Abstract
Introduction
Severe asthma is associated with airway inflammation and airway obstruction. In the phase 3 NAVIGATOR study, tezepelumab treatment significantly improved pre-bronchodilator forced expiratory volume in 1 s (FEV1) compared with placebo in patients with severe, uncontrolled asthma. This analysis assessed the effect of tezepelumab versus placebo on additional lung function parameters in patients from NAVIGATOR.
Methods
NAVIGATOR was a multicenter, randomized, double-blind, placebo-controlled study. Patients (12–80 years old) receiving medium- or high-dose inhaled corticosteroids and at least one additional controller medication, with or without oral corticosteroids, were randomized 1:1 to tezepelumab 210 mg or placebo subcutaneously every 4 weeks for 52 weeks. Changes from baseline to week 52 in pre-bronchodilator FEV1, post-bronchodilator FEV1, forced vital capacity (FVC), pre-bronchodilator FEV1/FVC ratio, pre-bronchodilator forced expiratory flow between 25 and 75% of vital capacity (FEF25–75), and morning and evening peak expiratory flow (PEF) were assessed.
Results
Tezepelumab treatment improved all evaluated lung function parameters over 52 weeks compared with placebo [least-squares mean difference (95% confidence interval): pre-bronchodilator FEV1, 0.13 (0.08, 0.18) L; post-bronchodilator FEV1, 0.12 (0.07, 0.16) L; FVC, 0.13 (0.07, 0.19) L; FEV1/FVC ratio, 2.06% (1.22%, 2.90%); FEF25–75, 0.13 (0.07, 0.19) L/s; morning PEF, 16.6 (8.1, 25.1) L/min; and evening PEF, 14.9 (6.3, 23.4) L/min]. Improvements were observed as early as weeks 1–2 and were maintained over 52 weeks. Greater improvements in lung function compared with placebo were observed in patients with a disease duration of less than 20 years, those with baseline post-bronchodilator FEV1 reversibility of at least 20%, and in patients with a baseline post-bronchodilator FEV1/FVC ratio of less than 0.7.
Conclusion
These findings further support the benefits of tezepelumab treatment in improving airflow limitation in patients with severe, uncontrolled asthma.
Clinical Trial Registration
NAVIGATOR (NCT03347279).
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02659-y.
Keywords: Biologics, Forced expiratory volume in 1 s (FEV1), Pulmonary function, Tezepelumab, Thymic stromal lymphopoietin (TSLP)
Key Summary Points
| Severe asthma has been associated with airway inflammation and remodeling, leading to the progressive decline of lung function over time and increased risk of permanent, irreversible airway obstruction. |
| Why carry out the study? |
| This analysis was performed to provide a more comprehensive evaluation of the effect of tezepelumab on lung function parameters in patients from NAVIGATOR. |
| Tezepelumab treatment improved all evaluated lung function parameters over 52 weeks compared with placebo. |
| What was learned from the study? |
| This study highlights the importance of early treatment of patients with a short disease duration and high baseline post-bronchodilator FEV1 reversibility to improve their lung function during the course of the disease, and further demonstrates the efficacy of tezepelumab in broadly improving lung function in patients with severe, uncontrolled asthma. |
Introduction
Severe asthma has been associated with airway inflammation and remodeling [1]. These structural changes can contribute to the progressive decline of lung function over time and increase the risk of permanent, irreversible airway obstruction [2, 3]. Mucus plugging, an important feature in asthma pathogenesis, has also been identified as a contributor to chronic airway obstruction [4, 5]. In addition to asthma severity, decreased lung function may also be linked to disease duration [6–8]. Duration of asthma (time since diagnosis) has been found to be inversely correlated with maximal forced expiratory volume in 1 s (FEV1) and percent predicted FEV1 (ppFEV1) [7, 8].
Low FEV1 and airway hyper-responsiveness are predictors of irreversible airflow obstruction and lung function decline [9, 10]. Obstruction of airflow is defined as a reduction in the FEV1/forced vital capacity (FVC) ratio [11]. Low post-bronchodilator FEV1/FVC ratios have been suggested as a marker of airway remodeling [12]. In addition, other lung function parameters may act as phenotypic or prognostic markers. Persistent reversible airway obstruction, when also associated with elevated blood eosinophil counts, can be a marker of accelerated lung function decline [13]. Furthermore, impaired forced expiratory flow between 25 and 75% of vital capacity (FEF25–75) has been associated with small-airway disease, bronchial hyperresponsiveness, and airway inflammation, and is a potential early marker of airway obstruction [14]. These observations highlight the importance of understanding the effect of therapies across multiple lung function parameters.
Thymic stromal lymphopoietin (TSLP) is an epithelial cytokine involved in the initiation and persistence of multiple downstream processes associated with asthma pathophysiology, including allergic inflammation, eosinophilic inflammation, and type 2 (T2)-independent effects on mast cells, airway smooth muscle, and airway hyperresponsiveness [15]. In patients with asthma, increased TSLP concentration in the bronchoalveolar lavage fluid has been shown to correlate with increased disease severity and reduced lung function [15, 16].
Tezepelumab is a human monoclonal antibody (immunoglobulin G2λ) that binds specifically to TSLP, blocking its interaction with its heterodimeric receptor [17]. In the phase 3 NAVIGATOR study (NCT03347279), tezepelumab treatment significantly reduced exacerbations over 52 weeks compared with placebo in adults and adolescents with severe, uncontrolled asthma. Additionally, tezepelumab treatment significantly improved pre-bronchodilator FEV1 by 0.13 L compared with placebo. Improvements were observed as early as week 2, were sustained throughout the treatment period, and were greater in patients with higher baseline blood eosinophil counts [18]. A more complete understanding of the lung function changes associated with tezepelumab treatment is required to determine the clinical benefits to patients and providers, and to provide mechanistic insights into the effects of blocking TSLP activity with tezepelumab.
This analysis was performed to provide a more comprehensive evaluation of the effect of tezepelumab on lung function parameters in patients from NAVIGATOR.
Methods
Study Design and Patients
NAVIGATOR was a phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. The full study design and inclusion and exclusion criteria have been described previously [18].
Eligible adolescents and adults (aged 12–80 years) were nonsmokers with physician-diagnosed asthma. Tezepelumab was administrated as an add-on therapy to patients who had been receiving medium- or high-dose inhaled corticosteroids (ICS; daily dose of ≥ 500 µg fluticasone propionate or equivalent) for at least 12 months before screening and at least one additional controller medication, with or without oral corticosteroids, for at least 3 months before the date of informed consent. No changes were permitted to background asthma medications throughout the duration of the study, and preferably 4 weeks after the final dose of tezepelumab, except during the treatment of an asthma exacerbation. Once and twice daily background asthma medications were withheld for at least 24 and 12 h, respectively, before scheduled spirometry and fractional exhaled nitric oxide (FeNO) measurements were taken. Patients were required to have a morning pre-bronchodilator FEV1 of below 80% of the predicted normal value (< 90% for patients aged 12–17 years) during the run-in period; a post-bronchodilator (albuterol/salbutamol) FEV1 reversibility of at least 12%, and at least 200 mL must have been documented during the 12 months before screening or during the run-in period. Participants must have experienced at least two asthma exacerbations (defined as a worsening of asthma symptoms that led to hospitalization, an emergency room visit that resulted in the use of systemic corticosteroids for ≥ 3 consecutive days, or the use of systemic corticosteroids for ≥ 3 consecutive days) in the 12 months before the date of informed consent. Patients were randomized 1:1 to receive tezepelumab 210 mg or placebo subcutaneously every 4 weeks for 52 weeks.
Procedures
Lung function (FEV1, FVC, and FEF25–75) was measured by spirometry using equipment provided by a central vendor. Spirometry was performed by the investigator or an authorized delegate in accordance with American Thoracic Society/European Respiratory Society guidelines [19]. Patients were required to withhold their usual maintenance therapies before lung function testing.
Patients were provided with an electronic handheld spirometer (AM3G+; eResearch Technology, Philadelphia, PA, USA) with which to take peak expiratory flow (PEF) measurements. Home PEF testing was performed daily by patients in the morning upon awakening and in the evening before sleeping (before taking their morning or evening asthma controller medication, respectively). Patients were required to record three successive PEF measurements during each morning and evening test, and had to perform all tests in the same position (either standing or sitting). The highest morning and evening PEF values were selected from the available individual measurements. If a patient used a short-acting β2 agonist as rescue medication, the PEF measurements were recorded at least 6 h after the last dose of this treatment. Home PEF measurements were recorded during the screening period up until 52 weeks after randomization. At each study visit, the investigator assessed patient adherence to correct spirometer use.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines and applicable regulatory requirements. Approvals from the Copernicus Central Institutional Review Board (Cary, NC, USA) and local independent ethics committees were obtained, and all patients or their guardians provided written informed consent in accordance with local requirements.
Outcomes
Prespecified secondary outcomes related to lung function included change from baseline in pre-bronchodilator FEV1 (minimum clinically important difference compared with placebo, 0.1 L) [20] and change from baseline in morning and evening PEF (minimal patient-perceivable improvement, 18.8 L/min) [21]. Prespecified exploratory outcomes included changes from baseline in post-bronchodilator FEV1, pre-bronchodilator ppFEV1, pre- and post-bronchodilator FVC, pre- and post-bronchodilator FEV1/FVC ratio, and pre-bronchodilator FEF25–75.
To provide further clinical insights into factors affecting the lung function improvements associated with tezepelumab, post hoc analyses were conducted to assess the pre-bronchodilator FEV1 and pre-bronchodilator FEF25–75 at time points up to and including week 52 in patients grouped by the following: disease duration (< 20 years and ≥ 20 years since asthma diagnosis; the threshold was based on the median duration of 18 years in NAVIGATOR patients), low and high baseline post-bronchodilator FEV1 reversibility (< 20 and ≥ 20%), and post-bronchodilator FEV1/FVC ratio (< 0.7 and ≥ 0.7). Although bronchodilator reversibility is a continuous variable, low bronchodilator reversibility has previously been defined using cutoffs in post-bronchodilator change in FEV1 of less than 12%, less than 16%, or less than 20% [22]. Post-bronchodilator FEV1 was assessed at time points up to and including week 52 in patients grouped by disease duration and by post-bronchodilator ppFEV1 [< 80% (abnormal lung function) and ≥ 80% (normal lung function)]. Pre-bronchodilator FEV1 was assessed in the following subgroups: patients receiving 1, 2, or at least 3 controller medications in addition to ICS at baseline in the overall NAVIGATOR population; patients grouped by the type of additional controller medications [long-acting β2 agonist (LABA), long-acting muscarinic antagonist (LAMA), or leukotriene receptor antagonist (LTRA)] that they were receiving at baseline in the overall NAVIGATOR population; and patients who were not receiving maintenance OCS at baseline grouped by the type of additional controller medications that they were receiving at baseline.
Statistical Analyses
Least-squares mean and adjusted mean changes from baseline in secondary and exploratory outcomes were assessed at multiple time points up to and including week 52 using a repeated measures model. All observations were included in the analyses, including those after discontinuation of tezepelumab or placebo.
Treatment group, region, age group (adolescent or adult), baseline lung function measurement, visit, and treatment-by-visit were included as covariates in the model. For subgroup analyses, subgroup (if not already included), treatment-by-subgroup, visit-by-subgroup, and treatment-by-visit-by-subgroup interactions were also included in the model, in addition to the covariates listed above. For the weekly PEF endpoints, each of the 52 weeks used for the weekly mean calculation replaced visit in the above model specification.
Results
Overall NAVIGATOR Population
Overall, 1059 patients received either tezepelumab (n = 528) or placebo (n = 531). Baseline demographics and clinical characteristics were generally balanced between treatment groups in the overall NAVIGATOR population (Table S1).
Improvements from baseline were observed with tezepelumab compared with placebo across all evaluated lung function parameters. Improvements were observed at the first post-baseline time point assessed and were sustained throughout the treatment period (Table 1; Figs. S1 and S2). Compared with placebo, tezepelumab treatment resulted in improvements of 0.12–0.13 L in pre-bronchodilator FEV1 [0.13 L; 95% confidence interval (CI): 0.08, 0.18], post-bronchodilator FEV1 (0.12 L; 95% CI: 0.07, 0.16), and pre-bronchodilator FVC (0.13 L; 95% CI: 0.07, 0.19), and 0.13 L/s in FEF25–75 (95% CI: 0.07, 0.19). Improvements from baseline in morning and evening PEF were observed with tezepelumab at week 1, with clinically meaningful improvements noted in morning PEF at week 2 and evening PEF at week 3.
Table 1.
Change from baseline to week 2 and week 52 in lung function parameters
| Lung function parameter | Week 2 | Week 52 | ||
|---|---|---|---|---|
| Tezepelumab 210 mg Q4W | Placebo | Tezepelumab 210 mg Q4W | Placebo | |
| Pre-bronchodilator FEV1, La | ||||
| Mean at baseline (SD) | 1.8 (0.7) | 1.9 (0.7) | 1.8 (0.7) | 1.9 (0.7) |
| n | 514 | 507 | 471 | 453 |
| LS mean change from baseline (SE) | 0.16 (0.02) | 0.05 (0.02) | 0.23 (0.02) | 0.10 (0.02) |
| LS mean difference (95% CI) | 0.11 (0.07, 0.15) | 0.13 (0.08, 0.18) | ||
| Pre-bronchodilator ppFEV1, % | ||||
| Mean at baseline (SD) | 62.8 (18.0) | 62.7 (18.0) | 62.8 (18.0) | 62.7 (18.0) |
| n | 514 | 507 | 471 | 453 |
| LS mean change from baseline (SE) | 5.71 (0.47) | 1.94 (0.47) | 7.86 (0.57) | 3.05 (0.58) |
| LS mean difference (95% CI) | 3.77 (2.46, 5.08) | 4.81 (3.22, 6.40) | ||
| Post-bronchodilator FEV1, Lb | ||||
| Mean at baseline (SD) | 2.1 (0.8) | 2.1 (0.8) | 2.1 (0.8) | 2.1 (0.8) |
| n | – | – | 480 | 460 |
| LS mean change from baseline (SE) | – | – | 0.15 (0.02) | 0.03 (0.02) |
| LS mean difference (95% CI) | – | 0.12 (0.07, 0.16) | ||
| Pre-bronchodilator FVC, L | ||||
| Mean at baseline (SD) | 2.9 (0.9) | 3.0 (0.9) | 2.9 (0.9) | 3.0 (0.9) |
| n | 514 | 507 | 471 | 453 |
| LS mean change from baseline (SE) | 0.16 (0.02) | 0.06 (0.02) | 0.24 (0.02) | 0.11 (0.02) |
| LS mean difference (95% CI) | 0.10 (0.05, 0.15) | 0.13 (0.07, 0.19) | ||
| Post-bronchodilator FVC, Lb | ||||
| Mean at baseline (SD) | 3.1 (1.0) | 3.2 (0.9) | 3.1 (1.0) | 3.2 (0.9) |
| n | – | – | 480 | 460 |
| LS mean change from baseline (SE) | – | – | 0.13 (0.02) | 0.04 (0.02) |
| LS mean difference (95% CI) | – | 0.09 (0.04, 0.14) | ||
| Pre-bronchodilator FEV1/FVC ratio, % | ||||
| Mean at baseline (SD) | 63.2 (13.3) | 62.5 (12.9) | 63.2 (13.3) | 62.5 (12.9) |
| n | 514 | 507 | 471 | 453 |
| LS mean change from baseline (SE) | 2.4 (0.3) | 0.5 (0.3) | 2.6 (0.3) | 0.5 (0.3) |
| LS mean difference (95% CI) | 1.89 (1.15, 2.63) | 2.06 (1.22, 2.90) | ||
| Post-bronchodilator FEV1/FVC ratio, %b | ||||
| Mean at baseline (SD) | 66.5 (13.7) | 66.1 (13.5) | 66.5 (13.7) | 66.1 (13.5) |
| n | – | – | 480 | 460 |
| LS mean change from baseline (SE) | – | – | 1.87 (0.3) | 0.08 (0.3) |
| LS mean difference (95% CI) | – | 1.78 (0.98, 2.59) | ||
| Pre-bronchodilator FEF25–75, L/s | ||||
| Mean at baseline (SD) | 1.1 (0.8) | 1.1 (0.9) | 1.1 (0.8) | 1.1 (0.9) |
| n | 514 | 507 | 471 | 453 |
| LS mean change from baseline (SE) | 0.16 (0.02) | 0.05 (0.02) | 0.21 (0.02) | 0.08 (0.02) |
| LS mean difference (95% CI) | 0.12 (0.06, 0.17) | 0.13 (0.07, 0.19) | ||
| Weekly mean morning PEF, L/min | ||||
| Mean at baseline (SD) | 261.7 (109.7) | 262.5 (105.8) | 261.7 (109.7) | 262.5 (105.8) |
| n | 519 | 519 | 414 | 391 |
| LS mean change from baseline (SE) | 20.3 (1.7) | 6.4 (1.7) | 34.6 (3.1) | 18.0 (3.1) |
| LS mean difference (95% CI) | 13.9 (9.15, 18.65) | 16.6 (8.07, 25.05) | ||
| Weekly mean evening PEF, L/min | ||||
| Mean at baseline (SD) | 276.9 (111.3) | 278.2 (106.9) | 276.9 (111.3) | 278.2 (106.9) |
| n | 517 | 519 | 405 | 390 |
| LS mean change from baseline (SE) | 17.4 (1.7) | 4.9 (1.7) | 23.9 (3.1) | 9.0 (3.1) |
| LS mean difference (95% CI) | 12.5 (7.69, 17.21) | 14.9 (6.31, 23.41) | ||
n is the number of patients with available data at the given time point. All patients who were randomized and received study treatment were included in the repeated measures model
CI confidence interval, FEF25–75 forced expiratory flow between 25 and 75% of vital capacity, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, LS least-squares, PEF peak expiratory flow, ppFEV1 percent predicted forced expiratory volume in 1 s, Q4W every 4 weeks, SD standard deviation, SE standard error
aFEV1 data have been published previously [18]
bPost-bronchodilator FEV1, post-bronchodilator FVC, and post-bronchodilator FEV1/FVC ratio were only assessed at week 24 and week 52
Disease Duration Subgroup
Baseline demographics were generally balanced in patients grouped by disease duration (Table S2). Compared with patients with a disease duration of 20 years or more, patients with a disease duration of less than 20 years had higher pre-bronchodilator ppFEV1 and FeNO levels at baseline, and comprised a lower proportion of patients who received high-dose ICS at baseline (Table S2).
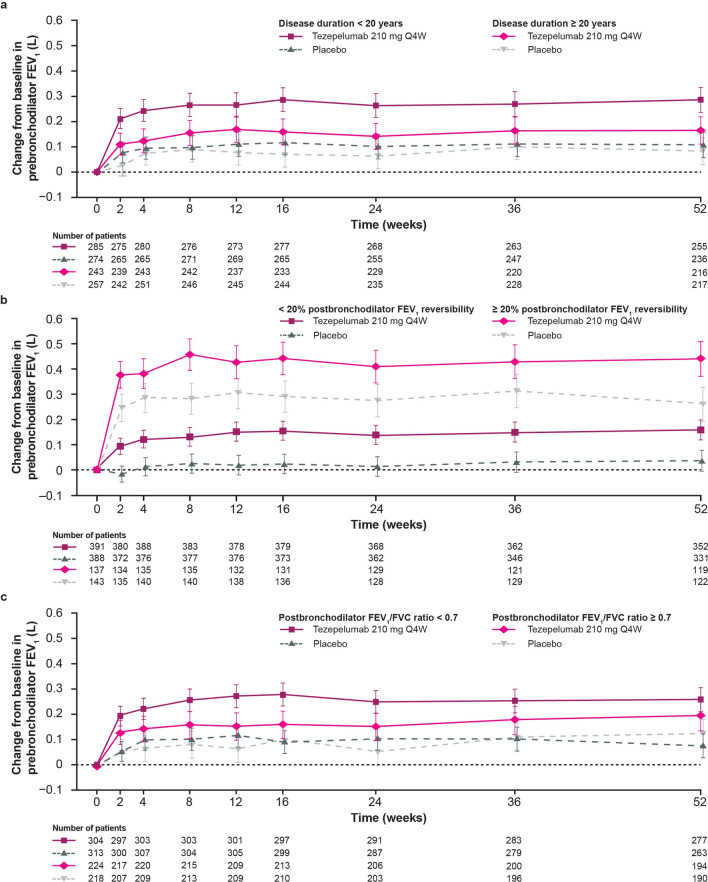
Among patients who received tezepelumab, improvements from baseline to week 52 compared with placebo in pre-bronchodilator FEV1, post-bronchodilator FEV1, and pre-bronchodilator FEF25–75 were greater in patients with a disease duration of less than 20 years than in those with a duration of 20 years or more [pre-bronchodilator FEV1: 0.18 L (95% CI: 0.11, 0.25) vs. 0.08 L (95% CI: 0.01, 0.15), respectively; post-bronchodilator FEV1: 0.13 L (95% CI: 0.07, 0.20) vs. 0.09 L (95% CI: 0.02, 0.16), respectively; pre-bronchodilator FEF25–75: 0.17 L (95% CI: 0.08, 0.25) vs. 0.09 L (95% CI: 0.00, 0.18), respectively] (Tables 2, S3 and S4; Figs. 1a and S3a).
Table 2.
Change from baseline to week 52 in pre-bronchodilator FEV1 in patients grouped by disease duration, baseline post-bronchodilator FEV1 reversibility, and baseline post-bronchodilator FEV1/FVC ratio
| Subgroup | Mean at baseline (SD), L | LS mean change from baseline (SE), L | LS mean difference versus placebo (95% CI), L | ||
|---|---|---|---|---|---|
| Tezepelumab 210 mg Q4W | Placebo | Tezepelumab 210 mg Q4W | Placebo | ||
| Baseline disease duration, years | |||||
| < 20 | 2.0 (0.8) | 2.0 (0.8) | 0.29 (0.03) | 0.11 (0.03) | 0.18 (0.11, 0.25) |
| n | 285 | 274 | 255 | 236 | |
| ≥ 20 | 1.7 (0.6) | 1.7 (0.6) | 0.16 (0.03) | 0.08 (0.03) | 0.08 (0.01, 0.15) |
| n | 243 | 257 | 216 | 217 | |
| Baseline post-bronchodilator FEV1 reversibility | |||||
| < 20% | 2.0 (0.7) | 1.9 (0.7) | 0.16 (0.02) | 0.03 (0.02) | 0.12 (0.07, 0.18) |
| n | 391 | 388 | 352 | 331 | |
| ≥ 20% | 1.5 (0.5) | 1.6 (0.6) | 0.44 (0.04) | 0.26 (0.03) | 0.18 (0.09, 0.27) |
| n | 137 | 143 | 119 | 122 | |
| Baseline post-bronchodilator FEV1/FVC ratio | |||||
| < 0.7 | 1.5 (0.5) | 1.6 (0.5) | 0.26 (0.02) | 0.08 (0.02) | 0.18 (0.12, 0.25) |
| n | 304 | 313 | 277 | 263 | |
| ≥ 0.7 | 2.2 (0.7) | 2.3 (0.7) | 0.19 (0.03) | 0.12 (0.03) | 0.07 (− 0.01, 0.15) |
| n | 224 | 218 | 194 | 190 | |
| Baseline combined post-bronchodilator FEV1 reversibility and post-bronchodilator FEV1/FVC ratio | |||||
| < 20% and < 0.7 | 1.6 (0.5) | 1.6 (0.5) | 0.21 (0.03) | 0.04 (0.03) | 0.18 (0.10, 0.25) |
| n | 209 | 224 | 191 | 185 | |
| ≥ 20% and < 0.7 | 1.4 (0.5) | 1.4 (0.5) | 0.41 (0.04) | 0.23 (0.04) | 0.19 (0.07, 0.30) |
| n | 95 | 89 | 86 | 78 | |
| < 20% and ≥ 0.7 | 2.4 (0.7) | 2.4 (0.7) | 0.09 (0.03) | 0.02 (0.03) | 0.06 (− 0.02, 0.15) |
| n | 182 | 164 | 161 | 146 | |
| ≥ 20% and ≥ 0.7 | 1.7 (0.5) | 2.0 (0.5) | 0.54 (0.06) | 0.33 (0.06) | 0.21 (0.04, 0.37) |
| n | 42 | 54 | 33 | 44 | |
CI confidence interval, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, LS least-squares, Q4W every 4 weeks, SD standard deviation, SE standard error
Fig. 1.
Change from baseline in pre-bronchodilator FEV1 over 52 weeks in patients grouped by (a) disease duration, (b) baseline post-bronchodilator FEV1 reversibility, and (c) post-bronchodilator FEV1/FVC ratio. “Number of patients” indicates the number of patients with available data at a given time point. Patients who received at least one dose of tezepelumab or placebo with at least one change from baseline assessment were included in the model. Data are adjusted means and 95% CIs. CI confidence interval, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, Q4W every 4 weeks
Among those who received tezepelumab, 75.1% (n = 214/285) and 90.5% (n = 220/243) of patients with a disease duration of less than 20 years and patients with a disease duration of 20 years or more, respectively, had abnormal lung function (pre-bronchodilator ppFEV1 < 80%) at baseline. Of these, 29.0% (n = 62/214) of patients with a disease duration of less than 20 years and 11.4% (n = 25/220) of patients with a duration of 20 years or more had normal lung function at week 52 (Table S5). The proportions of patients who achieved normal lung function at week 52 with tezepelumab were higher than those observed with placebo across disease duration subgroups, with the greatest difference observed in patients with a disease duration of less than 20 years. The proportion of patients who shifted from normal lung function at baseline to abnormal lung function at week 52 was lower with tezepelumab than with placebo in both disease duration subgroups (Table S5).
Post-bronchodilator FEV1 Reversibility Subgroup
Baseline demographics were generally balanced in patients grouped by post-bronchodilator FEV1 reversibility at baseline (Table S2). Patients with high post-bronchodilator FEV1 reversibility had lower pre-bronchodilator FEV1, higher blood eosinophil counts, and higher FeNO levels than those with low post-bronchodilator FEV1 reversibility (Table S2). Among patients who received tezepelumab, improvements from baseline to week 52 compared with placebo in pre-bronchodilator FEV1 and pre-bronchodilator FEF25–75 were generally similar between patients with high post-bronchodilator FEV1 reversibility and low post-bronchodilator FEV1 reversibility at baseline [pre-bronchodilator FEV1: 0.18 L (95% CI: 0.09, 0.27) vs. 0.12 L (95% CI: 0.07, 0.18), respectively; pre-bronchodilator FEF25–75: 0.13 L (95% CI: 0.01, 0.26) vs. 0.13 L (95% CI: 0.06, 0.20), respectively] (Tables 2 and S3; Figs. 1b and S3b).
Post-bronchodilator FEV1/FVC Ratio Subgroup
At baseline, compared with patients with an FEV1/FVC ratio of 0.7 or more, patients with a FEV1/FVC ratio of less than 0.7 had higher T2 inflammatory biomarker levels (blood eosinophils and FeNO) and comprised a higher proportion of patients who received high-dose ICS (Table S2).
Among patients who received tezepelumab, patients with a post-bronchodilator FEV1/FVC ratio of less than 0.7 had greater improvements from baseline to week 52 compared with placebo in pre-bronchodilator FEV1 and pre-bronchodilator FEF25–75 than those with a post-bronchodilator FEV1/FVC ratio of 0.7 or more [pre-bronchodilator FEV1: 0.18 L (95% CI: 0.12, 0.25) vs. 0.07 L (95% CI: − 0.01, 0.15), respectively; pre-bronchodilator FEF25–75: 0.15 L (95% CI: 0.07, 0.23) vs. 0.10 L (95% CI: 0.01, 0.20), respectively] (Tables 2 and S2; Figs. 1c and S3c).
FEV1 Reversibility and FEV1/FVC Ratio Subgroups
Among patients grouped by combined baseline post-bronchodilator FEV1 reversibility and post-bronchodilator FEV1/FVC ratio, patients with a high post-bronchodilator FEV1 reversibility and a post-bronchodilator FEV1/FVC ratio of less than 0.7 exhibited the highest baseline blood eosinophil counts and comprised the highest proportion of patients who received high-dose ICS at baseline (Table S2). Conversely, patients with low post-bronchodilator FEV1 reversibility and a post-bronchodilator FEV1/FVC ratio of 0.7 or more were younger, had a higher baseline pre-bronchodilator FEV1 level, a lower number of exacerbations in the year before study entry, and lower T2 biomarker levels than the other combined subgroups (Table S2).
Compared with placebo, improvements from baseline to week 52 in pre-bronchodilator FEV1 and pre-bronchodilator FEF25–75 with tezepelumab were similar across all subgroups, with the exception of slightly reduced improvements for both measures in patients with low post-bronchodilator FEV1 reversibility and a post-bronchodilator FEV1/FVC ratio of 0.7 or more (Tables 2 and S3).
Additional Asthma Controller Medications Subgroups
When grouped by additional asthma controller medication use, 492, 381, and 185 patients were receiving 1, 2, or ≥ 3 additional asthma controller medications, respectively. LABA was the most commonly received additional controller (n = 480), followed by LABA + LTRA (n = 224) (Table S6). Treatment with tezepelumab resulted in improvements to pre-bronchodilator FEV1 compared with placebo irrespective of the number or type of additional asthma controller medications that patients were receiving (Table S6). Improvements were also observed in patients who were not receiving maintenance OCS grouped by the type of additional asthma controller medications (Table S6).
Discussion
In this analysis of the phase 3 NAVIGATOR study in patients with severe, uncontrolled asthma, treatment with tezepelumab compared with placebo resulted in improvements in all lung function parameters evaluated, including small-airway improvements, as evidenced by changes in pre-bronchodilator FEV1, FEF25–75, FVC, and FEV1/FVC ratio. These improvements were observed as early as the first post-baseline measurement time point and were sustained through to week 52.
Tezepelumab treatment resulted in improvements in both pre- and post-bronchodilator FEV1, with an incremental pulmonary volume improvement similar to that observed for FVC and FEF25–75. The consistent pulmonary volume improvement across these measures suggests that these improvements could be due to reduced airway inflammation, as well as a reduction in airway mucus plugging and air trapping [5, 23, 24]. As with airway inflammation, a reduction of airway mucus plugging would be expected to increase pulmonary vital capacity and drive similar improvements across all of the measured lung function parameters.
Compared with placebo, improvements in lung function with tezepelumab were greater among patients with a short disease duration (< 20 years) than among those with a long disease duration (≥ 20 years). Additionally, among patients with abnormal lung function at baseline, a greater proportion of patients with a short disease duration achieved normal lung function (pre-bronchodilator ppFEV1 ≥ 80%) with tezepelumab treatment than those with a long disease duration. This finding highlights the importance of early treatment for improving lung function during the course of the disease. In addition, a large improvement in lung function was demonstrated among patients who received placebo with high baseline post-bronchodilator FEV1 reversibility (≥ 20%), which could be due to optimization of or increased adherence to standard-of-care treatment before study enrollment. In patients with low baseline post-bronchodilator FEV1 reversibility, improvements in lung function were minimal among patients who received placebo, which may indicate structural obstruction within this subgroup. Nevertheless, tezepelumab treatment resulted in early and sustained improvements in lung function compared with placebo in patients with high and low baseline post-bronchodilator FEV1 reversibility. A greater improvement in lung function with tezepelumab compared with placebo was also seen in patients with a post-bronchodilator FEV1/FVC ratio of less than 0.7 compared with those with an FEV1/FVC ratio of 0.7 or more. Among patients grouped by combined post-bronchodilator FEV1 reversibility and post-bronchodilator FEV1/FVC ratio, improvements with tezepelumab compared with placebo were similar across all subgroups. However, there were slightly reduced improvements compared with placebo in lung function in patients with low post-bronchodilator FEV1 reversibility and a post-bronchodilator FEV1/FVC ratio of 0.7 or more. This may be due to the patients’ pre-bronchodilator FEV1, post-bronchodilator FEV1 reversibility, and post-bronchodilator FEV1/FVC ratio being near normal at baseline. Among patients grouped by the number of additional controller medications, patients receiving three or more additional controllers or LABA + LAMA + LTRA had the greatest improvements in lung function following tezepelumab treatment compared with placebo. This may be because these patients had more severe asthma than those receiving one or two additional controllers.
Current biologic treatments for severe asthma include anti-immunoglobulin E (omalizumab), anti-interleukin (IL)-5 (mepolizumab, reslizumab), anti-IL-5 receptor α (benralizumab), and anti-IL-4 receptor α (dupilumab) monoclonal antibodies [25–27]. An indirect treatment comparison found these treatments to have similar efficacies for some patients with severe, uncontrolled asthma [28]; however, improvements across lung function parameters were inconsistent [25–27]. Certain studies have shown a small improvement in lung function in patients treated with omalizumab [29], although others have not. In the DREAM trial, no significant change in FEV1 was demonstrated with mepolizumab [30]. However, improvements in FEV1, FVC, and FEF25–75 were observed in patients treated with benralizumab [31]. In the aforementioned studies, a higher baseline eosinophil count was associated with an enhanced response to treatment. This and other clinical indicators could provide valuable insights to establish which treatment would give the most beneficial response.
This study demonstrates the importance of assessing the effects of treatment on multiple lung function parameters, and the subsequent effects on disease severity and lung function decline. In future studies, methods beyond spirometry could be used to provide additional insights. For example, computerized tomography scans and functional magnetic resonance imaging could be used to assess mucus plugs and their contribution to airway obstruction [32]; the lung clearance index could be used to assess severe airway obstruction, small-airway disease, and bronchoreversibility [33, 34]; and the forced oscillation technique could be employed to examine bronchodilator response and asthma control [35].
Limitations of this analysis included the requirement for patients with documented historical or on-site reversibility, meaning that the findings may not be generalizable to all patients with severe asthma. Additionally, whereas spirometry can capture phenomena occurring in the central airways, benefits in the small airways, improvement in air trapping, or changes in lung volume would require additional methods of measurement, such as plethysmography or oscillometry. Finally, this analysis was not prospectively powered to evaluate significant differences between all the subgroups of patients.
Conclusion
Tezepelumab treatment was associated with rapid and sustained improvements in lung function compared with placebo in patients with severe, uncontrolled asthma. In patients who received tezepelumab, greater improvements in lung function compared with placebo were observed in patients with a short disease duration, high post-bronchodilator FEV1 reversibility (≥ 20%), or a post-bronchodilator FEV1/FVC ratio of less than 0.7. Improvements in pre-bronchodilator FEV1 were accompanied by consistent improvements in FVC and FEF25–75, which may be due to reduced mucus plugging. Overall, these data further demonstrate the efficacy of tezepelumab in improving lung function in patients with severe, uncontrolled asthma.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing and Editorial Assistance
Medical writing support was provided by Eleni Tente, PhD, of PharmaGenesis London, London, UK, with funding from AstraZeneca and Amgen Inc.
Author Contributions
AM-G takes responsibility for the content of the manuscript, including the data and analysis. AstraZeneca are responsible for initiation of the study and the decision to publish. They reviewed drafts during development of the manuscript. All named authors contributed to the study design or data interpretation and drafting of the manuscript with support from a medical writer funded by AstraZeneca and Amgen Inc. All authors provided critical feedback and final approval for submission.
Funding
This study and the journal’s Rapid Service and Open Access Fees were funded by AstraZeneca (Cambridge, UK) and Amgen Inc. (Thousand Oaks, California, USA).
Data Availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Declarations
Conflict of Interest
Andrew Menzies-Gow has a new and additional affiliation of Respiratory and Immunology, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK; is an employee of AstraZeneca and has attended advisory board meetings for AstraZeneca, GSK, Novartis, Regeneron, Sanofi, and Teva Pharmaceuticals; has received speaker fees from AstraZeneca, Novartis, Sanofi, and Teva Pharmaceuticals; has participated in research with AstraZeneca, for which his institution has been remunerated; has attended international conferences with Teva Pharmaceuticals; and has consultancy agreements with AstraZeneca and Sanofi. Christopher S. Ambrose, Gene Colice, Gillian Hunter, and Bill Cook are employees of AstraZeneca and may own stock or stock options in AstraZeneca. Nestor A. Molfino and Jean-Pierre Llanos are employees of Amgen and own stock in Amgen. Elliot Israel has served as a consultant to and received personal fees from 4D Pharma, AB Science, Amgen, AstraZeneca, Avillion, Biometry, Cowen, Equillium, Genentech, GSK, Merck, Novartis, Pneuma Respiratory, PPS Health, Regeneron Pharmaceuticals, Sanofi, Sienna Biopharmaceuticals, and Teva Pharmaceuticals; has received nonfinancial support from Circassia, Teva Pharmaceuticals, and Vorso Corp; and has received clinical research grants from AstraZeneca, Avillion, Genentech, Gossamer Bio, Novartis, and Sanofi.
Ethical Approval
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines and applicable regulatory requirements. Approvals from the Copernicus Central Institutional Review Board (Cary, NC, USA) and local independent ethics committees were obtained, and all patients or their guardians provided written informed consent in accordance with local requirements.
Footnotes
Prior Presentation. This manuscript is based on work that was previously presented at the 2021 American College of Allergy, Asthma and Immunology Annual Meeting, November 4– 8, New Orleans, LA, USA; and at the 2021 American College of Chest Physicians Annual Meeting, October 17–20, 2021 [virtual presentation].
References
- 1.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30(3):452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 2.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116(3):477–486. doi: 10.1016/j.jaci.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Witt CA, Sheshadri A, Carlstrom L, Tarsi J, Kozlowski J, Wilson B, et al. Longitudinal changes in airway remodeling and air trapping in severe asthma. Acad Radiol. 2014;21(8):986–993. doi: 10.1016/j.acra.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang M, Elicker BM, Henry T, Gierada DS, Schiebler ML, Huang BK, et al. Mucus plugs persist in asthma, and changes in mucus plugs associate with changes in airflow over time. Am J Respir Crit Care Med. 2022;205(9):1036–1045. doi: 10.1164/rccm.202110-2265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128(3):997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown PJ, Greville HW, Finucane KE. Asthma and irreversible airflow obstruction. Thorax. 1984;39(2):131–136. doi: 10.1136/thx.39.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little SA, MacLeod KJ, Chalmers GW, Love JG, McSharry C, Thomson NC. Association of forced expiratory volume with disease duration and sputum neutrophils in chronic asthma. Am J Med. 2002;112(6):446–452. doi: 10.1016/S0002-9343(02)01047-1. [DOI] [PubMed] [Google Scholar]
- 8.Cassino C, Berger KI, Goldring RM, Norman RG, Kammerman S, Ciotoli C, et al. Duration of asthma and physiologic outcomes in elderly nonsmokers. Am J Respir Crit Care Med. 2000;162(4):1423–1428. doi: 10.1164/ajrccm.162.4.9912140. [DOI] [PubMed] [Google Scholar]
- 9.Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58(4):322–327. doi: 10.1136/thorax.58.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sears MR. Lung function decline in asthma. Eur Respir J. 2007;30(3):411–413. doi: 10.1183/09031936.00080007. [DOI] [PubMed] [Google Scholar]
- 11.Eschenbacher WL. Defining airflow obstruction. Chron Obstruct Pulmonary Dis. 2016;3(2):515. doi: 10.15326/jcopdf.3.2.2015.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae EJ, Kim T-B, Cho YS, Park C-S, Seo JB, Kim N, et al. Airway measurement for airway remodeling defined by post-bronchodilator FEV1/FVC in asthma: investigation using inspiration-expiration computed tomography. Allergy Asthma Immunol Res. 2011;3(2):111–117. doi: 10.4168/aair.2011.3.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sposato B, Scalese M, Ricci A, Rogliani P, Paggiaro P, et al. Persistence of both reversible airway obstruction and higher blood eosinophils may predict lung function decline in severe asthma. Clinl Respir J. 2021;15(2):237–243. doi: 10.1111/crj.13325. [DOI] [PubMed] [Google Scholar]
- 14.Malerba M, Radaeli A, Olivini A, Damiani G, Ragnoli B, Sorbello V, et al. Association of FEF25–75% impairment with bronchial hyperresponsiveness and airway inflammation in subjects with asthma-like symptoms. Respiration. 2016;91(3):206–214. doi: 10.1159/000443797. [DOI] [PubMed] [Google Scholar]
- 15.Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi: 10.1080/14728222.2020.1783242. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Wang W, Lv Z, Li Y, Chen Y, Huang K, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol. 2018;200(7):2253–2262. doi: 10.4049/jimmunol.1701455. [DOI] [PubMed] [Google Scholar]
- 17.Gauvreau GM, O'Byrne PM, Boulet L-P, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 18.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Tepper RS, Wise RS, Covar R, Irvin CG, Kercsmar CM, Kraft M, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3 Suppl):S65–87. doi: 10.1016/j.jaci.2011.12.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santanello N, Zhang J, Seidenberg B, Reiss T, Barber B. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999;14(1):23–27. doi: 10.1034/j.1399-3003.1999.14a06.x. [DOI] [PubMed] [Google Scholar]
- 22.Busse WW, Holgate ST, Wenzel SW, Klekotka P, Chon Y, Feng J, et al. Biomarker profiles in asthma with high vs low airway reversibility and poor disease control. Chest. 2015;148(6):1489–1496. doi: 10.1378/chest.14-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura K, Shirai T, Hirai K, Nakayasu H, Takahashi S, Kishimoto Y, et al. Mucus plugs and small airway dysfunction in asthma, COPD, and asthma-COPD overlap. Allergy Asthma Immunol Res. 2022;14(2):196. doi: 10.4168/aair.2022.14.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diver S, Khalfaoui L, Emson C, Wenzel SE, Menzies-Gow A, Wechsler ME, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312. doi: 10.1016/S2213-2600(21)00226-5. [DOI] [PubMed] [Google Scholar]
- 25.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:10834. doi: 10.1002/14651858.CD010834.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:3559. doi: 10.1002/14651858.CD003559.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zayed Y, Kheiri B, Banifadel M, Hicks M, Aburahma A, Hamid K, et al. Dupilumab safety and efficacy in uncontrolled asthma: a systematic review and meta-analysis of randomized clinical trials. J Asthma. 2019;56(10):1110–1119. doi: 10.1080/02770903.2018.1520865. [DOI] [PubMed] [Google Scholar]
- 28.Menzies-Gow A, Steenkamp J, Singh S, Erhardt W, Rowell J, Rane P, et al. Tezepelumab compared with other biologics for the treatment of severe asthma: a systematic review and indirect treatment comparison. J Med Econ. 2022;25(1):679–690. doi: 10.1080/13696998.2022.2074195. [DOI] [PubMed] [Google Scholar]
- 29.Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 30.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 31.Mathur SK, Modena BD, Coumou H, Barker P, Kreindler JL, Zangrilli JG. Postbronchodilator lung function improvements with benralizumab for patients with severe asthma. Allergy. 2020;75(6):1507. doi: 10.1111/all.14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svenningsen S, Haider E, Boylan C, Mukherjee M, Eddy RL, Capaldi DP, et al. CT and functional MRI to evaluate airway mucus in severe asthma. Chest. 2019;155(6):1178–1189. doi: 10.1016/j.chest.2019.02.403. [DOI] [PubMed] [Google Scholar]
- 33.Lubrano L, Volpi S, Andreatta E, Borruso A, Spinelli E, Vassanelli C, et al. Lung clearance index in asthmatic patients. Eur Respir Soc. 2016;2:2. [Google Scholar]
- 34.Cherrez-Ojeda I, Robles-Velasco K, Osorio MF, Calderon J, Bernstein JA. Current needs assessment for using lung clearance index for asthma in clinical practice. Curr Allergy Asthma Rep. 2022;22(2):13–20. doi: 10.1007/s11882-022-01025-2. [DOI] [PubMed] [Google Scholar]
- 35.Cottee AM, Seccombe LM, Thamrin C, King GG, Peters MJ, Farah CS. Bronchodilator response assessed by the forced oscillation technique identifies poor asthma control with greater sensitivity than spirometry. Chest. 2020;157(6):1435–1441. doi: 10.1016/j.chest.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.