Abstract
Acute pulmonary embolism (PE) can present with non-specific signs and symptoms and thus the diagnosis and management might not be as straightforward. This review outlines the new PE management guidelines in the Indian context. The exact prevalence in the Indian population is not well defined; despite recent studies suggesting an increasing trend in the Asian population. A delay in treatment can be fatal, especially in massive PE. The nuances associated with stratification and management have led to heterogeneity in acute PE management. The purpose of the review is.
1
To highlight the principles of stratification, diagnosis and management of acute PE with a special attention towards the Indian population.
2
To aid patient selection for newer catheter based therapies.
To conclude, formulation of pulmonary embolism guidelines in the Indian setting is required underlying the role of further research in this area.
Keywords: Pulmonary, Embolism, Thrombolysis, Anticoagulation
1. Introduction
Venous thromboembolism (VTE), with its spectrum of deep venous thrombosis (DVT) and PE, represents a common health problem in patients worldwide. It accounts for a significant economic burden.1 As per the American Heart Association, about 1,220,000 cases of VTE occur in the USA annually.2 Initially, it was thought that the rates of VTE are lower in the Asian population compared to the western population. The annual incidence of VTE in India is not well defined. A retrospective study reviewing data from 2007 to 2018 showed 812 patients with a diagnosis of VTE.3 Population based studies are needed to assess the incidence of VTE in India.
2. Management
The management approach in acute PE involves risk stratification, diagnosis and treatment. Numerous tools have been developed and used in clinical practice to classify patients into high, intermediate and low risk for PE. Computed tomography pulmonary angiography (CTPA) is the most commonly used test to confirm or exclude the diagnosis whereas treatment options range from anticoagulation, thrombolysis (intravenous or catheter based) and catheter based thrombus aspiration. Fig. 1 shows an overview of PE stratification, diagnosis and management.
Fig. 1.
Overview of pulmonary embolism stratification, diagnosis and management.,9 CTPA: Computed tomography pulmonary angiography, V/Q: Ventilation perfusion scan, US: Ultrasound, PESI: Pulmonary embolism severity index, sPESI: Simplified pulmonary embolism severity index, IVC: Inferior vena cava, VTE: Venous thromboembolism, CTEPH: chronic thromboembolism pulmonary hypertension.
3. Triage for imaging/diagnosis
The diagnostic workup begins with an assessment of the patient's hemodynamic status. In hemodynamically stable patients with suspected acute PE, the assessment starts with evaluating clinical or pretest probability. This can be done by clinical assessment or using validated scores such as Wells score, Modified wells score and Modified Geneva score.4 These assessment scores integrate the patient's baseline characteristics with the patient's history, thus allowing classification into three categories: high, intermediate, and low. Clinical estimation and validated scores have an almost similar sensitivity when used in conjunction with D-dimer testing.
While the approach is straightforward in patients with high clinical probability going for computed tomography pulmonary angiography (CTPA), those with low and intermediate probability require additional considerations. D-dimer levels and pulmonary embolism rule-out criteria (PERC) are commonly used in these patients. The PERC criteria are particularly useful in low risk PE patients, albeit it has a low specificity.5 No further testing is indicated in low risk patients meeting PERC criteria, whereas testing with high sensitive D-dimer is done when PERC criteria are not met.
Although a D-dimer <500 ng/mL safely excludes PE in these patients, the specificity of D-dimer is low and hence if levels are >500 ng/mL, a CTPA is usually required to confirm the diagnosis. Additionally, the specificity of D-dimer decreases with age >50 years, and PE being much more common in the elderly population, age-adjusted D-dimers are increasingly being used. A recent meta-analysis showed that both conventional and age adjusted D-dimers have high sensitivity. But, compared to age adjusted D-dimer, conventional D-dimer use is associated with higher false positive results.6 The formula to calculate age-adjusted D-dimer is Age (if over 50 years) x 10 = cutoff value in ng/mL (fibrinogen equivalent units).
The preferred imaging modality for diagnosis is CTPA. In patients with contraindications to CTPA, such as a history of a contrast allergy, increased risk of contrast induced nephropathy (glomerular filtration rate <30 mL/min/1.73 m2), or inability to tolerate CT scanning due to severe obesity or inability to lie flat), a ventilation/perfusion (V/Q) scan is performed. CTPA is >90% sensitive and specific for diagnosing PE, especially in low and intermediate-risk groups. In the future, artificial intelligence (AI) might be used to assess radiology images. A recent study comparing AI image reading vs. radiologist's image reading showed that sensitivity and negative predictive values were greater with AI. In contrast, specificity and positive predictive values were greater with radiologists.7 A lower extremity ultrasound doppler can be considered when neither a CTPA nor a V/Q scan can be performed. A new onset DVT in patients with symptoms of PE is highly suggestive but not diagnostic of PE. Doppler can be used as an initial test to evaluate suspected PE, and anticoagulation can be initiated if the results are positive (Fig. 2). But given its low sensitivity, it can not definitively rule out PE.8
Fig. 2.
Use of Doppler ultrasound to evaluate suspected acute PE.8
Magnetic resonance pulmonary angiography (MRPA) is an alternative in patients who can neither undergo a CTPA nor a V/Q scan. Compared to CTPA, MRPA is less sensitive for diagnosing acute PE and is not widely available. A diagnosis of acute PE can rarely be made on 2-D Echocardiography if the thrombus is visualized in the pulmonary arteries. Presence of a clot in the right heart or a new right heart strain on echocardiography aid in the diagnosis as well. These findings, especially in unstable patients, may be helpful if a preemptive emergency diagnosis is needed for the use of thrombolytic therapy.9 However, in hemodynamically stable patients, echocardiography is generally insensitive and nonspecific and mainly used for prognosis purposes. Newer modalities such as single photon emission computed tomography (SPECT), dual-energy CT, and multiorgan ultrasound are being investigated to diagnose PE accurately.
4. Stratification
Clinically, acute PE is classified based on hemodynamic status and estimated early death risk. Identifying patients with high-risk, intermediate-risk (intermediate high and intermediate low), and low-risk is essential as the approach differs. It is well known that the presence of right ventricular (RV) dysfunction and RV failure from PE-induced pressure overload is associated with adverse patient outcomes.10 High-risk or massive PE is defined as the presence of shock from florid RV failure or hypotension, i.e. systolic blood pressure (SBP) < 90 mm Hg, drop in SBP> 40 mm Hg from baseline for 15 min, or hypotension requiring vasopressor support. In these patients, mortality risk is exceptionally high in the first 2 h and remains increased for up to 72 h after the presentation. Therefore, urgent treatment with circulatory and respiratory support is paramount. Catastrophic PE is defined as PE with progressive shock despite on multiple pressors/inotropes, impending or active cardiac arrest or persistent shock despite thrombolysis.11
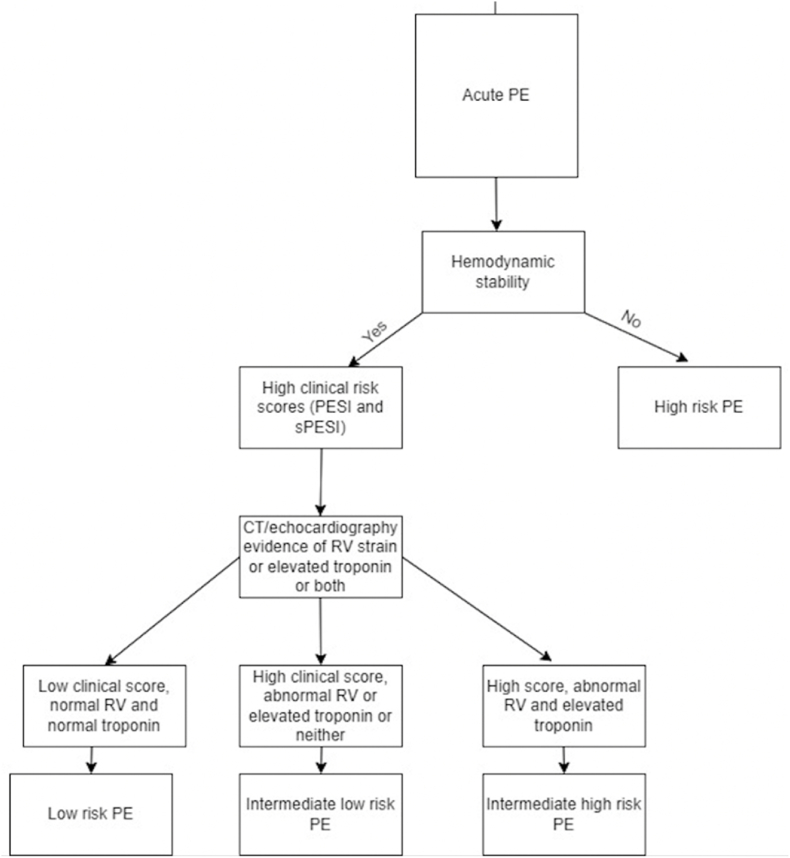
Fig. 3 shows an approach to risk stratification. Fig. 4 shows an approach to management. Low-risk PE is defined as hemodynamically stable PE with no RV strain. Within this group, the challenge remains to identify patients who can be discharged early and treated as outpatient versus those who need close in-hospital monitoring. Validated risk stratification tools such as Pulmonary Embolism Severity Index (PESI) and simplified pulmonary embolism severity index (sPESI) have been developed.12,13 These are prognostic bedside scoring systems to estimate the risk of adverse outcomes and aid in decision-making. Both PESI and sPESI scores predict mortality in patients with PE. PESI risk strata I-II have a lower 30-day mortality; while sPESI score have showed high negative predictive value in ruling out early adverse events after discharge.13Adding biomarkers such as high-sensitivity troponin to sPESI score further increases the negative predictive value and can help in the early discharge of these patients. One recent study showed that addition of echocardiography parameters to PESI score further improves the mortality prediction in patients with acute PE.14
Fig. 3.
Approach to risk stratification.11
Fig. 4.
Approach to management.11
Sub-massive/intermediate risk PE is defined as PE that does not meet high risk criteria but is associated with RV strain. Given that use of imaging and lab tests has shown to have prognostic importance, the 2019 European Society of Cardiology guidelines recommend the use of clinical prognostic indicators, cardiac biomarkers and evidence of RV dysfunction either on Echocardiography or CT to systematically stratify intermediate-risk PE.11,12 Based on these guidelines, intermediate-risk PE is sub-classified into high and low based on imaging and biomarkers (Table 1).
Table 1.
Acute PE risk stratification.11
| Risk Category | Hemodynamic status | PESI or sPESI | RV dysfunction |
|---|---|---|---|
| High | Unstable | High | Abnormal RV on imaging, elevated troponin or both |
| Intermediate high | Stable | High | Abnormal RV on imaging and elevated troponin |
| Intermediate low | Stable | High | May have abnormal RV on imaging or elevated troponin but not both |
| Low | Stable | Low | None |
5. Management
The initial approach to management depends on the patient's hemodynamic status. In hemodynamically unstable patients, the goal is to restore perfusion with intravenous fluids and vasopressors, maintain oxygenation above 90%, and airway protection if needed. Careful assessment of volume status is essential before starting intravenous fluids as some studies have shown excessive fluids cause RV stretch and worsening failure whereas a small volume of fluids might help in increasing the cardiac index. Therefore, a trial of intravenous fluid volume is likely to be successful in patients without signs of increased right-sided preload. Norepinephrine is the preferred pressor, whereas dobutamine, dopamine, and epinephrine are the alternatives. In unstable patients with high suspicion of PE who can not be transferred for CTPA, bedside echocardiography to assess RV overload can be performed. Regardless of whether the patient needs advanced therapies, if there are no contraindications to anticoagulation, it should be started promptly. If there are no contraindications for thrombolysis, anticoagulation is held, the patient is thrombolysed, and then anticoagulation is resumed. Anticoagulation is discussed below. Due to heterogeneity in consensus guidelines, the 2019 European Society of Cardiology guidelines encourage the Pulmonary Embolism Response Team (PERT) concept in the management of PE, mainly intermediate and high-risk PE which has shown promise in initial studies.15 Fig. 5, Fig. 6 show management approach to high risk PE and intermediate risk PE respectively.
Fig. 5.
Management of high risk PE, CBT = catheter based thrombectomy, MCS = mechanical circulatory support, RHC = right heart catheterisation.
Fig. 6.
Management of intermediate high risk PE, CBT = catheter based thrombectomy, MCS = mechanical circulatory support + indicates success and - indicates failure.
Anticoagulation is the mainstay of treatment in hemodynamically stable patients. Empiric anticoagulation depends on the bleeding risk and clinical risk of PE. Thrombolysis is not preferred in these patients. Anticoagulation can be initiated in patients with low bleeding risk and high suspicion (Wells score >6) or those with moderate suspicion (Wells score 2–6) and in whom diagnostic evaluation can take >4 h. In patients with absolute contraindications to anticoagulation, diagnostic therapies should be expedited so that if PE is confirmed, other therapies, such as inferior vena cava (IVC) filter, can be initiated. For patients with intermediate high-risk PE, the PEITHO trial compared Tenecteplase plus heparin with heparin and placebo. In the former group, all-cause death and hemodynamic death within seven days occurred less frequently, whereas there was a higher chance of hemorrhage.16 Thus in this subgroup of patients, parenteral heparin with close monitoring is preferred with rescue fibrinolysis considered if signs of hemodynamic collapse appear.12
5.1. Systemic thrombolysis
It is primarily indicated in patients with massive PE to reverse hemodynamic compromise and in select patients with intermediate high-risk PE to prevent hemodynamic collapse. In these patients, fibrinolysis is life-saving.12 Commonly used drugs include streptokinase, urokinase, Tenecteplase and alteplase. Table 2 shows the doses used in acute PE. The PEITHO trial was conducted in intermediate-risk PE patients and compared the efficacy and safety of Tenecteplase plus heparin with heparin alone. Although fibrinolysis reduced all-cause mortality (2.6% vs 5.6%, p 0.015), it was associated with intracranial hemorrhage (6.3% vs 1.5%, p < 0.001).16 Given the risks of life-threatening bleeding with thrombolysis, the concept of reduced-dose thrombolysis and catheter directed aspiration were designed.
Table 2.
Thrombolytic doses.
| Thrombolytic | Dose |
|---|---|
| Streptokinase | Loading dose: 250,000 IU infused over 30 min Maintenance dose: 100,000 IU/hour for 24 h |
| Urokinase | Loading dose: 4400 units/kg over 10 min Maintenance dose: 4400 units/kg/hour for 12 h |
| Tenecteplase | Systemic: 30–50 mg single bolus over 5–10 s |
| Alteplase | Systemic: 100 mg infused over 2 h Catheter directed: 0.5–2 mg/h for 2–15 h |
Low dose urokinase thrombolysis was studied in a retrospective study of 81 intermediate risk PE patients. The in-hospital mortality, 9-month mortality, and long-term mortality at the last follow-up were comparable for the low-dose urokinase group and the anticoagulant therapy group (6.45% vs. 0%, p 0.144, 9.68% vs. 8.16%, p 0.815, and 12.90% vs. 12.24%, p 0.931, respectively). There were no differences with regards to major bleeding and the incidence of minor bleeding was not significantly different between the two groups (3.23% vs. 6%, p 0.974).17 A recent study on sub-massive pulmonary embolism compared safety and efficacy of half dose thrombolytics versus LMWH only. The half dose thrombolytic group had lower deaths/hemodynamic decompensation in the first 7 days (p 0.028) and at 30 days (p 0.009). There was no significant difference in the bleeding complications.18 Table 3 indicates the contraindications for thrombolysis.
Table 3.
Contraindications for thrombolysis.
| ABSOLUTE | RELATIVE |
|---|---|
| Ischemic stroke in last 6 months | Transient ischemic attack in the last 6 months |
| Central nervous system neoplasm | Pregnancy or first postpartum week |
| History of hemorrhagic stroke | Oral anticoagulation |
| Major trauma, surgery, head injury in last 3 weeks | Traumatic resuscitation |
| Bleeding diathesis/active bleeding | Use of ECMO |
| Advanced liver disease | |
| Infective endocarditis | |
| Active peptic ulcer | |
| Refractory hypertension (systolic BP > 180) |
ECMO indicates extracorporeal membrane oxygenation.
5.2. Catheter directed therapies (CDT)
CDT for PE were introduced in the 1990s, but have gained interest recently. These include catheter-directed thrombolysis, catheter based thrombectomy (CBT) with/without local thrombolysis and catheter directed clot fragmentation. Currently, CBT is indicated in high risk PE patients in whom thrombolysis is contraindicated or has failed.10 Table 4 shows the indications for CBT.
Table 4.
Indications for CBT.
| Intermediate high risk PE | High risk PE |
|---|---|
| Worsening hemodynamics despite being on anticoagulation (treatment failure) and contraindications for systemic thrombolysis. | Contraindications for systemic thrombolysis |
| As a rescue treatment in the case of failure of systemic thrombolysis. | As a rescue treatment in the case of failure of systemic thrombolysis. |
In patients with high risk PE, CBT should be considered if there is no improvement in 2–4 h after full dose thrombolysis. In intermediate risk PE, CBT is considered if there is no improvement 24–48 h after starting therapeutic anticoagulation.10
Increased thrombus resolution efficacy due to higher local drug concentrations combined with reduced hemorrhagic complications are the potential advantages of catheter directed thrombolysis. Although theoretically correct, randomized controlled trials are needed to establish the efficacy and safety of these modalities.19 A systematic review and meta-analysis of 65,589 patients compared safety and efficacy of catheter directed therapies (catheter directed thrombolysis and CBT) compared to systemic thrombolysis. Thirty-day mortality was lower in the intervention group, compared to the control group (7.3 vs. 13.6%; p < 0.001). Complications, including myocardial injury, stroke and cardiac arrest were also lower in the catheter directed therapy group (p < 0.001 for all). Rates of major bleeding including intracranial bleeding was found to be lower in the catheter directed therapy group (p < 0.01).20 Ultrasound-facilitated, catheter-directed techniques have also been extensively studied. In a recent RCT on intermediate-risk PE, this technique was reviewed in 46 patients. Fewer patients in the intervention group (ultrasound-facilitated, catheter-directed fibrinolysis with alteplase 0.5 mg plus heparin) experienced a 3-month composite of death or RV/LV ratio greater than 0.9 (indicating improved RV function), however the results were statistically non significant (p 0.48).21 The role of thrombolysis in intermediate high risk PE is unclear. Whereas guidelines recommend against systemic thrombolysis in hemodynamically stable patients, the studies on catheter directed thrombolysis have shown mixed results. A recent meta-analysis of 9789 intermediate high risk PE patients comparing catheter directed thrombolysis and anticoagulation showed significant lower in-hospital mortality (p < 0.00001), 30 day mortality (p 0.04) and 90 day mortality (p 0.004) in the former group. Additionally, the rates of major bleeding, minor bleeding and blood transfusion were similar in both groups.22
Isolated mechanical thrombectomy without thrombolysis is ideal for patients with contraindications to thrombolysis. In intermediate-risk acute PE, mechanical thrombectomy has been shown to improve RV function. The EXTRACT PE trial evaluated the safety and efficacy of the Indigo aspiration system (Penumbra device) without thrombolysis in patients with acute sub-massive PE. In a cohort of 119 patients, mean RV/LV reduction from baseline to 48 h post aspiration was 0.43 (p < 0.0001) whereas just 1.7% patients had major bleeding and 0.8% of the patients had catheter related death.23 In a prospective study on aspiration thrombectomy in intermediate risk PE patients, use of aspiration thrombectomy was associated with significant reduction in the pulmonary artery pressures (55; 44–66 mmHg vs. 42; 34–53 mmHg; p = 0.0015) and improvement in RV/LV ratio (p < 0.0001).24
However, there are no standardized guidelines on patient selection, procedural protocols and definitions for procedural success or failure of CDT. Therefore, further large scale studies are needed to establish the safety and efficacy of these modalities.
Surgical embolectomy is usually indicated in intermediate-high risk and high-risk acute PE in whom thrombolysis has failed or is contraindicated. It has also been indicated in a clot in transit, paradoxical embolism, and hemodynamic collapse requiring cardiopulmonary resuscitation. It is usually most effective in patients with large centrally located PE and in experienced centers has shown to be safe and effective. A retrospective study evaluated the outcomes of patients undergoing surgical embolectomy as a first line treatment for massive and sub-massive PE. Three out of twenty four massive PE patients died, whereas no deaths occurred in 17 sub-massive PE patients. Surgical embolectomy was associated with improved RV function. Pulmonary artery pressures reduced significantly 3 months post operatively (73.3 ± 25.9 mm Hg vs 32.9 ± 11.5, p < 0.05).25
Mechanical circulatory support (MCS) is indicated in patients with refractory shock and severe RV failure. Isolated RV assist devices (Impella RP, ProtekDuo) and ECMO are the available modalities. Whereas RV assist devices work best in patients with RV failure and reduction in thrombus burden with thrombolysis or aspiration thrombectomy, VA ECMO can be initiated in patients without clot reduction (Fig. 5).11 In the past, ECMO was used mainly as a bridging therapy for surgical embolectomy. However, recent data suggest that these patients might recover from anticoagulation alone.26 IVC filter is recommended in patients with acute PE who have contraindications to anticoagulation or recurrent PE despite anticoagulation.13 A recent meta-analysis on 1274 patients evaluated the effect of IVC filters on pulmonary embolism mortality and major complications. No significant difference was found in PE related mortality between the intervention and control groups at the end of 3 months (0.94% vs 1.10%; p 0.81). No significant difference was found in major bleeding rates in the IVC filter group and the control group (20.39% vs 20.29%; p 0.88).27
6. Indian perspective
In resource limited settings, use of CDT or MCS is not possible in all the cases. In these patients, anticoagulation is paramount. The use of sPESI and PESI scores can be increased to stratify patients eligible for discharge. In patients with shock, goal should be to maintain perfusion with intravenous fluids and vasopressors, maintenance of oxygen saturation >90% and mechanical ventilation for airway protection. After initial stabilization, transfer to a higher center should be considered on a case-by-case basis. In tertiary care centers, CDTs and/or MCS can be initiated based on hemodynamic status and initial therapy received (Fig. 5, Fig. 6). Additionally, given that CTPA is not emergently available at all centers, Indian doctors can be trained to do a cardiac point of care ultrasound to assess signs of acute PE such as RV dilation.
Funding
None.
Declaration of competing interest
None.
References
- 1.Setyawan J., Mu F., Yarur A., et al. Risk of thromboembolic events and associated risk factors, including treatments, in patients with immune-mediated diseases. Clin Therapeut. 2021 Aug;43(8):1392–1407.e1. doi: 10.1016/j.clinthera.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021 Feb 23;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 3.Basavanthappa R.P., Jp V.V., Gangadharan A.N., et al. RAVS study: one Indian single center analysis of patients with VTE. Ann Vasc Dis. 2019 Jun 25;12(2):205–209. doi: 10.3400/avd.oa.18-00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucassen W., Geersing G.J., Erkens P.M.G., et al. Clinical decision rules for excluding pulmonary embolism: a meta-analysis. Ann Intern Med. 2011 Oct 4;155(7):448–460. doi: 10.7326/0003-4819-155-7-201110040-00007. [DOI] [PubMed] [Google Scholar]
- 5.Kothare A., Abahussain M., Svirkov-Vainberg N., O'Kelly P., Nfila G., Gilligan P. A retrospective application of the pulmonary embolism rule out criteria (PERC) of the American College of Physicians would reduce the number of CTPAS by 6% without a false negative in an Irish hospital. Ir J Med Sci. 2021 Aug;190(3):1189–1193. doi: 10.1007/s11845-020-02398-x. [DOI] [PubMed] [Google Scholar]
- 6.Iwuji K., Almekdash H., Nugent K.M., et al. Age-adjusted D-dimer in the prediction of pulmonary embolism: systematic review and meta-analysis. J Prim Care Community Health. 2021;12 doi: 10.1177/21501327211054996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheikh A.B., Gorincour G., Nivet H., et al. How artificial intelligence improves radiological interpretation in suspected pulmonary embolism. Eur Radiol. 2022 Sep;32(9):5831–5842. doi: 10.1007/s00330-022-08645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falster C., Jacobsen N., Coman K.E., et al. Diagnostic accuracy of focused deep venous, lung, cardiac and multiorgan ultrasound in suspected pulmonary embolism: a systematic review and meta-analysis. Thorax. 2022 Jul;77(7):679–689. doi: 10.1136/thoraxjnl-2021-216838. [DOI] [PubMed] [Google Scholar]
- 9.Alerhand S., Sundaram T., Gottlieb M. What are the echocardiographic findings of acute right ventricular strain that suggest pulmonary embolism? Anaesth Crit Care Pain Med. 2021 Apr;40(2) doi: 10.1016/j.accpm.2021.100852. [DOI] [PubMed] [Google Scholar]
- 10.Pruszczyk P., Klok F.A., Kucher N., et al. Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC working group on pulmonary circulation and right ventricular function and the European association of percutaneous cardiovascular interventions. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2022 Oct 7;18(8):e623–e638. doi: 10.4244/EIJ-D-22-00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll B.J., Larnard E.A., Pinto D.S., Giri J., Secemsky E.A. Percutaneous management of high-risk pulmonary embolism. Circ Cardiovasc Interv. 2023 Feb;16(2) doi: 10.1161/CIRCINTERVENTIONS.122.012166. [DOI] [PubMed] [Google Scholar]
- 12.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020 Jan 21;41(4):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita Y., Morimoto T., Amano H., et al. Validation of simplified PESI score for identification of low-risk patients with pulmonary embolism: from the COMMAND VTE Registry. Eur Heart J Acute Cardiovasc Care. 2020 Jun;9(4):262–270. doi: 10.1177/2048872618799993. [DOI] [PubMed] [Google Scholar]
- 14.Burgos L.M., Scatularo C.E., Cigalini I.M., et al. The addition of echocardiographic parameters to PESI risk score improves mortality prediction in patients with acute pulmonary embolism: PESI-Echo score. Eur Heart J Acute Cardiovasc Care. 2021 May 11;10(3):250–257. doi: 10.1093/ehjacc/zuaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera-Lebron B.N., Rali P.M., Tapson V.F. The PERT concept: a step-by-step approach to managing pulmonary embolism. Chest. 2021 Jan;159(1):347–355. doi: 10.1016/j.chest.2020.07.065. [DOI] [PubMed] [Google Scholar]
- 16.Meyer G., Vicaut E., Danays T., et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014 Apr 10;370(15):1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 17.Weng C., Wang X., Huang L., Lin X., Liu Q. Low-dose urokinase thrombolytic therapy for patients with acute intermediate-high-risk pulmonary embolism: a retrospective cohort study. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yilmaz E.S., Uzun O. Low-dose thrombolysis for submassive pulmonary embolism. J Investig Med Off Publ Am Fed Clin Res. 2021 Dec;69(8):1439–1446. doi: 10.1136/jim-2021-001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giri J., Sista A.K., Weinberg I., et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American heart association. Circulation. 2019 Nov 12;140(20):e774–e801. doi: 10.1161/CIR.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 20.Pietrasik A., Gąsecka A., Szarpak Ł., et al. Catheter-based therapies decrease mortality in patients with intermediate and high-risk pulmonary embolism: evidence from meta-analysis of 65,589 patients. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.861307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadeghipour P., Jenab Y., Moosavi J., et al. Catheter-directed thrombolysis vs anticoagulation in patients with acute intermediate-high-risk pulmonary embolism: the CANARY randomized clinical trial. JAMA Cardiol. 2022 Dec 1;7(12):1189–1197. doi: 10.1001/jamacardio.2022.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismayl M., Machanahalli Balakrishna A., Aboeata A., et al. Meta-analysis comparing catheter-directed thrombolysis versus systemic anticoagulation alone for submassive pulmonary embolism. Am J Cardiol. 2022 Sep 1;178:154–162. doi: 10.1016/j.amjcard.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Sista A.K., Horowitz J.M., Tapson V.F., et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv. 2021 Feb 8;14(3):319–329. doi: 10.1016/j.jcin.2020.09.053. [DOI] [PubMed] [Google Scholar]
- 24.Araszkiewicz A., Sławek-Szmyt S., Jankiewicz S., et al. Continuous aspiration thrombectomy in high- and intermediate-high-risk pulmonary embolism in real-world clinical practice. J Intervent Cardiol. 2020 Aug 21 doi: 10.1155/2020/4191079. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.QiMin W., LiangWan C., DaoZhong C., et al. Clinical outcomes of acute pulmonary embolectomy as the first-line treatment for massive and submassive pulmonary embolism: a single-centre study in China. J Cardiothorac Surg. 2020 Oct 21;15(1):321. doi: 10.1186/s13019-020-01364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoreishi M., DiChiacchio L., Pasrija C., et al. Predictors of recovery in patients supported with venoarterial extracorporeal membrane oxygenation for acute massive pulmonary embolism. Ann Thorac Surg. 2020 Jul;110(1):70–75. doi: 10.1016/j.athoracsur.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Lu H., Bai H., Liu Q., Chen R. Effect of inferior vena cava filters on pulmonary embolism-related mortality and major complications: a systematic review and meta-analysis of randomized controlled trials. J Vasc Surg Venous Lymphat Disord. 2021 May;9(3):792–800.e2. doi: 10.1016/j.jvsv.2021.02.008. [DOI] [PubMed] [Google Scholar]