Abstract
This study investigated the colorimetric response of standard glucose, serum glucose, and nucleic acid assays on various paper surfaces with different wettability, including hydrophilic, hydrophobic, and nearly superhydrophobic surfaces. Water contact angles (WCA) formed by water droplets on each surface were measured using ImageJ software. The hydrophilic surface showed no contact angle, while the hydrophobic and nearly superhydrophobic surfaces exhibited contact angles of 115.667° and 133.933°, respectively. The colorimetric sensitivity of the standard glucose assay was analyzed on these surfaces, revealing enhanced sensitivity on the nearly superhydrophobic surface due to the high molecular crowding effect owing to its non-wetting behavior and eventually confined reaction product at the sample loading zone. The hydrophobic nature of the surface restricts the spreading and diffusion of the reaction product, leading to a controlled and localized concentration of the assay product leading to moderate colorimetric intensity. On the other hand, the hydrophilic surface showed the least enhancement in colorimetric sensitivity; this is attributed to the high wettability of the hydrophilic surface causing the reaction product to spread extensively, resulting in a larger area of dispersion and consequently a lower colorimetric intensity. The measured limit of detection (LOD) for nucleic acid on nearly superhydrophobic surfaces was found to be 16.15 ng/µL, which was almost four-fold lower than on hydrophilic surfaces (60.08 ng/µL). Additionally, the LODs of standard glucose and clinical serum samples were two-fold lower on nearly superhydrophobic surfaces compared to hydrophilic surfaces. Our findings clearly highlight the promising potential of utilizing superhydrophobic surfaces to significantly enhance colorimetric sensitivity in paper-based diagnostic applications. This innovative approach holds promise for advancing point-of-care diagnostics and improving disease detection in resource-limited settings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00216-023-04921-2.
Keywords: Paper-based analytical device, LAMP amplicon, Serum glucose, Hydrophobicity, Colorimetric enhancement
Introduction
In the pursuit of improving diagnostic capabilities, researchers have been harnessing the power of biomarkers; the measurable indicators of biological processes or disease states, to revolutionize disease detection methods [1–7]. The past century has seen a rise in various diagnostic strategies for measuring disease-specific signatures in patient samples (e.g., blood, urine, and tissue) [8, 9]. These indicators are typically classified into different types, including genetic, proteomic, and metabolomic biomarkers [10]. In clinical practice, conventional methods such as immunoassays, polymerase chain reaction (PCR), mass spectrometry, and imaging modalities have been the cornerstone of biomarker detection [11, 12]. However, they require complex laboratory procedures and specialized equipment thereby hindering rapid on-site testing [13, 14]. Recently, paper-based point-of-care (POC) diagnostics integrating colorimetric detection modalities have revolutionized biomarker detection by offering unique advantages that address the aforementioned limitations [15–19].
By combining the colorimetric detection modalities, paper-based colorimetric POC diagnostics offer distinct advantages such as affordability, portability, and visual readout, enabling easy interpretation of results without the need for additional pieces of equipment [20–22]. Despite their significant advantages, colorimetric assays on paper do have certain limitations. One of the primary challenges is their inherent limitation in sensitivity, particularly when detecting biomarkers at low concentrations [23]. To overcome these limitations and to enhance the sensitivity of colorimetric assays on paper, researchers have pioneered sophisticated techniques that incorporate signal amplification strategies driven by rapid advancements in novel materials and nanotechnology [24]. A significant focus has been placed on enhancing the signal output through a meticulous control of the physicochemical properties of nanoparticles, such as their size and shape and functionalization of nanocomposites with polymers and the utilization of enzyme-mimicking noble metal nanoparticles as an effective means to amplify the colorimetric signal [25].
Furthermore, in order to enhance colorimetric signals on paper, other techniques such as liquid evaporation and electrokinetic methods have been utilized. Electrokinetic approaches majorly comprise field-amplified sample stacking (FASS), ion concentration polarization (ICP), and isotachophoresis (ITP) [26]. However, these aforementioned techniques are complex and time-consuming and require specialized equipment and expertise[27–29]. Furthermore, the need for reagents and additional steps for electrode preparation (in the case of electrokinetic methods) and maintenance adds further complexity and cost [30, 31]. These limitations highlight the urgent need to devise simplified and resilient strategies for sensitivity enhancement in colorimetric paper-based diagnostics[32, 33].
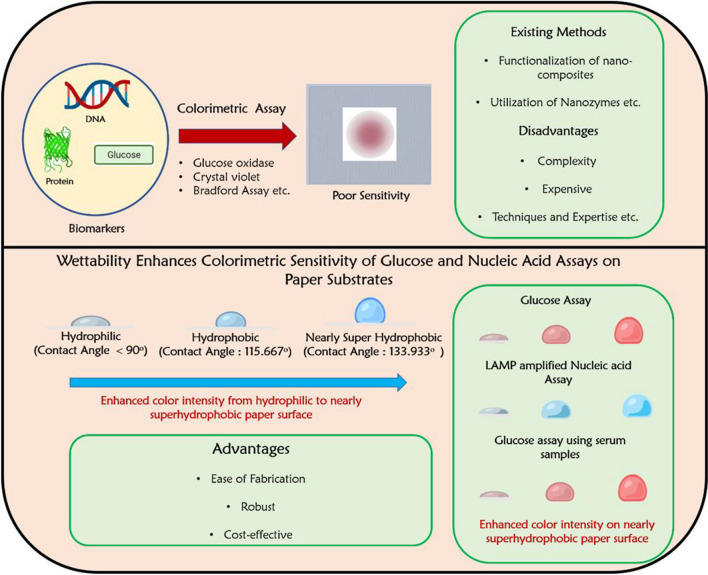
In this manuscript, for the first time, we present an innovative approach to enhance the sensitivity of colorimetric assays, specifically for the detection of glucose and Candida albicans DNA. We achieve this by precisely tuning the wettability of paper-based devices. Modifying the surface properties of the paper substrate allows us to achieve hydrophobic and nearly superhydrophobic characteristics, resulting in remarkable improvements in assay performance. To create hydrophobic surfaces, we employ candle scratching and heating techniques thereby carefully controlling the surface roughness. Additionally, we utilized commercially available spray onto the candle-scratched paper to create nearly superhydrophobic surfaces. Through glucose oxidase and colorimetric nucleic acid assays conducted on the modified surfaces (tuning wettability), we observed a significant enhancement in colorimetric sensitivity (Fig. 1). Furthermore, we assess the clinical applicability of this approach using serum samples from patients with varying glucose levels. The simplicity of our method allows for easy implementation, even by non-experts, and the visual readout enables rapid interpretation of results without the need for expensive equipment. Besides, the approach enables the detection of target biomolecules even at low concentrations, which is of paramount importance for early disease diagnosis and monitoring. Besides, the modified paper surfaces also exhibit enhanced stability and robustness, ensuring reliable assays under varying environmental conditions [34–37]. Furthermore, the improved sensitivity reduces the required sample volume for accurate detection, which is beneficial for handling limited or precious samples. By addressing these limitations, our study aims to bridge the gap between sophisticated, expensive diagnostic methods and the urgent need for accessible and affordable diagnostics, particularly in resource-limited settings.
Fig. 1.
Schematic illustration showing the wettability tuning of paper-spot devices for enhanced colorimetric sensitivity of glucose and nucleic acid assays
Materials and methods
Materials
Grade 1 Whatman (R) filter paper (thickness 180 µm and pore size, 11 µm) was obtained from GE Life Sciences. Scotch BOPP transparent (50.8 mm × 50 meter) tape manufactured by 3 M™, India, was procured from a local stationary shop. Candle sticks were purchased from a local vendor. Neverwet superhydrophobic spray manufactured by Rust-Oleum was purchased from Amazon, India. Glucose (S.L) reagent was purchased from Agappe Diagnostics Ltd. Crystal violet and nuclease-free water were purchased from Himedia. Sodium sulfite (Na2SO3) was purchased from SRL, India. The Candida albicans (ATCC 24433) culture was collected from the Department of Microbiology, Kasturba Medical College, Manipal. Anonymized clinical serum samples (total of 12 samples comprising triplicates of each concentration, specifically, 60 mg/dL, 90 mg/dL, 120 mg/dL, and 150 mg/dL) validated using EM 360 autoanalyzer were collected from the Department of Biochemistry, Kasturba Medical College, Manipal.
Methods
Device fabrication
Whatman® filter paper (grade 1) was used in this study. Paper-spot devices were created by punching the paper using a 4 mm diameter single-hole punching machine. In order to introduce a hydrophobic coating, the paper devices were gently scratched with a candle and subsequently placed inside a hot air oven set at 100 °C for 15 min. This step ensured the penetration of paraffin wax into the perforated pores of the paper. Consequently, these devices were affixed onto a scotch transparent tape. To impart a nearly superhydrophobic coating to the paper devices, a fresh set of 4 mm diameter holes were punched, and the paper spots were gently scratched with a candle as before and incubated at 100 °C for 15 min. After the incubation, these devices were taped. To further enhance the water-repellent properties, and achieve a nearly superhydrophobic surface, 3 μL of Neverwet superhydrophobic spray was sprayed onto these paper devices. The devices were then allowed to air dry at room temperature for 30 min.
Contact angle measurement
To evaluate the hydrophilic, hydrophobic, and nearly superhydrophobic properties of the paper devices, a contact angle measurement was performed. A water droplet of 10 µL was carefully placed on each surface, and horizontal images of the water droplets formed on the paper devices were captured using a DSLR camera (Canon EOS 80D). The contact angle was quantitatively analyzed using Fiji (ImageJ) software. In the analysis, five reference points were manually selected along the periphery of the droplets, and an ellipse was created using the manual point procedure. Furthermore, the contact angle was determined using the following equation:
where E represents the angle measured from the created ellipse. All the trials were done in triplicate. The mean contact angle and the standard deviation (SD) for hydrophilic, hydrophobic, and nearly superhydrophobic paper surfaces were calculated and tabulated.
Glucose oxidase assay with standard glucose samples
To evaluate the colorimetric sensitivity enhancement of the glucose assay on the fabricated paper spots, we performed the glucose oxidase assay following the established protocol by Agappe, India [38]. Initially, we prepared a series of standard glucose concentrations, specifically 60 mg/dL, 80 mg/dL, 100 mg/dL, 120 mg/dL, and 150 mg/dL. In amber-colored vials, we added 1000 µL of glucose (S.L) reagent and subsequently added 10 µL of each standard glucose concentration to the respective vials. The vials were then incubated at 37 °C for 10 min in a dry bath. After the incubation period, 10 µL of the reaction product was added to each hydrophilic, hydrophobic, and nearly superhydrophobic paper-spot device. To ensure consistent and uniform image capturing of the fabricated paper-spot devices, a Canon EOS 80D DSLR camera set to AV mode was used. The picture style was set in “daylight” to portray the true colors of the colorimetric reaction products, avoiding any artificial color shifts or biases that could influence our analysis. The ISO rate was fixed at 600 to maintain uniform sensitivity to light and consistent exposure levels across all captured images. To achieve the desired focus on the paper-spot devices and control the depth of field, the aperture was set to f/3.5. Additionally, the color saturation and contrast settings were left untouched to preserve the vividness and dynamic range of the images. The images of the colorimetric test samples, including the controls, were inverted, and processed using the ImageJ software. The inverted images were split into red (R), green (G), and blue (B) channels, and a line zone was selected in the blue (B) channel image to quantify the colorimetric response, specifically in the region where the colorimetric reaction product is concentrated [39, 40]. The intensity measurements were performed in triplicate for each glucose concentration.
Loop-mediated isothermal amplification (LAMP)
The conserved sequence of Candida albicans, the Internal Transcribed Spacer-2 (ITS-2) sequence, was amplified by loop-mediated isothermal amplification (LAMP). The primer sequence for Candida albicans ITS-2 was obtained from previous studies (Supplementary Table 1). All the samples were prepared in triplicates. The LAMP reaction was carried out in a final volume of 50 μL comprising five different primers comprising of forward inner primer (FIP) (2 μM), backward inner primer (BIP) (2 μM), loop backward (LB) (0.33 μM), forward (F3) (0.167 μM) and backward (B3) (0.167 μM) (Proteogen Biosciences), 1.4 mM dNTPs (New England Biolabs, USA), 1 × isothermal amplification buffer (New England Biolabs, USA), 6 mM MgSO4 (New England Biolabs, USA), 8 U of Bst 2.0 Warm Start DNA polymerase (New England Biolabs, USA), and 2 μL of DNA template. The reaction was run at 65 °C for 60 min and was terminated at 80 °C for 5 min in a thermocycler.
Nucleic acid assay
Firstly, for the nucleic acid assay, ITS-2 amplicon sequences of different concentrations, specifically, 80 ng/µL, 100 ng/µL, 200 ng/µL, and 500 ng/µL from Candida albicans, were prepared using nuclease-free water and stored at 4οC. Crystal violet (CV) solution was prepared using 0.5 mM CV and 50 mM Tris–Hcl (pH 8.8). From these solutions, 40 µL of CV and 10 µL of 30 mM sodium sulfite were mixed to yield a leucocrystal violet (LCV) solution. Next, the LCV control solution was prepared by mixing 50 μL of LCV solution with an equivalent volume of distilled water and reacting at room temperature for 10 min. The LCV + DNA solution was prepared using the same protocol as that for the LCV solution, with the only difference being the utilization of previously prepared amplicon samples at different concentrations instead of using distilled water. Ten microliters of these reaction products was added to different paper-spot devices having hydrophilic, hydrophobic, and nearly superhydrophobic surfaces. For consistent and uniform image capturing of the paper-spot devices, the same imaging parameters (as described previously) were set in the camera. After capturing the images of the colorimetric test samples, including the controls, image inversion was performed and processed using the ImageJ software. These inverted images were split into red (R), green (G), and blue (B) channels, and selected a line zone in the green (G) channel image to quantify the colorimetric response, specifically in the region where the colorimetric reaction product is concentrated [39, 40]. To ensure accuracy, the intensity measurements were performed in triplicate for each nucleic acid concentration.
Evaluation of serum glucose concentration on varying hydrophobic paper spots
To assess the clinical applicability of our developed device, a glucose oxidase assay was conducted (as described before) by collecting serum samples from patients with varying glucose levels and analyzing them using our devices having different hydrophobicities. The assay was carried out on anonymized clinical serum samples from patients having different concentrations of fasting blood glucose (FBG), specifically, 60 mg/dL, 90 mg/dL, 120 mg/dL, and 150 mg/dL. To achieve consistent and uniform image capturing of the fabricated paper-spot devices, similar imaging parameters as previously described were set in the camera. After capturing the images of the colorimetric test samples, including the controls, we performed image inversion and processed them using the ImageJ software. Subsequently, the inverted images of the clinical serum samples were split into red (R), green (G), and blue (B) channels and selected a line zone in the blue (B) channel image to quantify the colorimetric response, particularly in the region where the colorimetric reaction product is concentrated [39, 40]. To ensure accuracy, intensity measurements were performed in triplicate for each serum concentration.
Statistical analysis
Experimental data obtained from standard glucose; nucleic acids and serum glucose assays were represented as mean ± standard deviation (SD). The statistical significance of B channel intensities exhibited by glucose droplets (both standard and serum glucose) and G channel intensities of the nucleic acid samples on all hydrophilic, hydrophobic, and nearly superhydrophobic paper-spot devices were determined using the one-way ANOVA test with Tukey’s post hoc analysis in GraphPad Prism 8 software. A significance level of p < 0.05 was considered statistically significant.
Calculating limit of detection (LOD)
The limit of detection was calculated by using the below-given equation.
where SD is the standard deviation; σ is the slope.
Results and discussion
Water contact angle measurement of hydrophobic and nearly superhydrophobic surfaces
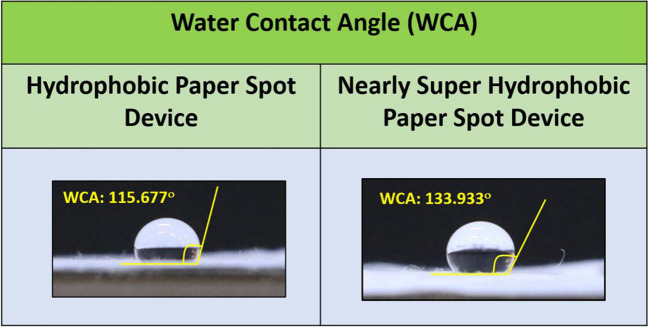
In our study, we aimed to investigate the behavior of glucose and nucleic acid assays on different paper surfaces, including hydrophilic, hydrophobic, and nearly superhydrophobic surfaces. The water contact angle (WCA) formed by water droplets on each of these paper surfaces was measured using ImageJ software. No contact angle was formed for the hydrophilic paper surface, whereas the hydrophobic paper surface and nearly superhydrophobic paper surface have shown the contact angles 115.667° and 133.933° respectively (Table 1). The observed contact angles align with the theoretical understanding of contact angle formation, which is based on the intermolecular forces between the water droplet and the paper surfaces [41]. The contact angle, also known as a wetting angle, is the angle formed at the point, where a liquid drop contacts a solid surface [42]. This phenomenon is influenced by various factors, including the surface tension of the liquid, the surface energy of the solid, and the topography of the surface [43].
Table 1.
The measured mean contact angle and standard deviation (SD) values for hydrophilic, hydrophobic, and nearly superhydrophobic paper surfaces
| Sl. no | Type of paper surface | Mean contact angle | SD |
|---|---|---|---|
| 1. | Hydrophilic | No contact angle was observed | - |
| 2. | Hydrophobic | 115.667ο | 5.23 |
| 3. | Nearly superhydrophobic | 133.933ο | 3.72 |
The hydrophilic paper surface exhibited a smaller contact angle, indicating a high affinity between the water droplet and the surface. This is in line with the concept that hydrophilic surfaces have a higher surface energy than the surface tension of the liquid [44]. Consequently, the water droplet spreads out and wets the surface, leading to a relatively smaller contact angle. On the other hand, the hydrophobic paper surface showed a contact angle of 115.667° (Fig. 2). This larger contact angle compared to the hydrophilic paper surface suggests that the surface energy of the paper is lower than the surface tension of the water droplet [45]. As a result, the droplet tends to bead up and form a more spherical shape, minimizing contact with the hydrophobic surface. Nearly superhydrophobic paper surface exhibited the largest contact angle of 133.933° (Fig. 2). This is attributed to the surface possessing relatively low surface energy compared to the surface tension of the water and intricate surface topography [46]. These parameters together contribute to a non-wetting behavior. Consequently, the water droplet sits on top of the surface structure, minimizing the contact area and resulting in a larger contact angle.
Fig. 2.
Water contact angle (WCA) measured for hydrophobic and nearly superhydrophobic paper surfaces
Colorimetric standard glucose assay on hydrophilic, hydrophobic, and nearly superhydrophobic surfaces
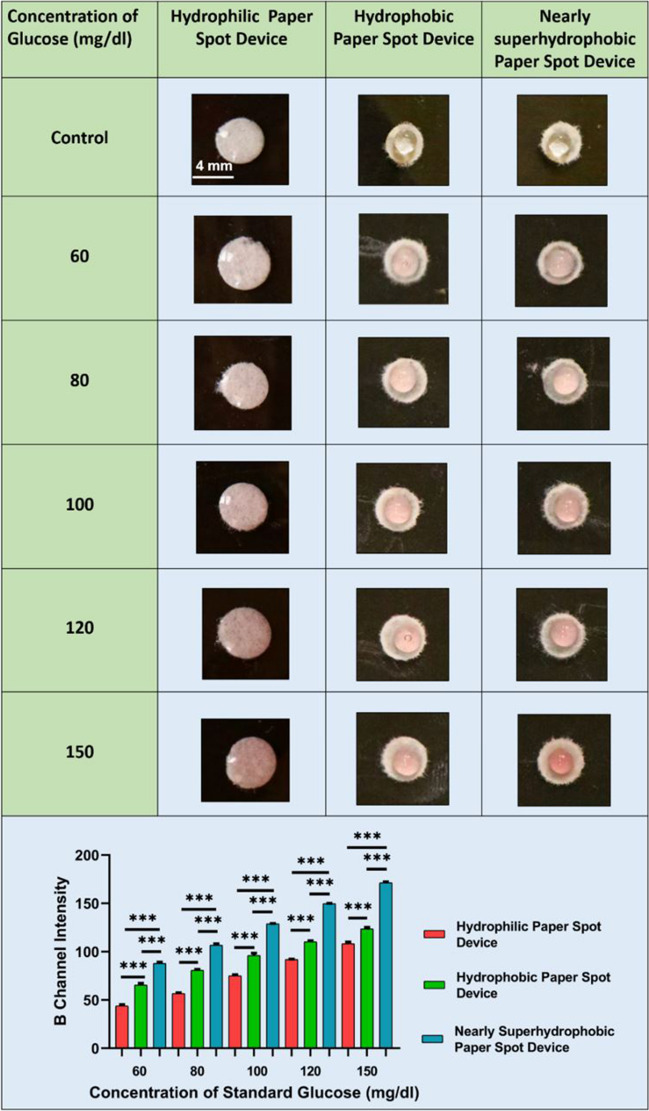
Glucose, a vital energy source for the body, is crucial in the diagnosis and management of diabetes mellitus. Plasma and serum glucose levels are commonly assessed using a colorimetric assay that involves the conversion of glucose and oxygen to gluconate and hydrogen peroxide (H2O2) by glucose oxidase. The presence of H2O2 leads to the formation of a colored complex, quinonimine, through a reaction with 4-amino antipyrine and a phenolic compound [38]. Typically, this colored complex is measured spectrophotometrically within the wavelength range of 490–550 nm. In our study, we sought to enhance the colorimetric sensitivity of the glucose oxidase assay by adjusting the wettability of paper surfaces using different concentrations of standard glucose. Our findings revealed that among the selected concentrations of glucose, the blue channel intensity was observed in the order hydrophilic < hydrophobic < nearly superhydrophobic paper surfaces.
The hydrophilic paper surface displayed the lowest blue channel intensity, indicating a lower colorimetric sensitivity. This difference can be attributed to the high wettability of the hydrophilic surface, causing the reaction product to spread and disperse over a larger area [35], resulting in a lower intensity. Furthermore, the hydrophobic paper surface exhibited a relatively higher blue channel intensity compared to the hydrophilic paper devices, indicating a moderate enhancement in colorimetric sensitivity (Fig. 3, top). This enhancement is corroborated by the molecular crowding effect, which restricts the spreading and diffusion of the glucose reaction product to a controlled extent [47]. As a result, the reaction product remains more confined within the paper surface, leading to a noticeable increase in the intensity. We also hypothesize that pore blockage on the paper surface (by hydrophobic agents) restricts the axial movement of the reaction product, allowing it to be more concentrated within the hydrophobic paper surface. The accumulation of the analyte at the sample loading zone contributes to a stronger colorimetric signal, thereby enhancing the color intensity and sensitivity [48]. The nearly superhydrophobic paper surfaces exhibited the highest colorimetric sensitivity, which can be attributed to several factors [49]. Firstly, a very low surface energy of the nearly superhydrophobic surface combined with its intricate surface topography creates a non-wetting behavior. This behavior confines the reaction product to remain localized in a smaller area upon addition, resulting in higher intensity measurements.
Fig. 3.
Glucose oxidase assay performed for different standard glucose concentrations on different paper surfaces and their respective blue (B) channel intensities. Level of significance *p < 0.05, **p < 0.01, and ***p < 0.001 for comparison between the samples
Generally, distinguishing narrow concentrations of glucose solely based on intensity is challenging in paper-based colorimetric assays [50, 51]. This limitation leads to false negative or false positive results. However, by incorporating superhydrophobic materials, it becomes more viable to differentiate close glucose concentrations solely based on color intensity. Additionally, the statistical analysis conducted on the blue channel intensities of different concentrations of glucose droplets added to hydrophilic, hydrophobic, and nearly superhydrophobic paper surfaces revealed significant differences in intensity (Fig. 3, bottom). Specifically, the intensity of droplets on both hydrophobic and nearly superhydrophobic paper surfaces was significantly higher compared to the hydrophilic surface across all glucose concentrations.
Colorimetric nucleic acid assay on hydrophilic, hydrophobic, and nearly superhydrophobic surfaces
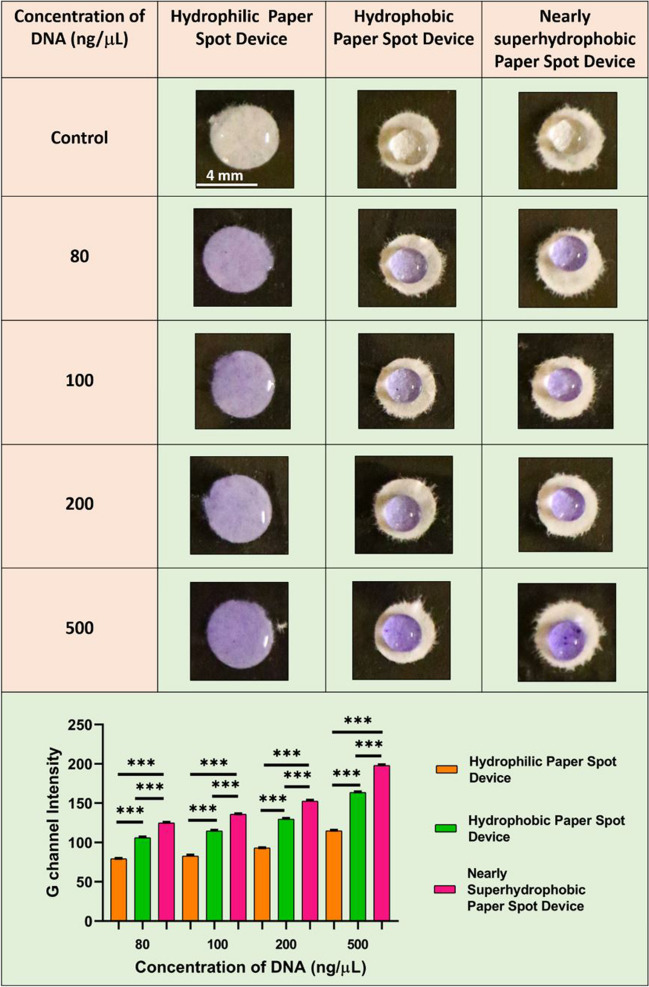
Furthermore, to assess the potential application of our approach in colorimetric nucleic acid assays, we conducted experiments using ITS-2 amplicons isolated from Candida albicans [52]. In this assay, crystal violet (CV) was utilized as an indicator to detect the presence of dsDNA. Crystal violet, a triphenylmethane dye with a p-quinoid group acting as a chromophore, has a pronounced affinity for double-stranded DNA (dsDNA). Initially, when sodium sulfite (Na2SO3) is added to crystal violet, CV undergoes a conversion into leucocrystal violet (LCV), which is colorless [53]. Upon introducing dsDNA, the positively charged quinoid of CV interacts with the dsDNA through electrostatic attraction which results in a violet color [54]. This colorimetric response serves as a reliable and sensitive method for detecting the presence of dsDNA.
Hydrophilic surfaces characterized by high wettability cause the LCV-DNA mixture to spread extensively, leading to a larger area of dispersion and consequently a lower color intensity (Fig. 4, top). Whereas, hydrophobic surfaces exhibit a moderate intensity compared to hydrophilic surfaces. This enhancement in intensity is corroborated by the previously described molecular crowding effect, which restricts the spreading and diffusion of the LCV-DNA mixture [47]. As a result, the reaction product remains more confined within the paper surface, leading to a noticeable increase in intensity. Similarly, the nearly superhydrophobic surfaces exhibit the most pronounced enhancement in colorimetric response and improved detection sensitivity. This is primarily due to the higher contact angle and reduced spreading of the LCV-DNA mixture on the superhydrophobic surface. The low surface energy and intricate surface topography of the superhydrophobic surface create a non-wetting behavior, allowing the reaction product to remain localized in a smaller area [55]. These observations were further validated through statistical analysis of green (G) channel intensities of droplets added to these three distinct surfaces (Fig. 4, bottom).
Fig. 4.
Nucleic acid assay for different concentrations of dsDNA on different paper surfaces and their respective green (G) channel intensities. Level of significance *p < 0.05, **p < 0.01, and ***p < 0.001 for comparison between the samples
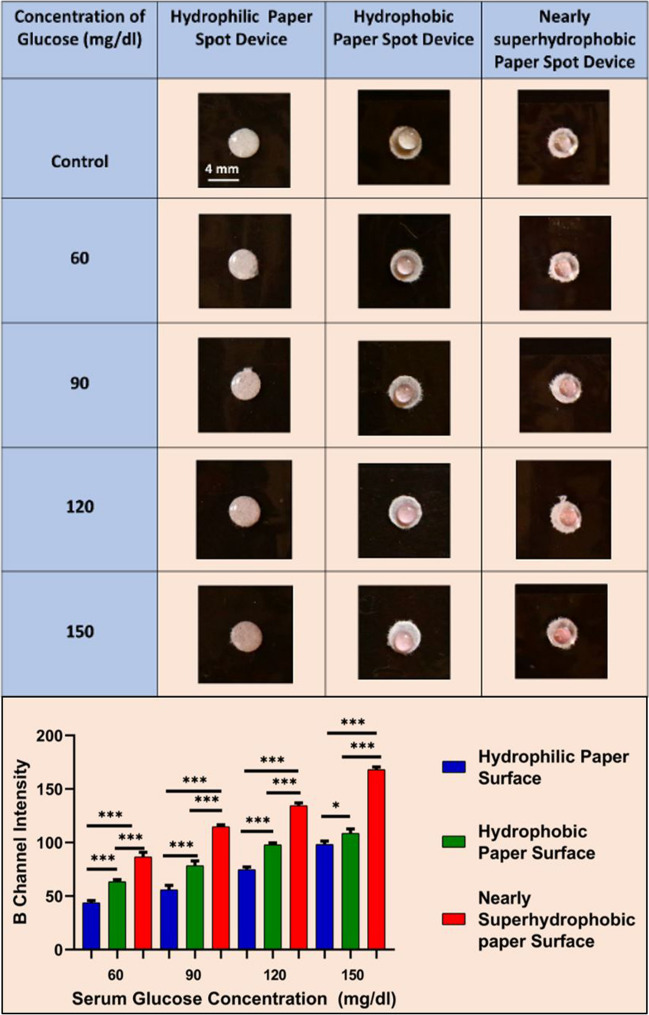
Furthermore, the practicality of this approach was validated using anonymized clinical serum samples (undergone prior validation using EM 360 autoanalyzer) of normal and diabetic patients. It was observed that the nearly superhydrophobic paper surfaces outperformed hydrophobic and hydrophilic paper surfaces (Fig. 5, top). More importantly, the nearly superhydrophobic surface yielded better distinguishable color intensity for narrow glucose concentrations. This was supported through statistical analysis of respective blue (B) channel intensities exhibited by serum samples on hydrophilic, hydrophobic, and nearly superhydrophobic paper surfaces (Fig. 5, bottom).
Fig. 5.
Glucose oxidase assay for different concentrations of glucose in serum samples on different paper surfaces and their respective blue (B) channel intensities. Level of significance *p < 0.05, **p < 0.01, and ***p < 0.001 for comparison between the samples
Limit of detection (LOD)
The limit of detection (LOD) for nucleic acid, standard glucose, and clinical serum samples was determined from the standard calibration plot. The change in the B and G channel intensities was plotted against respective concentrations of the analytes, ranging from 80 to 500 ng/µL for nucleic acid and 60 to 150 mg/dL for both standard glucose and serum glucose samples. Supplementary Fig. 1 illustrates a linear relationship between the concentration of analytes and their respective intensities. The LOD of nucleic acid on the nearly superhydrophobic surfaces (16.15 ng/µL) was found to be almost four-fold lower than that on the hydrophilic surfaces (60.08 ng/µL). Similarly, the LODs of standard glucose and serum glucose samples revealed a two-fold lower value on the nearly superhydrophobic surfaces compared to the hydrophilic surfaces. The calculated LODs of all the analytes on various surfaces are summarized in Table 2.
Table 2.
Measured LOD for nucleic acid, standard glucose, and serum glucose on different paper surfaces
| Analytes | Surface | LOD | R2 |
|---|---|---|---|
| Nucleic acid | Hydrophilic | 60.08 ng/μL | 0.969 |
| Hydrophobic | 36.52 ng/μL | 0.982 | |
| Nearly superhydrophobic | 16.15 ng/μL | 0.989 | |
| Standard glucose | Hydrophilic | 12.56 mg/dL | 0.985 |
| Hydrophobic | 10.03 mg/dL | 0.992 | |
| Nearly superhydrophobic | 6.19 mg/dL | 0.989 | |
| Serum glucose | Hydrophilic | 14.37 mg/dL | 0.976 |
| Hydrophobic | 9.23 mg/dL | 0.986 | |
| Nearly superhydrophobic | 7.52 mg/dL | 0.982 |
Conclusion
For the first time, we have demonstrated that harnessing the wettability of the paper surfaces in colorimetric assays holds great promise for improving the intensity. For nearly superhydrophobic surfaces, we have achieved a two-fold increase in the sensitivity of standard and serum glucose concentrations. Similarly, we have accomplished a four-fold increase in the sensitivity of LAMP amplicons of the fungus, Candida albicans. The superior performance of the nearly superhydrophobic paper surfaces in differentiating narrow analyte concentrations is due to its water-repelling nature, which promotes localized concentration of the assay product which in turn leads to the accumulation of reaction product with better colorimetric signal. Furthermore, the relatively lower surface energy compared to the surface tension of the reaction products and intricate surface topology of nearly superhydrophobic surfaces resulted in amplified colorimetric signals. This approach presents a practical and reliable solution for distinguishing analyte concentrations using simulated as well as clinical samples. Exploring the compatibility and applicability of superhydrophobic surfaces in diverse diagnostic scenarios can lead to the development of innovative and reliable diagnostic devices with improved performance and accuracy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our special thanks to the Department of Biotechnology, Manipal Institute of Technology. N. K. M. acknowledges Dr. Praveen Kumar and Dr. Vijendra Prabhu for their fruitful discussions. N.K.M also thanks Dr. Revathi P. Shenoy, Department of Biochemistry, Kasturba Medical College, Manipal for providing serum samples.
Funding
Open access funding was provided by Manipal Academy of Higher Education, Manipal. N. K. M. received financial support from the Vision Group on Science and Technology, Government of Karnataka, under RGS/F Scheme [Sanction Letter No.: KSTePS/VGSTRGS/F/GRD No. 711/2017–18]. N. K. M. also received financial support from the Science and Engineering Research Board (SERB), Department of Science and Technology, Govt of India, under the Core Research Grant (CRG) Scheme (File Number: CRG/2020/003060). S.S acknowledges Indian Council of Medical Research (ICMR) for providing Senior Research Fellowship (File Number: 5/3/8/67/ITR-F/2022-ITR).
Declarations
Ethics approval
The study was approved by the Manipal Academy of Higher Education with IEC No: 318–2021; “Paper-based and thread-based device for detection of liver and glucose biomarkers.”
Competing interests
The authors declare no competing interests.
Source of biological material
Anonymized serum samples were collected from the Department of Biochemistry, Kasturba Medical College, Manipal 576104.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sujesh Sudarsan and Prashil Shetty contributed equally to this study.
References
- 1.Sobsey CA, Ibrahim S, Richard VR, Gaspar V, Mitsa G, Lacasse V, Zahedi RP, Batist G, Borchers CH. Targeted and untargeted proteomics approaches in biomarker development. Proteomics. 2020;20:1900029. doi: 10.1002/pmic.201900029. [DOI] [PubMed] [Google Scholar]
- 2.Mani NK, Prabhu A, Biswas SK, Chakraborty S. Fabricating paper based devices using correction pens. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-018-38308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray R, Prabhu A, Prasad D, Garlapati V kumar, Aminabhavi TM, Mani NK, Simal-Gandara J. Paper-based microfluidic devices for food adulterants: cost-effective technological monitoring systems. Food Chem. 2022;390:133173 [DOI] [PubMed]
- 4.Ray R, Noronha C, Prabhu A, Mani NK. Latex-based paper devices with super solvent resistance for on-the-spot detection of metanil yellow in food samples. Food Anal Methods. 2022;15:2664–2674. doi: 10.1007/s12161-022-02322-2. [DOI] [Google Scholar]
- 5.Hasandka A, Singh AR, Prabhu A, Singhal HR, Nandagopal MSG, Mani NK. Paper and thread as media for the frugal detection of urinary tract infections (UTIs) Anal Bioanal Chem. 2022;414:847–865. doi: 10.1007/s00216-021-03671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal HR, Prabhu A, GiriNandagopal MS, Dheivasigamani T, Mani NK. One-dollar microfluidic paper-based analytical devices: do-it-yourself approaches. Microchem J. 2021;165:106126. doi: 10.1016/j.microc.2021.106126. [DOI] [Google Scholar]
- 7.Prabhu A, Giri Nandagopal MS, Peralam Yegneswaran P, Singhal HR, Mani NK. Inkjet printing of paraffin on paper allows low-cost point-of-care diagnostics for pathogenic fungi. Cellulose. 2020;27:7691–7701. doi: 10.1007/s10570-020-03314-3. [DOI] [Google Scholar]
- 8.Laigle L, Chadli L, Moingeon P. Biomarker-driven development of new therapies for autoimmune diseases: current status and future promises. Expert Rev Clin Immunol. 2023;19:305–314. doi: 10.1080/1744666X.2023.2172404. [DOI] [PubMed] [Google Scholar]
- 9.Kelkar N, Prabhu A, Prabhu A, GiriNandagopal MS, Mani NK. Sensing of body fluid hormones using paper-based analytical devices. Microchem J. 2022;174:107069. doi: 10.1016/j.microc.2021.107069. [DOI] [Google Scholar]
- 10.García-Gutiérrez MS, Navarrete F, Sala F, Gasparyan A, Austrich-Olivares A, Manzanares J. Biomarkers in psychiatry: concept, definition, types and relevance to the clinical reality. Front psychiatry. 2020;11:432. doi: 10.3389/fpsyt.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam H, Gopinath SCB, Arshad MKM, Adam T, Hashim U, Sauli Z, Fakhri MA, Subramaniam S, Chen Y, Sasidharan S. Integration of microfluidic channel on electrochemical-based nanobiosensors for monoplex and multiplex analyses: an overview. J Taiwan Inst Chem Eng. 2023;146:104814. doi: 10.1016/j.jtice.2023.104814. [DOI] [Google Scholar]
- 12.Khosla NK, Lesinski JM, Colombo M, Bezinge L, DeMello AJ, Richards DA. Simplifying the complex: accessible microfluidic solutions for contemporary processes within in vitro diagnostics. Lab Chip. 2022;22:3340–3360. doi: 10.1039/D2LC00609J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son MH, Park SW, Sagong HY, Jung YK. Recent advances in electrochemical and optical biosensors for cancer biomarker detection. BioChip J. 2023;17:44–67. doi: 10.1007/s13206-022-00089-6. [DOI] [Google Scholar]
- 14.Boonkaew S, Jang I, Noviana E, Siangproh W, Chailapakul O, Henry CS. Electrochemical paper-based analytical device for multiplexed, point-of-care detection of cardiovascular disease biomarkers. Sensors Actuators B Chem. 2021;330:129336. doi: 10.1016/j.snb.2020.129336. [DOI] [Google Scholar]
- 15.Hasandka A, Prabhu A, Prabhu A, Singhal HR, Nandagopal MSG, Shenoy R, Mani NK. “Scratch it out”: carbon copy based paper devices for microbial assays and liver disease diagnosis. Anal Methods. 2021;13:3172–3180. doi: 10.1039/D1AY00764E. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava SK, Oggu GS, Rayaprolu A, Adicherla H, Rao CM, Bhatnagar I, Asthana A. Chitosan reduced in-situ synthesis of gold nanoparticles on paper towards fabricating highly sensitive, stable uniform SERS substrates for sensing applications. Int J Biol Macromol. 2023;239:124240. doi: 10.1016/j.ijbiomac.2023.124240. [DOI] [PubMed] [Google Scholar]
- 17.Parween S, Asthana A, Nahar P. Fundamentals of image-based assay (IBA) system for affordable point of care diagnostics. Microchem J. 2023;186:108345. doi: 10.1016/j.microc.2022.108345. [DOI] [Google Scholar]
- 18.Walia S, Bhatnagar I, Liu J, Mitra SK, Asthana A. A novel method for fabrication of paper-based microfluidic devices using BSA-ink. Int J Biol Macromol. 2021;193:1617–1622. doi: 10.1016/j.ijbiomac.2021.10.224. [DOI] [PubMed] [Google Scholar]
- 19.Parween S, Bhatnagar I, Bhosale S, Paradkar S, Michael IJ, Rao CM, Asthana A. Cross-linked chitosan biofunctionalized paper-based microfluidic device towards long term stabilization of blood typing antibodies. Int J Biol Macromol. 2020;163:1233–1239. doi: 10.1016/j.ijbiomac.2020.07.075. [DOI] [PubMed] [Google Scholar]
- 20.Trinh TND, Thai DA, Lee NY. Pop-up paper-based and fully integrated microdevice for point-of-care testing of vancomycin-resistant Enterococcus. Sensors Actuators B Chem. 2021;345:130362. doi: 10.1016/j.snb.2021.130362. [DOI] [Google Scholar]
- 21.Luo Z, Lv T, Zhu K, Li Y, Wang L, Gooding JJ, Liu G, Liu B. Paper-based ratiometric fluorescence analytical devices towards point-of-care testing of human serum albumin. Angew Chemie. 2020;132:3155–3160. doi: 10.1002/ange.201915046. [DOI] [PubMed] [Google Scholar]
- 22.Prabhu A, Singhal H, Giri Nandagopal MS, Kulal R, Peralam Yegneswaran P, Mani NK. Knitting thread devices: detecting Candida albicans using napkins and tampons. ACS Omega. 2021;6:12667–12675. doi: 10.1021/acsomega.1c00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D-M, Xu B, Dong C. Recent advances in colorimetric strategies for acetylcholinesterase assay and their applications. TrAC Trends Anal Chem. 2021;142:116320. doi: 10.1016/j.trac.2021.116320. [DOI] [Google Scholar]
- 24.Umapathi R, Sonwal S, Lee MJ, Rani GM, Lee E-S, Jeon T-J, Kang S-M, Oh M-H, Huh YS. Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: new horizons, perspectives, and challenges. Coord Chem Rev. 2021;446:214061. doi: 10.1016/j.ccr.2021.214061. [DOI] [Google Scholar]
- 25.Hoang TX, Phan LMT, Vo TAT, Cho S. Advanced signal-amplification strategies for paper-based analytical devices: a comprehensive review. Biomedicines. 2021;9. 10.3390/biomedicines9050540 [DOI] [PMC free article] [PubMed]
- 26.Mani NK, Das SS, Dawn S, Chakraborty S. Electro-kinetically driven route for highly sensitive blood pathology on a paper-based device. Electrophoresis. 2020;41:615–620. doi: 10.1002/elps.201900356. [DOI] [PubMed] [Google Scholar]
- 27.da Silva VAOP, de Freitas RC, de Oliveira PR, Moreira RC, Marcolino-Júnior LH, Bergamini MF, Coltro WKT, Janegitz BC. Microfluidic paper-based device integrated with smartphone for point-of-use colorimetric monitoring of water quality index. Measurement. 2020;164:108085. doi: 10.1016/j.measurement.2020.108085. [DOI] [Google Scholar]
- 28.de Castro LF, de Freitas SV, Duarte LC, de Souza JAC, Paixão TRLC, Coltro WKT. Salivary diagnostics on paper microfluidic devices and their use as wearable sensors for glucose monitoring. Anal Bioanal Chem. 2019;411:4919–4928. doi: 10.1007/s00216-019-01788-0. [DOI] [PubMed] [Google Scholar]
- 29.Rocha DS, Duarte LC, Silva-Neto HA, Chagas CLS, Santana MHP, AntoniosiFilho NR, Coltro WKT. Sandpaper-based electrochemical devices assembled on a reusable 3D-printed holder to detect date rape drug in beverages. Talanta. 2021;232:122408. doi: 10.1016/j.talanta.2021.122408. [DOI] [PubMed] [Google Scholar]
- 30.Alahmad W, Varanusupakul P, Varanusupakul P. Recent developments and applications of microfluidic paper-based analytical devices for the detection of biological and chemical hazards in foods: a critical review. Crit Rev Anal Chem. 2023;53:233–252. doi: 10.1080/10408347.2021.1949695. [DOI] [PubMed] [Google Scholar]
- 31.Ray R, Goyal A, Prabhu A, Parekkh S, Maddasani S, Mani NK. Paper-based dots and smartphone for detecting counterfeit country eggs. Food Chem. 2023;403:134484. doi: 10.1016/j.foodchem.2022.134484. [DOI] [PubMed] [Google Scholar]
- 32.Silva-Neto HA, Cardoso TMG, McMahon CJ, Sgobbi LF, Henry CS, Coltro WKT. Plug-and-play assembly of paper-based colorimetric and electrochemical devices for multiplexed detection of metals. Analyst. 2021;146:3463–3473. doi: 10.1039/d1an00176k. [DOI] [PubMed] [Google Scholar]
- 33.Sousa LR, Duarte LC, Coltro WKT. Instrument-free fabrication of microfluidic paper-based analytical devices through 3D pen drawing. Sensors Actuators B Chem. 2020;312:128018. doi: 10.1016/j.snb.2020.128018. [DOI] [Google Scholar]
- 34.Chi J, Zhang X, Wang Y, Shao C, Shang L, Zhao Y. Bio-inspired wettability patterns for biomedical applications. Mater Horizons. 2021;8:124–144. doi: 10.1039/D0MH01293A. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Zhang L, Huang J, Deng Z, Yuan Y, Zou J, Nie J, Zhang Y. Enhanced functional DNA biosensor for distance-based read-by-eye quantification of various analytes based on starch-hydrolysis-adjusted wettability change in paper devices. RSC Adv. 2020;10:28121–28127. doi: 10.1039/D0RA04619A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aydemir C, Karademir A, İmamoğlu S, Altay BN, Fleming PD, Tutak D. Investigation of the evolution of hydrophobicity and wettability of paper in multi-color printing process. Cellul Chem Technol. 2019.
- 37.Wang X, Liu Y, Cheng H, Ouyang X. Surface wettability for skin-interfaced sensors and devices. Adv Funct Mater. 2022;32:2200260. doi: 10.1002/adfm.202200260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glucose SL. Onboard calibration stability glucose ( S . L ) quality control. 2016;7151:2015–2016.
- 39.Types I, Tool C FIJI (FIJI is just ImageJ) Contents. 1–30
- 40.Sathyanesan A, Ogura T, Lin W. Automated measurement of nerve fiber density using line intensity scan analysis. J Neurosci Methods. 2012;206:165–175. doi: 10.1016/j.jneumeth.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Omari H, Ablouh E, Brouillette F, Taourirte M, Belfkira A. New method for determining paper surface energy per contact angle. Cellulose. 2019;26:9295–9309. doi: 10.1007/s10570-019-02695-4. [DOI] [Google Scholar]
- 42.Drelich JW, Boinovich L, Chibowski E, Della Volpe C, Hołysz L, Marmur A, Siboni S. Contact angles: history of over 200 years of open questions. Surf Innov. 2019;8:3–27. doi: 10.1680/jsuin.19.00007. [DOI] [Google Scholar]
- 43.Edachery V, Ravi S, Badiuddin AF, Tomy A, Kailas SV, Suvin PS. Wetting behaviour of a Green cutting fluid (GCF); influence of surface roughness and surface energy of AA5052, Ti6Al4V and EN31. Mater Today Proc. 2022;62:7605–7609. doi: 10.1016/j.matpr.2022.04.835. [DOI] [Google Scholar]
- 44.Huang F, Motealleh B, Wang D, Cornelius CJ. Tailoring intrinsic hydrophobicity and surface energy on rough surface via low-T Cassie-Wenzel wetting transition method. AIChE J. 2023;69:e17908. doi: 10.1002/aic.17908. [DOI] [Google Scholar]
- 45.Wang X, Zhang Q. Role of surface roughness in the wettability, surface energy and flotation kinetics of calcite. Powder Technol. 2020;371:55–63. doi: 10.1016/j.powtec.2020.05.081. [DOI] [Google Scholar]
- 46.Parvate S, Dixit P, Chattopadhyay S. Superhydrophobic surfaces: insights from theory and experiment. J Phys Chem B. 2020;124:1323–1360. doi: 10.1021/acs.jpcb.9b08567. [DOI] [PubMed] [Google Scholar]
- 47.Khoshbin Z, Housaindokht MR, Izadyar M, Bozorgmehr MR, Verdian A. Temperature and molecular crowding effects on the sensitivity of T30695 aptamer toward Pb2+ ion: a joint molecular dynamics simulation and experimental study. Mol Simul. 2020;46:592–603. doi: 10.1080/08927022.2020.1751842. [DOI] [Google Scholar]
- 48.Qin X, Liu J, Zhang Z, Li J, Yuan L, Zhang Z, Chen L. Microfluidic paper-based chips in rapid detection: current status, challenges, and perspectives. TrAC Trends Anal Chem. 2021;143:116371
- 49.Backholm M, Molpeceres D, Vuckovac M, Nurmi H, Hokkanen MJ, Jokinen V, Timonen JVI, Ras RHA. Water droplet friction and rolling dynamics on superhydrophobic surfaces. Commun Mater. 2020;1:64. doi: 10.1038/s43246-020-00065-3. [DOI] [Google Scholar]
- 50.He F, Wang H, Du P, Li T, Wang W, Tan T, Liu Y, Ma Y, Wang Y, Abd El-Aty AM. Personal glucose meters coupled with signal amplification technologies for quantitative detection of non-glucose targets: recent progress and challenges in food safety hazards analysis. J Pharm Anal. 2023. [DOI] [PMC free article] [PubMed]
- 51.Bollella P, Katz E. Biosensors—recent advances and future challenges. Sensors. 2020;20:6645. doi: 10.3390/s20226645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sudarsan S, Prabhu A, Prasad D, Mani NK. DNA compaction enhances sensitivity of fluorescence-based nucleic acid assays: game changer in point of care sensors? Analyst. 2023. [DOI] [PubMed]
- 53.Roy S, Mohd-Naim NF, Safavieh M, Ahmed MU. Colorimetric nucleic acid detection on paper microchip using loop mediated isothermal amplification and crystal violet dye. ACS Sensors. 2017;2:1713–1720. doi: 10.1021/acssensors.7b00671. [DOI] [PubMed] [Google Scholar]
- 54.Miyamoto S, Sano S, Takahashi K, Jikihara T. Method for colorimetric detection of double-stranded nucleic acid using leuco triphenylmethane dyes. Anal Biochem. 2015;473:28–33. doi: 10.1016/j.ab.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 55.Li A, Wang G, Zhang Y, Zhang J, He W, Ren S, Xu Z, Wang J, Ma Y. Preparation methods and research progress of superhydrophobic paper. Coord Chem Rev. 2021;449:214207. doi: 10.1016/j.ccr.2021.214207. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.