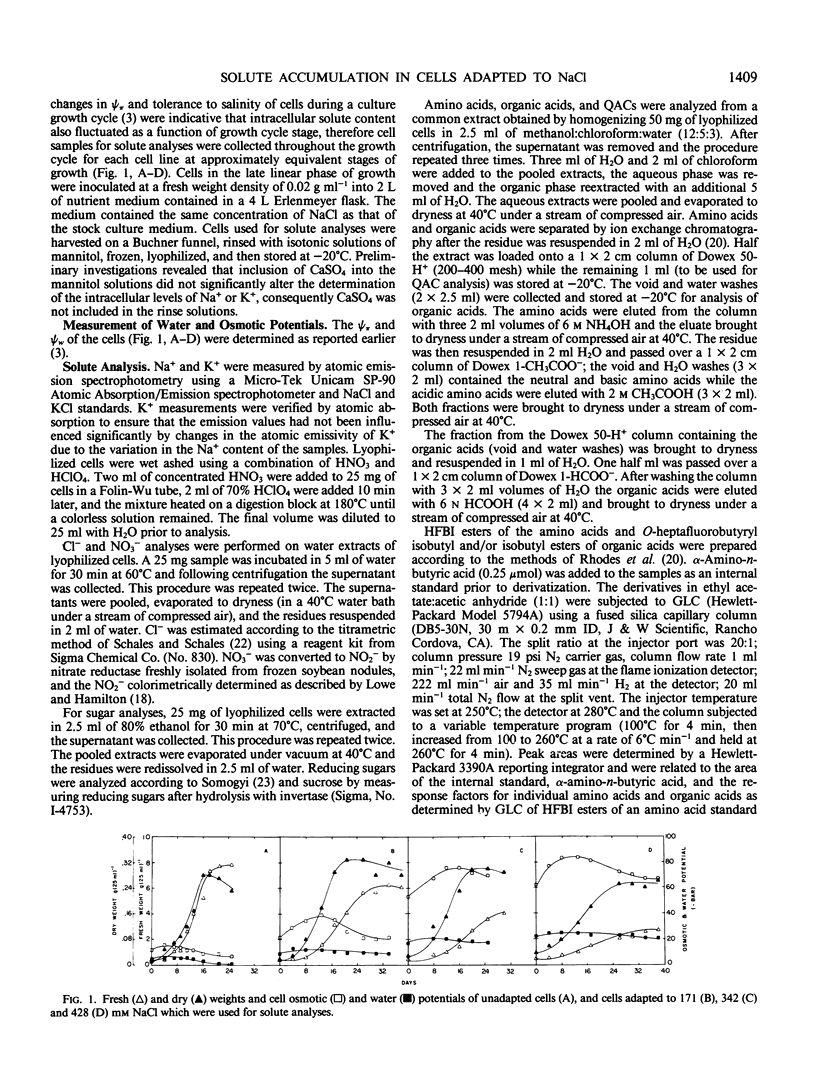
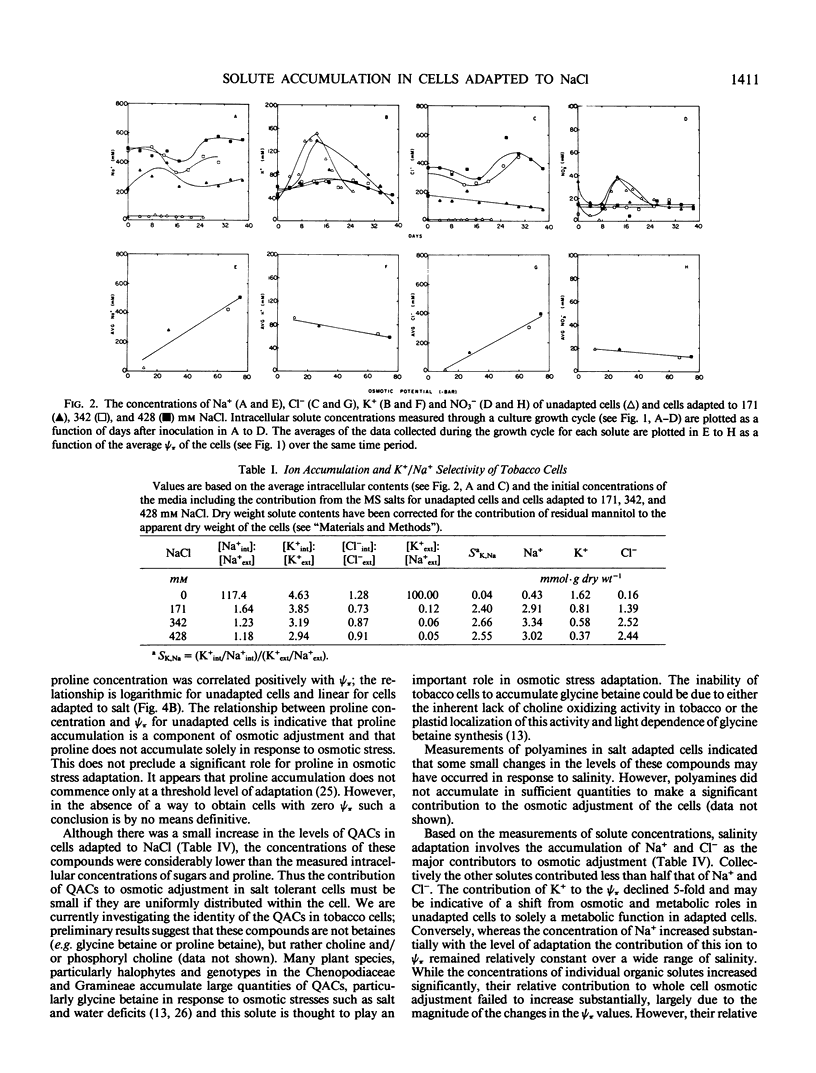
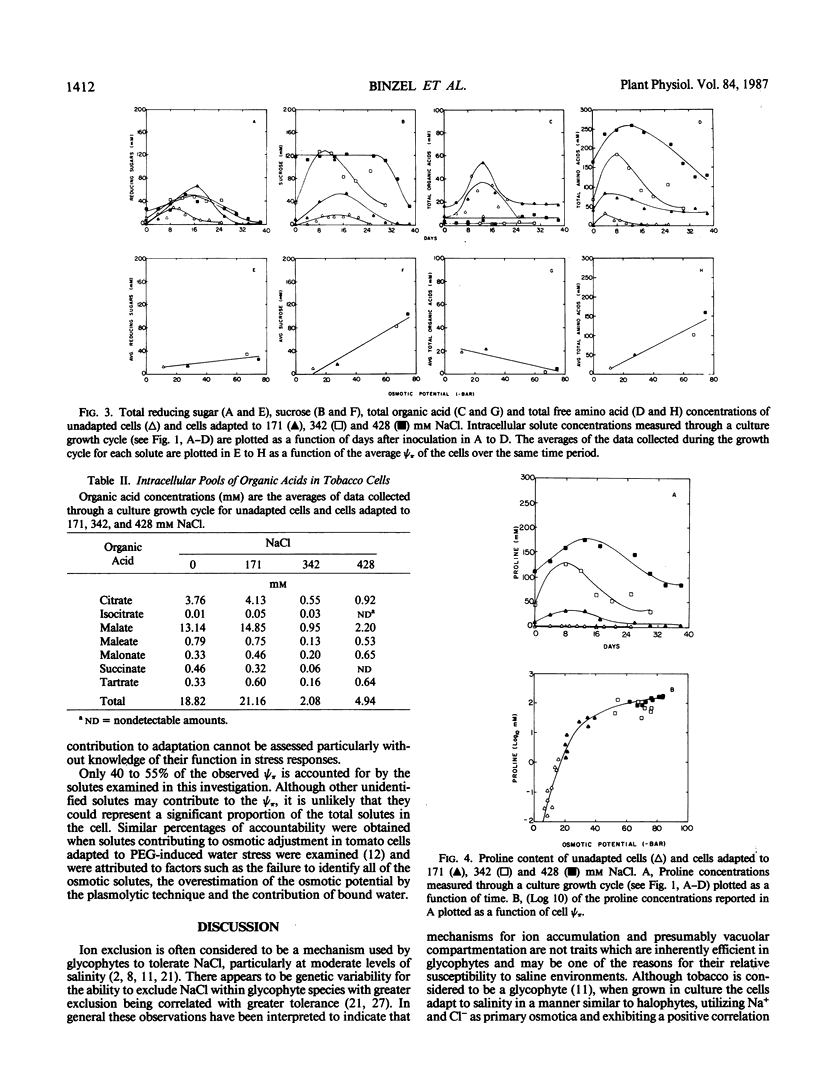
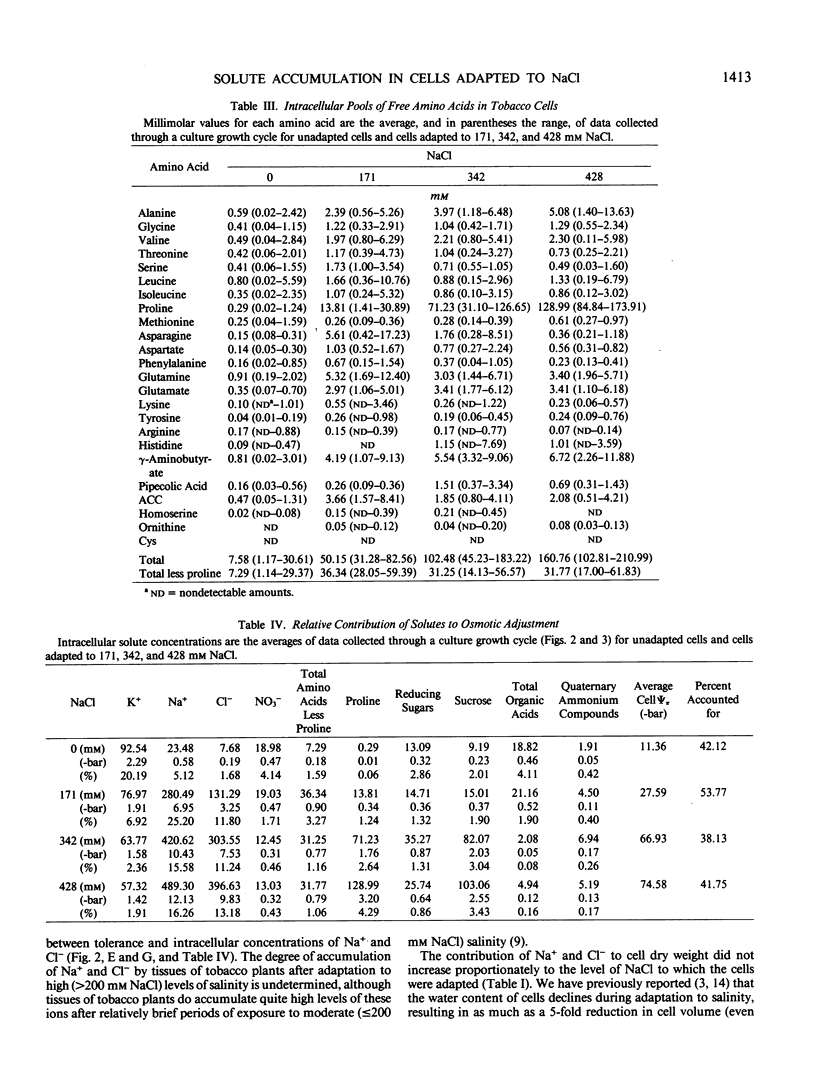
Cells of Nicotiana tabacum L. var Wisconsin 38 adapted to NaCl (up to 428 millimolar) which have undergone extensive osmotic adjustment accumulated Na+ and Cl− as principal solutes for this adjustment. Although the intracellular concentrations of Na+ and Cl− correlated well with the level of adaptation, these ions apparently did not contribute to the osmotic adjustment which occurred during a culture growth cycle, because the concentrations of Na+ and Cl− did not increase during the period of most active osmotic adjustment. The average intracellular concentrations of soluble sugars and total free amino acids increased as a function of the level of adaptation; however, the levels of these solutes did not approach those observed for Na+ and Cl−. The concentration of proline was positively correlated with cell osmotic potential, accumulating to an average concentration of 129 millimolar in cells adapted to 428 millimolar NaCl and representing about 80% of the total free amino acid pool as compared to an average of 0.29 millimolar and about 4% of the pool in unadapted cells. These results indicate that although Na+ and Cl− are principal components of osmotic adjustment, organic solutes also may make significant contributions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Hayyim G., Kochba J. Aspects of Salt Tolerance in a NaCl-Selected Stable Cell Line of Citrus sinensis. Plant Physiol. 1983 Jul;72(3):685–690. doi: 10.1104/pp.72.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hayyim G., Spiegel-Roy P., Neumann H. Relation between Ion Accumulation of Salt-Sensitive and Isolated Stable Salt-Tolerant Cell Lines of Citrus aurantium. Plant Physiol. 1985 May;78(1):144–148. doi: 10.1104/pp.78.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa S., Bressan R. A., Handa A. K., Carpita N. C., Hasegawa P. M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983 Nov;73(3):834–843. doi: 10.1104/pp.73.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., May A. M., Grumet R., Bode J., Jamieson G. C., Rhodes D. Betaine synthesis in chenopods: Localization in chloroplasts. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3678–3682. doi: 10.1073/pnas.82.11.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser J. W., Nabors M. W. Osmotic Adjustment of Cultured Tobacco Cells (Nicotiana tabacum var. Samsum) Grown on Sodium Chloride. Plant Physiol. 1981 Apr;67(4):720–727. doi: 10.1104/pp.67.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Myers A. C., Jamieson G. Gas Chromatography-Mass Spectrometry of N- Heptafluorobutyryl Isobutyl Esters of Amino Acids in the Analysis of the Kinetics of [N]H(4) Assimilation in Lemna minor L. Plant Physiol. 1981 Nov;68(5):1197–1205. doi: 10.1104/pp.68.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Watad A. E., Reinhold L., Lerner H. R. Comparison between a Stable NaCl-Selected Nicotiana Cell Line and the Wild Type : K, Na, and Proline Pools as a Function of Salinity. Plant Physiol. 1983 Nov;73(3):624–629. doi: 10.1104/pp.73.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]


