Abstract
Background:
Cardiac transplants increasingly occur following placement of ventricular assist devices (VADs). A strong association exists between human leukocyte antigen (HLA) sensitization and VAD placement; however, desensitization protocols that utilize therapeutic plasma exchange (TPE) are fraught with technical challenges and are at increased risk of adverse events. In response to increased VAD utilization in our pre-transplant population, we developed a new institutional standard for TPE in the operating room.
Methods:
Through a multidisciplinary effort, we developed an institutional protocol for intraoperative TPE immediately prior to cardiac transplantation after cannulation onto cardiopulmonary bypass (CPB). All procedures used the standard TPE protocol on the Terumo Optia® (Terumo BCT, Lakewood, CO, USA), but incorporated multiple modifications to limit patients’ bypass times, and to coordinate with the surgical teams. These modifications included deliberate misidentification of replacement fluid and maximization of the citrate infusion rate.
Results:
These adjustments allowed the machine to run at maximal inlet speeds, minimizing duration of TPE. To date, eleven patients have been treated with this protocol. All survived their cardiac transplantation operation. Hypocalcemia and hypotension were noted; however, none of these adverse events appeared to have clinical impact. Technical complications included unexpected fibrin deposition in the TPE circuit and air in the inlet line due to surgical manipulation of the CPB cannula. No thromboembolic complications occurred in any patient.
Conclusion:
We feel that this procedure can be rapidly and safely performed in HLA sensitized pediatric patients on CPB to limit the risk of antibody mediated rejection of their heart transplant.
Keywords: Therapeutic plasma exchange (TPE), cardiopulmonary bypass (CPB), heart transplant, allosensitization, ventricular assist device (VAD), donor specific crossmatch, antibody mediated rejection (AMR)
INTRODUCTION
The number of children currently awaiting heart transplantation is 438 nationally (as of April 6, 2022) and rising.1 One of the most well-studied risk factors contributing to worse post-operative outcomes after orthotopic heart transplant (OHT) in children, especially with respect to early post-transplant mortality, is allosensitization.2 Allosensitization is generally defined as a panel reactive antibody (PRA) value of >10%.3 Prior studies have shown that the risks of mortality and graft failure increase significantly when PRA exceeds 20%.2 The suspected physiological mechanism for allosensitization as a risk factor for post-transplant mortality is an increased likelihood of circulating donor-specific, cytotoxic anti-human leukocyte antigen (HLA) I and anti-HLA II antibodies in recipient serum, which can bind to HLA molecules expressed on donor vascular endothelial cells. Formation of antigen-antibody complexes in the graft’s vascular endothelium leads to inflammation, platelet recruitment, microthrombosis, interstitial hemorrhage, and, in some cases, cardiac allograft vasculopathy. 3
Ventricular-assist devices (VADs) are being increasingly used as a bridge to OHT in children with heart failure.4–5 It has been well established in the adult literature that VAD use is a risk factor for allosensitization.6–8 This has been attributed to the inflammatory response to foreign mechanical elements, increased blood product exposure, and the risk of infection associated with VAD implantation. The same trend is now being observed in children as the pediatric VAD experience evolves.9–11
One retrospective study demonstrated an increased incidence of acute rejection in allosensitized children with VAD support compared to sensitized peers who were not exposed to VADs.11 High PRA, then, may not only occur with more frequency in the VAD population, but may be of more clinical significance to those children requiring mechanical circulatory support. In addition, children are more likely than adults to utilize a pulsatile-flow VAD, which, when compared to continuous-flow VADs, portend a higher likelihood of allosensitization.12
In response to the increase in VAD use in our pre-transplant population, we sought to establish an institutional protocol for intra-operative therapeutic plasma exchange (TPE) to occur in tandem with cardiopulmonary bypass (CPB), with the goal of desensitizing children with high PRA or a positive predicted crossmatch immediately preceding OHT. The goal was to perform the procedure only after the recipient had been placed on CPB, as this was when the patient was felt to be the most stable and able to tolerate the volume shifts associated with TPE. Additionally, due to the risks associated with prolonged bypass time, minimizing the duration of TPE was prioritized. This demanded a concerted, multidisciplinary effort from stakeholders across several disciplines including therapeutic apheresis (managed by transfusion medicine), transplant cardiology, cardiothoracic surgery, perfusionists, and anesthesiology to ensure patient safety and to proactively anticipate potential interventions to avoid adverse events.
Here, we describe the creation and successful implementation of a TPE protocol to manage allosensitization in the operating room immediately preceding OHT in children in conjunction with CPB.
MATERIALS AND METHODS
Study design
This single-center retrospective case series was approved by the Institutional Review Board at Children’s National Hospital. A waiver of informed consent and parental permission was obtained.
Identification of key stakeholders
In order to maximize patient safety and efficiency, input was obtained from members of several key stakeholder groups. Cardiologists and transplant coordinators were included so that the apheresis team could be notified of transplant opportunities in a timely fashion. Cardiac surgeons needed to be aware of the plan to incorporate TPE into the CPB plan prior to cannulation. Perfusionists and the apheresis team worked together to place the TPE and CPB circuits in parallel. Cardiac anesthesiologists needed to be aware of the increased risks of hypotension, hypocalcemia, and hypothermia adopted by adding TPE to the CPB circuit. Roles and responsibilities were discussed. Adverse events were anticipated, tolerable limits were discussed, and appropriate pre-emptive interventions were agreed upon.
Patient selection
Starting in 2017, patients were included if they had at least one of the following risk factors: allosensitization at the time of their most recent PRA (defined as >10% PRA), a predicted positive crossmatch, or personal history of VAD use. Due to the relatively rare incidence of heart transplantation and the absence of equivalently sensitized patients, no control cohort was established. Procedure times were compared to TPE performed post-operatively for treatment of antibody-mediated rejection.
Modifications to TPE settings
Our goals when designing our protocol were to maximize exchange efficiency and minimize total procedure time without increasing adverse events. The ideal protocol would therefore utilize the highest inlet flow rate tolerable during apheresis. The Optia® (Terumo BCT, Lakewood, CO, USA) system is a centrifugal apheresis platform and is the standard device for TPE at our institution. The Optia® software is pre-programmed with recommended safety limits for citrate infusion rates to avoid toxicity. For our desensitization protocol to run as fast as possible, we anticipated the need to bypass several pre-programmed limitations of the Optia®.
In all procedures, 1.5 – 3 plasma volumes inclusive of the CPB circuit volume were processed. As the team gained experience, the number of plasma volumes exchanged decreased with subsequent patients which maximized efficiency. In order to achieve the highest flow rates possible and minimize procedure duration, “albumin/saline” was deliberately selected as the replacement fluid, even though plasma was exclusively used (with the exception of patient 11, in which 500mL of 5% albumin was used initially due to paucity of ABO compatible plasma due to shortages related to COVID-19). The anticoagulation (AC) infusion rate was maximized at 2.5mL/min in all cases. The inlet rate was maximized to 142mL/min as the AC infusion rate allowed. Initially, to facilitate order placement, the CPB circuit volume estimate was standardized for all patients at 185mL. Improved collaboration across multidisciplinary teams over time allowed for more specific volume estimates of the CPB circuit, which allowed for more precise exchange planning. Due to the presence of heparin in the CPB circuit, inlet:AC ratio was maintained at 50:1 in all cases except in the event of fibrin accumulation in the TPE circuit. Activated clotting time (ACT) was monitored frequently in all cases to ensure adequate anticoagulation. Heparin sulfate was bolused as needed to maintain ACT >900 seconds. A separate blood warmer for the Optia® was not necessary due to the configuration with the CPB circuit which includes an oxygenator and warmer. A summary of the modifications described above is provided in Table 1.
Table 1:
Summary of modifications made to the standard therapeutic plasma exchange procedure for use in parallel with cardiopulmonary bypass.
| TPE in Parallel with CPB | Standard TPE Procedure | |
|---|---|---|
| Priming solution for tubing set | 100% saline | ACD-A and saline |
| Target processed blood volumes | 1 – 1.5 blood volumes, or as ordered by physician | 1 – 1.5 blood volumes |
| Replacement fluid | 100% FFP | FFP or albumin as determined by indication |
| Replacement fluid input | Select Albumin/Saline | Select appropriate fluid |
| Anticoagulation infusion rate | 2.5mL/min/L | Maximum rate 1.2mL/min/L |
| Inlet | 142 mL/min | As calculated by machine |
| Inlet AC ratio | 50:1 | 10:1 |
| TBV | Increase as per physician order to including CPB circuit | As calculated by machine |
| Inlet and return connections |
Inlet connects to venous (draw) line. Return connects to cardiotomy. |
Sites determined by central access or vein assessment |
| Calcium replacement | 3x the standard dose, as calcium chloride or calcium gluconate at the anesthesiologist’s discretion | Calcium gluconate 20mg/kg/hour |
| Calcium goals | iCal goal range 0.8–1.3mmol/L | iCal goal range 1.1–1.3mmol/L |
| Additional materials | Extra accessory waste bag available due to high volumes removed | - |
| Additional personnel | Secondary operator/provider present to assist with rapid exchange rate and documentation | - |
Abbreviations: TPE: therapeutic plasma exchange, CPB: cardio-pulmonary bypass, TBV: total blood volume, ACD-A: anticoagulant citrate dextrose solution, AC: anticoagulation, FFP: fresh frozen plasma, mL: milliliter, mg: milligram, kg: kilogram, min: minute, L: liter, mmol: millimole, hr; hour.
Extra-TPE modifications
The TPE circuit was connected to the CPB circuit in parallel. The inlet line was connected directly to the venous (draw) line of the CPB circuit and the return line was connected to the cardiotomy (Figure 1a and 1b). An additional waste bag was added to the standard TPE setup due to the large volumes of waste plasma. For the same reason, a secondary apheresis team member was required to be present in the OR to hang replacement fluids during the exchange. Finally, patients were empirically treated with a continuous infusion of calcium gluconate or calcium chloride, with additional boluses of calcium given as needed, by anesthesia at the anesthesiologists’ discretion. Calcium was titrated to maintain ionized calcium measurement greater than 0.8 mmol/L and to mitigate hypotension.
Figure 1A:
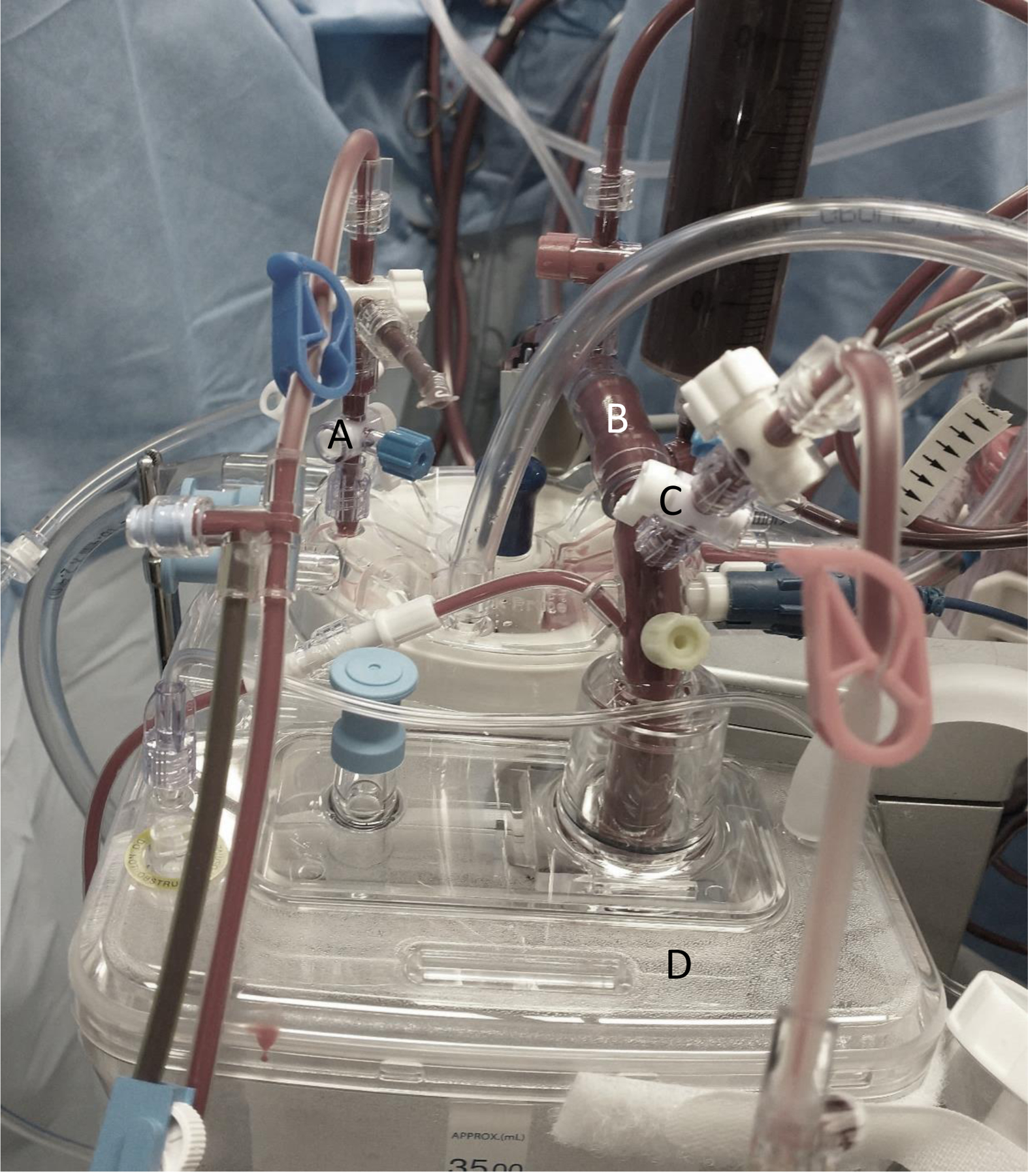
Connection of therapeutic plasma exchange (TPE) inlet/return lines to cardiopulmonary bypass (CPB). A. Apheresis return line connected to cardiotomy; B. CPB venous (draw) line; C. Apheresis inlet line connected to venous line; D. Cardiotomy.
Figure 1B:
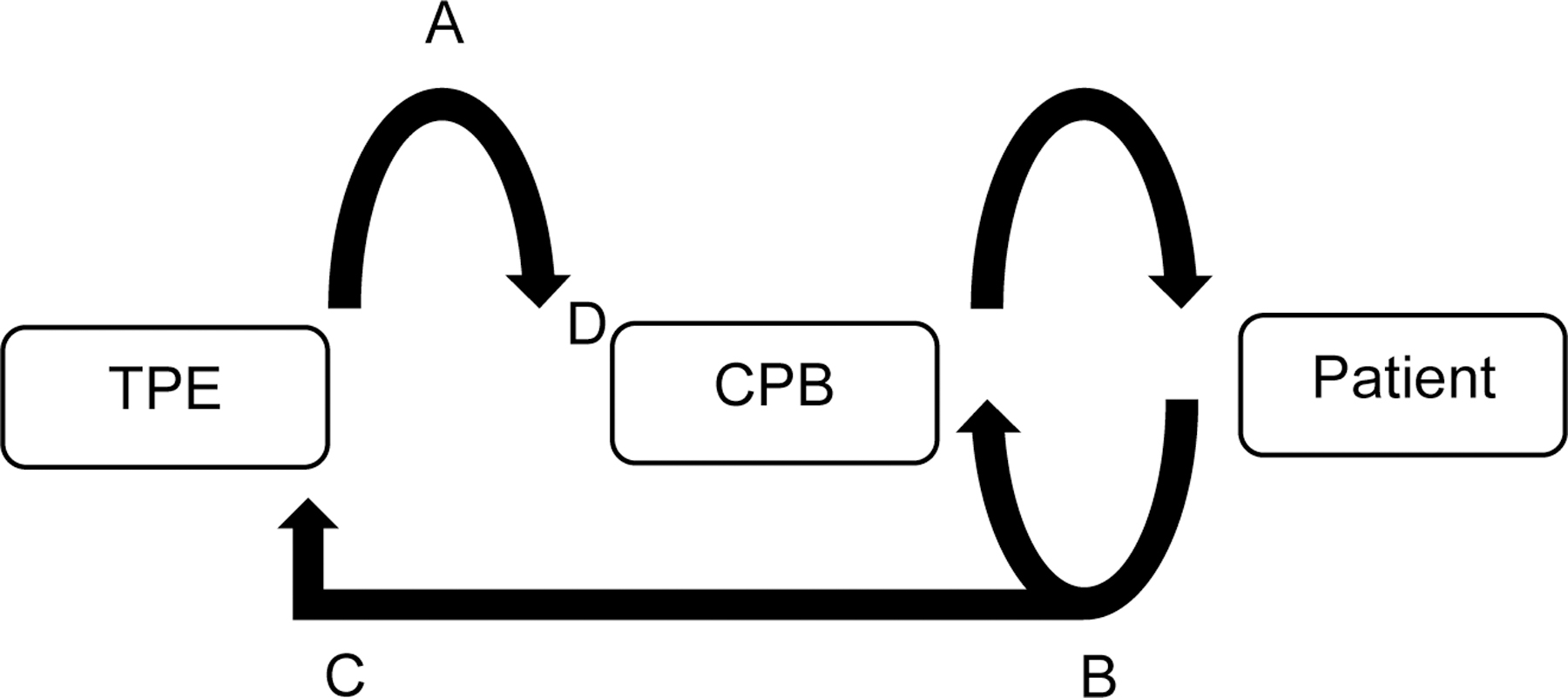
Schematic representation of the connection of therapeutic plasma exchange (TPE) inlet/return lines to cardiopulmonary bypass (CPB). A. Apheresis return line connected to cardiotomy; B. CPB venous (draw) line; C. Apheresis inlet line connected to venous line; D. Cardiotomy.
RESULTS
Patient characteristics
To date, eleven children have been treated with this protocol at our institution. Of these, seven (64%) had PRA evidence of allosensitization with PRA >10%. Two (18%) had a B-cell incompatible but T-cell compatible crossmatch, both without evidence of donor specific antibodies (DSA) at the time of transplant. One (9%) had a T-cell and B-cell incompatible crossmatch along with weakly positive DSA. Three (27%) had evidence of DSA at the time of transplant. Three patients had no positive PRA, DSA, or crossmatch, but did have a history of transfusion while on VAD support which placed them at risk of developing antibodies. Given potential risk of HLA sensitization in this population and the urgent nature of cardiac transplant, TPE was performed empirically when a recent PRA was not available.
The most common cause of heart failure in our cohort was dilated cardiomyopathy (6/11 = 54%), followed by hypoplastic left heart syndrome (2/11= 18%). Nine patients (82%) were supported with a VAD (8 LVADs, 1 BiVAD). Of these, four were bridged to VAD from extracorporeal membranous oxygenation (ECMO). The duration of mechanical circulatory support (MCS) ranged from 16 days to 10 months. Patient age ranged from four months to 15 years. A slight majority (64%) of the patients were female.
Patient outcomes
All eleven children treated with this protocol survived their cardiac transplant operation (Table 2). Three (27%) patients died after transplant, all within 2 months of their operation. Of these, one mortality was associated with antibody mediated rejection (AMR), which occurred at one month post-transplant. The two remaining cases died of causes unrelated to graft failure or rejection (pseudomonal sepsis and renal failure, respectively). Of the eight surviving cases to date, one (12%) developed stable chronic rejection and one (12%) developed positive DSA which resolved with treatment including additional TPE procedures. The remainder are free of DSA.
Table 2:
Patient characteristics and outcomes
| PATIENT 1 | PATIENT 2 | PATIENT 3 | PATIENT 4 | PATIENT 5 | PATIENT 6 | PATIENT 7 | PATIENT 8 | PATIENT 9 | PATIENT 10 | PATIENT 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | 11yo female 51kg |
14yo male 54kg |
10yo male 41kg |
4mo female 5kg |
4yo female 15kg |
8yo male 18.8kg |
6yo male 26kg |
12yo female 86.4kg |
15yo female 55kg |
7yo female 29kg |
12 yo female 57.8kg |
| Pre-Transplant Diagnosis | Dilated Cardiomyopathy | Ischemic Cardiomyopathy | Restrictive Cardiomyopathy | Dilated cardiomyopathy | Myocarditis s/p OHT, graft failure | HLHS (MA/AA) | Dilated cardiomyopathy | Dilated cardiomyopathy | HLHS (MA/AA) | Dilated cardiomyopathy | Dilated cardiomyopathy |
| Pre-Transplant Course | ECMO x 10 days, Heartware LVAD x 6 days | ECMO x 10 days, Heartware LVAD x 6 months | Heartware LVAD x 6 months | Berlin Heart LVAD x 5 weeks | ECMO x 25 days, Berlin Heart BiVAD x 3 months | Fontan | Heartware LVAD x 5 months | ECMO x 4 days, Heartware LVAD x 4 months | Fontan | HeartMate LVAD x 5 weeks | Heartware LVAD x 10 months |
| Pre-TPE PRA | 0% | 27% | 97% | 0% | 72% | 68% | 24% | 65% | 0% | 11% | 0% |
| Duration of Intraoperative TPE (min) | 136 | 80 | 45 | 50 | 21 | 40 | 40 | 59 | 49 | 47 | 62 |
| Plasma Volumes Processed | 2.8 | 1.5 | 1.2 | 1.7 | 1.4 | 2.2 | 1.3 | 1.4 | 1.1 | 1.6 | 1.5 |
| Transplant Crossmatch | B cell and T cell compatible, no DSAs | T cell compatible, B cell incompatible, no DSAs | B cell and T cell incompatible, weakly positive DSA (A23, B49) | T cell compatible, B cell compatible, no DSAs | T cell compatible, B cell incompatible, no DSAs | T cell compatible, B cell compatible, weakly positive DSA (A31, DR4) | T cell incompatible, B cell compatible, no DSAs | T cell compatible, B cell compatible, no DSAs | T cell compatible, B cell compatible, weakly positive DSA (A2) | T cell compatible, B cell compatible, no DSAs | T cell compatible, B cell compatible, no DSAs |
| Additional TPE Performed | None | 6 | 4 | None | 5 | 8 | None | None | 3 | None | None |
| Dates of Additional TPE | POD#1 – POD#6 | POD#1 – POD#5 | POD#1 – POD#5 | POD#19 – POD#22, POD#36-POD#38 | POD#13, 14, 16 | ||||||
| Indication for Additional TPE | Weakly positive DSA (Bw6) on POD#4 | Positive crossmatch | 72% PRA | Antibody mediated rejection | Positive DSA (A2) | ||||||
| Latest DSA | Negative 2 months post-transplant | Negative 37 months post-transplant | DQ8 (1:64) 39 months post-transplant | Negative 37 months post-transplant | Negative 5 days post-transplant | A31 (1:16), DR4 (1:64) 5 weeks post-transplant | Negative 12 months post-transplant | Negative 11 months post-transplant | Negative 7 months post-transplant | Negative 2 months post-transplant | Negative 1 month post-transplant |
| Outcome | Deceased 2 months post-transplant due to pseudomonal sepsis | Doing well 4 years post-transplant | Stable chronic rejection 3 years post-transplant | Doing well 37 months post-transplant | Deceased 17 days post-transplant due to renal failure | Deceased 7 weeks post-transplant due to AMR | Doing well 14 months post-transplant | Doing well 1 year post-transplant | Doing well 10 months post-transplant | Doing well 4 months post-transplant | Doing well 1 month post-transplant |
Abbreviations: ECMO: extracorporeal membranous oxygenation, LVAD: left ventricular assist device, BiVAD: biventricular assist device, HLHS: hypoplastic left heart syndrome, MA/AA: mitral atresia/aortic atresia, TPE: therapeutic plasma exchange, PRA: panel reactive antibodies, DSA: donor specific antigen, AMR: antibody mediated rejection
In the first two performances of the modified protocol, the procedure lasted >70 minutes. The remaining sessions ranged from 21 – 62 minutes in length. The average duration of the exchange was 57 minutes. The median duration was 49 minutes. The average plasma volumes processed per patient was 1.6. Notably, the expected duration of the procedure when performed without the modifications described is 120 – 180 minutes.
Patient-centered complications
Hypocalcemia was the most commonly encountered procedural complication, occurring in 8/11 (73%) of patients. Ionized calcium levels were obtained prior to the initiation of TPE and approximately every 5 minutes throughout the duration of the TPE. The lowest ionized calcium level observed was “undetectable”, the remainder of the values ranged from 0.23 to 0.6 mmol/L (institutional normal range: 1.12 – 1.37 mmol/L). Of note, the CPB protocol used at our institution is intentionally hypocalcemic, so lower levels of ionized calcium were tolerated. No adverse events were attributed to hypocalcemia. Intra-procedural hypotension was recorded as a complication in 2/11 (18%) procedures. Hypotension was defined as per the American Heart Association for the Pediatric Advanced Life Support guidelines.13 Hypotension was treated with increasing vasoactive support in these cases and was well tolerated. In all cases, ideal mean arterial pressure was maintained with vasoactive support in conjunction with CPB.
Technical complications
In 2 patients (18%), a small amount of air was introduced into the TPE circuit during manipulation of the CPB cannula, leading to pressure alarms. This was handled with a brief pause in centrifugation to allow air to escape into the reservoir. No air reached the patients. In two cases, fibrin was noted in the TPE reservoir, requiring the addition of anticoagulant citrate dextrose-A (ACDA) to the local TPE circuit through adjustment of inlet:AC to 20:1. No thromboembolic events occurred, and no fibrin deposition was noted in the CPB circuits.
DISCUSSION
The impact of allosensitization on overall patient survival and graft survival in pediatric OHT is a subject of active research, with some studies describing an insignificant impact and others noting increased mortality associated with high PRA 14,2. This discrepancy may exist because the PRA is a nonspecific screening tool reflective of an entire population, and therefore its value cannot be used to estimate any one child’s ability to tolerate an individual graft. Data associated with positive donor-specific crossmatches (DSC) are more compelling, as children with both a high PRA and positive DSC are at an increased risk of post-transplant mortality compared to those with either a high PRA or positive DSC alone.2 Regardless of actual mortality-driven outcomes, children with higher PRAs are more likely to receive augmented immunosuppression therapy, which may increase their risk of immunosuppression-related morbidity.
It should be noted that PRA values can fluctuate during the pre-transplant course. The most recent PRA at the time of transplant is of the most clinical significance, as opposed to the peak PRA.3 Efforts to reduce allosensitization, then, will be most efficient when implemented as close as possible to the time of transplant. Several desensitization regimens prior to transplant have been described, including TPE, intravenous immunoglobulin (IVIG), anti-B cell therapies (rituximab, cyclophosphamide), and the proteasome inhibitor bortezomib.12,15 However, the timing of OHT can never be precisely predicted, as a potential donor may become available at any time, which makes the ideal timing of these interventions a clinical challenge.
TPE performed in the operating room at the time of OHT has been described as early as 1999 for desensitization in adults and is used with the goal of reducing the incidence of hyperacute rejection in patients with a high PRA 15–16. The benefits of intra-operative TPE are the ideal timing with respect to transplant due to the immediacy of effect and short duration of procedure. Potential complications of TPE include hypotension and hypocalcemia during the procedure, and increased risk of thrombosis. For patients awaiting OHT who are bridged with a VAD, TPE outside of the OR can be technically challenging as VADs rely on adequate preload to prevent dangerous suction events. Fluid shifts due to TPE can cause acute drops in VAD filling and cardiac output, as well as life-threatening arrhythmias. Thus, the advantage of conducting TPE in the operating room is that patients have already been stabilized on CPB, which can augment cardiac output and reduce adverse events associated with hypotension.
We created and implemented an institutional protocol for desensitization with TPE in allosensitized children for use in the operating room immediately preceding OHT. Through the concerted effort of multidisciplinary stakeholders, this protocol was designed to maximize efficiency, minimize run time, and be hemodynamically tolerated across a wide range of patient characteristics, including VAD patients. We found that TPE performed in this manner did not significantly extend CPB time beyond clinically tolerable limits and did not precipitate adverse events. We conclude that this protocol can be safely performed in that select group of patients placed at high risk for allosensitization due to personal history of VAD use, positive PRA, or positive DSC. Despite hemodynamic shifts due to rapid TPE, all of our patients tolerated the procedure well. We attribute this success to both the simultaneous use of CPB for hemodynamic support and the deliberate preparation of the multidisciplinary team. Notably, this protocol was also well tolerated across pediatric age groups in patients ranging from infancy to adolescence.
Institutional experience
As our team became more familiar with the protocol, run times tended to become shorter. In all eleven patients the procedure was completed without the need to abort due to complications. Again, we attribute this to a combination of CPB efficacy and the anticipatory training of the multidisciplinary teams involved. By anticipating the need for an additional apheresis team member to hang replacement fluid, an additional waste bag, and modified AC parameters in the setting of CPB, we were able to avoid unnecessary pauses in TPE. Multidisciplinary agreement on greater tolerance of hypocalcemia in the setting of CPB was also helpful.
Despite a high rate of sensitization in our patient population (64%), there was a low rate of AMR observed post-transplant (18%). One patient succumbed to acute AMR at one month post-transplant, representing the only mortality associated with graft failure in our cohort. One patient is currently tolerating stable, chronic, antibody mediated rejection as an outpatient at three years post-transplant. Though there is paucity of evidence to suggest a strong linear relationship between sensitization and AMR in pediatric OHT recipients, there is sufficient equipoise to support desensitization of patients with high-PRAs, especially those with a history of VAD support. This is especially reasonable when the potential benefits outweigh the clinical risks. Here, we have demonstrated that modified rapid TPE with CPB support can be used safely and in optimal temporal proximity to OHT without increasing bypass time or perioperative adverse events.
We found that while the Optia® could achieve the increased flow rates desired, this was only achievable by deliberately manipulating settings on the instrument. These modifications necessarily increase the technical complexity of the procedure and required dedicated training and multidisciplinary support. The success of this protocol relied heavily on the collaborative engagement of the apheresis, perfusion, anesthesiology, surgery, cardiology, and transplant coordination teams.
CONCLUSIONS
The standard TPE protocol can be modified to safely achieve rapid plasma exchange in children with risk factors for allosensitization prior to OHT when used in conjunction with CPB in the operating room. Expected hypocalcemia and hypotension events during TPE were well tolerated in the setting of CPB. The success of these modifications relies on the engagement and commitment of a multidisciplinary care team, as well as institutional support for increased training and staffing. Our protocol represents a new institutional standard of care for a subset of patients receiving a heart transplant following mechanical circulatory support or allosensitization.
Practitioner Points.
A cohort of children awaiting heart transplant with personal use of ventricular assist device (VAD) may be at high risk of HLA allosensitization.
Therapeutic plasma exchange (TPE), which is technically challenging in patients with VADs, can be performed safely once a patient has transitioned to a cardiopulmonary bypass (CPB) circuit in the operating room.
In order to minimize total bypass time when TPE is performed in the operating room, rapid TPE can be safely achieved and well tolerated with certain modifications described herein.
ACKNOWLEDGEMENTS
The authors acknowledge Nina Deutsch, MD and Mark Nuszkowski, MPS, CCP, FPP for their significant clinical contributions to this work.
Funding Statement:
Salary support for ED was provided by a Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant awarded to the Children’s Research Institute Hematology Training Program by the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (5T32HL110841-08).
Abbreviations:
- TPE
therapeutic plasma exchange
- OHT
orthotopic heart transplant
- HLA
human leukocyte antigen
- PRA
panel reactive antibodies
- DSA
donor-specific antibodies
- CPB
cardiopulmonary bypass
- ACT
activated clotting time
- ACDA
anticoagulant citrate dextrose-A
- CAV
cardiac allograft vasculopathy
- VAD
ventricular assist device
- LVAD
left ventricular assist device
- BiVAD
biventricular assist device
- ECMO
extracorporeal membranous oxygenation
- MCS
mechanical circulatory support
- HLHS
hypoplastic left heart syndrome
Footnotes
Conflict of Interest:
None of the authors have a conflict of interest to disclose.
Ethics Approval Statement:
This single-center case series was approved by the Institutional Review Board at Children’s National Hospital. A waiver of informed consent and parental permission was obtained. The work contained herein and each of the authors are in accordance with Wiley’s Best Practice Guidelines on Research Integrity and Publishing Ethics.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES:
- 1.Organ Procurement and Transplantation Network (OPTN). US Department of Health and Human Services Website https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Updated March 3, 2022. Accessed March 3, 2022.
- 2.Mahle WT, Tresler MA, Edens E et al. Allosensitization and outcomes in pediatric heart transplantation. J Heart Lung Transplant 2011;30:1221–7. doi: 10.1016/j.healun.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 3.Al-Mohaissen MA, Virani SA. Allosensitization in heart transplantation: an overview. Can J Cardiol 2014;30:161–172. doi: 10.1016/j.cjca.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Puri K, Anders MM, Tume SC, et al. Characteristics and outcomes of pediatric patients supported with ventricular assist device—a multi-institutional analysis. Pediatr Crit Care Med 2019;20(8):744–752. doi: 10.1097/PCC.0000000000001966. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal DN, Almond CS, Jaquiss RD, et al. Adverse events in children implanted with ventricular assist devices in the us: data from the pediatric interagency registry for mechanical circulatory support (Pedimacs). J Heart Lung Transplant 2016; 35(5): 569–577. doi: 10.1016/j.healun.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massad MG, Cook DJ, Schmitt SK, et al. Factors influencing HLA sensitization in implantable LVAD recipients. Ann Thorac Surg 1997;64(4):1120–5. doi: 10.1016/s0003-4975(97)00807-2. [DOI] [PubMed] [Google Scholar]
- 7.Ko BS, Drakos S, Kfoury AG, et al. Immunologic effects of continuous-flow left ventricular assist devices before and after heart transplant. J Heart Lung Transplant 2016;35:1024–1030. doi: 10.1016/j.healun.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Schuster M, Kocher A, John R, et al. B-cell activation and allosensitization after left ventricular assist device implantation is due to T-cell activation and CD40 ligand expression. Hum Immunol 2002;63(3):211–220. doi: 10.1016/s0198-8859(01)00380-9. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor MJ, Menteer J, Chrisant MR, et al. Ventricular assist device-associated anti-human leukocyte antigen antibody sensitization in pediatric patients bridged to heart transplantation. J Heart Lung Transplant 2010;29(1):109–116. doi: 10.1016/j.healun.2009.08.028 [DOI] [PubMed] [Google Scholar]
- 10.Grutter G, Amodeo A, Brancaccio G, Parisi F. Panel reactive antibody monitoring in pediatric patients undergoing ventricle assist device as a bridge to heart transplantation. Artif Organs 2013;37(5):435–438. doi: 10.1111/aor.12016 [DOI] [PubMed] [Google Scholar]
- 11.Magdo HS, Schumacher KR, Yu S, Gajarski RJ, Friedland-Little JM. Clinical significance of anti-HLA antibodies associated with ventricular assist device use in pediatric patients: A United Network for Organ Sharing database analysis. Pediatr Transplant 2017;21(5): 10.1111/petr.12938. doi: 10.1111/petr.12938. [DOI] [PubMed] [Google Scholar]
- 12.Asante-Korang A, Jacobs JP, Ringewald J, et al. Management of children undergoing cardiac transplantation with high Panel Reactive Antibodies. Cardiol Young 2011;21 Suppl 2:124–132. doi: 10.1017/S1047951111001703. [DOI] [PubMed] [Google Scholar]
- 13.American Heart Association. ECC guidelines part 10: Pediatric advanced life support. Circulation 2000;102 Suppl 1: I-291–I-342. doi: 10.1161/circ.102.suppl_1.I-291 [DOI] [PubMed] [Google Scholar]
- 14.Casarez TW, Perens G, Williams RJ, et al. Humoral rejection in pediatric orthotopic heart transplantation. J Heart Lung Transplant 2007;26(2):114–119. doi: 10.1016/j.healun.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Lick SD, Beckles DL, Piovesana G, et al. Transplantation of high panel-reactive antibody left ventricular assist device patients without crossmatch using on-bypass pheresis and alemtuzumab. Ann Thorac Surg 2011;92(4):1428–1434. doi: 10.1016/j.athoracsur.2011.04.064 [DOI] [PubMed] [Google Scholar]
- 16.Larson DF, Elkund DK, Arabia F, Copeland JG. Plasmapheresis during cardiopulmonary bypass: a proposed treatment for presensitized cardiac transplantation patients. J Extra Corpor Technol 1999;31(4):177–183. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


