Abstract
Introduction/Case report
We describe the case of a 6-month-old female infant who received the equivalent of 6 adult doses of the COVID-19 Pfizer vaccine due to an immunization error. The patient underwent clinical and laboratory evaluations from the time of vaccination error (January 2022) until November 2022. In the first three days after immunization, she presented with low-grade fever (38 °C) and mild pain and induration at the injection site. She showed no other symptoms afterwards. Laboratory tests were within normal limits for age, except for an elevated D-dimer (3.71 ug/mL; normal: up to 0.5 ug/mL) and as the echocardiogram and electrocardiogram were within normal limits as well, no interventions were instituted at that moment. On the tenth day, immune response evaluation showed a strong expression of cytokines related to the Th2 profile and a well-controlled inflammatory state. Forty-three days after the vaccine administration inflammation status remained, with a predominance of cellular immune response, IFN-γ expression increased compared to the previous evaluation, and a robust antiviral state was in place. After 90 days, immune response evaluation showed a significant reduction in the inflammatory state, still with a predominance of the cellular immune response. Clinically, the patient remained well, with no other noteworthy intercurrences, until the last appointment in November 2022. This child has had no evidence of a severe adverse effect associated to the vaccine overdose.
Conclusion
The close follow-up of this case of vaccination error demonstrated that the COVID-19 Pfizer was safe and immunogenic in this individual, noting careful monitoring and followup of these vaccine administration errors is crucial.
Keywords: Covid 19 vaccines, Vaccines, Immune system, Immunization, Antibodies neutralizing
Introduction
Vaccination is the most important prevention strategy capable of causing a significant impact on the control or elimination of vaccine-preventable diseases [1]. Safety is a worldwide concern in immunization strategies and a determining factor in the success or failure of vaccination programs. Vaccination administration errors (VAEs) are adverse events caused by improper handling, prescription, or vaccine administration by health professionals [1]. In Brazil, from 2014 to 2016, among completely notified vaccine adverse reactions, 10 % of 1.622 cases were considered VAEs [2]. In the United States, from 2000 to 2013, VAEs represented 7 % of 311.185 cases [3].
Different factors can increase the risk of VAEs. Among them, the different types of vaccines, the number of doses administered, the introduction of new products, and the eventual changes in guidelines and standards are the most important factors [1], [4]. All these factors, in association with a large number of people demanding for vaccines, contribute to possible human mistakes during vaccine administration.
These VAEs probably occurred worldwide during the vaccination campaign against COVID-19, initiated in 2021, as several types of new vaccines were used, from different platforms, guidelines for use, dilutions according to the age of the vaccine, specific storage and handling. In Brazil, from January 2021 to June 2022, 179.469 adverse events related to the COVID-19 vaccination were reported and 45.285 (25.2 %) were considered VAEs [5].
The errors most commonly reported to surveillance services during this campaign were: a) administration of an incorrect dose; b) conservation of the vaccine at an inadequate temperature; c) inoculation by the subcutaneous route; d) administration in an age group other than the current recommendations; e) administration of subsequent doses with an interval shorter than the minimum recommended by the manufacturing laboratory [4]. It is necessary to describe and investigate the impact of these adverse events on human health to make sure these vaccines are safe and thus, to improve the population’s confidence in using these new vaccines [4], [6]. Here, we describe a case of an VAEs and evaluation of the patient’s clinical and immunological parameters following an accidental overdose of the COVID-19 Pfizer vaccine (adult type), administered to a 6-month-old infant.
Case report
In January 2022, a previously healthy six-month-old female infant was brought to a health clinic to receive the routine vaccines recommended for her age. A dose of injectable poliomyelitis vaccine (IPV) was administered in the vastus lateralis muscle of the right leg. Then, instead of injecting the pentavalent vaccine, the staff, inadvertently, diluted the COVID-19 Pfizer vaccine, intended for adult use, in 0.5 mL of sterile 0.9 % Sodium Chloride and administered it into the vastus lateralis muscle of the left leg, which was equivalent to six adult doses of the product (180 µg mRNA encoding the viral spike glycoprotein of SARS-CoV-2). Immediately after the vaccination, the nurse aide realized her mistake and communicated it to the family. The incident was also reported to the epidemiological surveillance system of the city of Altinopolis, in the state of São Paulo, Brazil. Shortly after the incident, a pediatrician evaluated the child and did not find any clinical abnormalities. After an observational period, the child was discharged with instructions to return to the health clinic if necessary. Fifteen hours later, she developed a low-grade fever (38 °C), hyperemia, a mild nodule and pain at the vaccine administration site. The following day, she returned for clinical evaluation, maintaining fever, pain in the left leg, hyperemia, warmth, and edema at the injection site. She was then referred to the Hospital das Clínicas of Ribeirão Preto (HCRP), for specialized medical assessment, given the unprecedented nature of the event in a young child and the necessity to perform a strict clinical observation. Upon admission, physical examination did not reveal any systemic or neurological abnormalities, only pain when moving the left leg, a mild increase in the leg circumference, and the presence of edema and hyperemia at the injection site. The local reactions were not significant and were consistent with other site reactions expected with any intramuscular viral vaccine. Postmarketing data of the Pfizer vaccine indicated adverse reactions such as thrombocytopenia, thrombotic events, increased risks of myocarditis, pericarditis, and muscle pain. Although she was clinically well, laboratory tests were ordered to rule out these inflammatory alterations. The laboratory results were within the normal range for her age (see Table 1), except for D-dimer (3.71 µg/mL; normal values: up to 0.5 µg/mL). Echocardiogram and electrocardiogram were normal.
Table 1.
Laboratory tests performed during the evaluation of the immunization error in an infant of 6 months old (HCRP, 2022).
| Day 1 | Day2 | Day4 | Day15 | Day71 | |
|---|---|---|---|---|---|
|
D-DIMER (ug/mL) |
1.81 | 3.71 | 0.53 | 0.24 |
0.4 |
|
NT-proBNP (pg/ml) |
419 | ||||
| INR | 0.87 | 1.09 | 1.25 | ||
|
CRP (mg/dL) |
53.9 (<2.8) |
6.39 (<1.0) |
1.54 (<1.0) |
||
| TROPONIN | Negative | ||||
|
CKMB (u/L) |
20 (<25,0) | ||||
| CBC | HB 11,3 HT 33 WBC: 9900 neut: 9,7%(1,9) lymph 70,5% (7,0) eos 3 % (0,3) PLT 491.000 |
HB 10.7 HT 32 WBC: 7800 neut:13 %(1,0) lymph:75 %(5,9) eos 5.5 % (0,4) PLT: 519.000 |
HB 10,2 HT 30,2 WBC: 9000 neut:23 %(2,0) lymph:70 %(6,3) eos 3 % (0,27) PLT: 463.000 |
HB 10,2 HT 30 WBC: 6100 neut:50 %(2,1) lymph:36 %(2,1) eos: 0 % PLT:224.000 |
|
|
LIVER ENZYMES (U/L) CREATININE (mg/dL) |
AST 72 0.19 |
AST 72 ALT 27 0.20 |
|||
| FIBRINOGEN (mg/dL) | 279 | 241 | |||
The patient experienced no clinical intercurrence during hospitalization. Due to progressive improvement in laboratory tests, resolution of fever after 3 days and decrease in local symptoms, she was discharged for outpatient follow-up. The infant maintained monthly appointments, always in good clinical condition, without inflammatory signs at the site of vaccine administration or any systemic symptoms. In March 2022, there was a report of daily fever for five days (37.8 °C to 38.5 °C) without other clinical findings, and the fever subsided with paracetamol. She was assessed by a local pediatrician who diagnosed a probable common cold, with complete resolution of the symptoms after a few days. She received pentavalent and MMR vaccines without incident. Since there were few studies reporting the characteristics of the immune response elicited by the Pfizer vaccine in infants, especially in the case of an elevated dosage, we conducted a complete cytokine and antibody evaluation to assess the inflammatory state and the long-term protection against COVID-19. The child's parents were deeply concerned about the elevated vaccine dosage and promptly agreed to a complete evaluation. Blood samples were collected after obtaining signatures of the informed consent.
Evaluation of cytokine gene expression after 10 days of immunization error
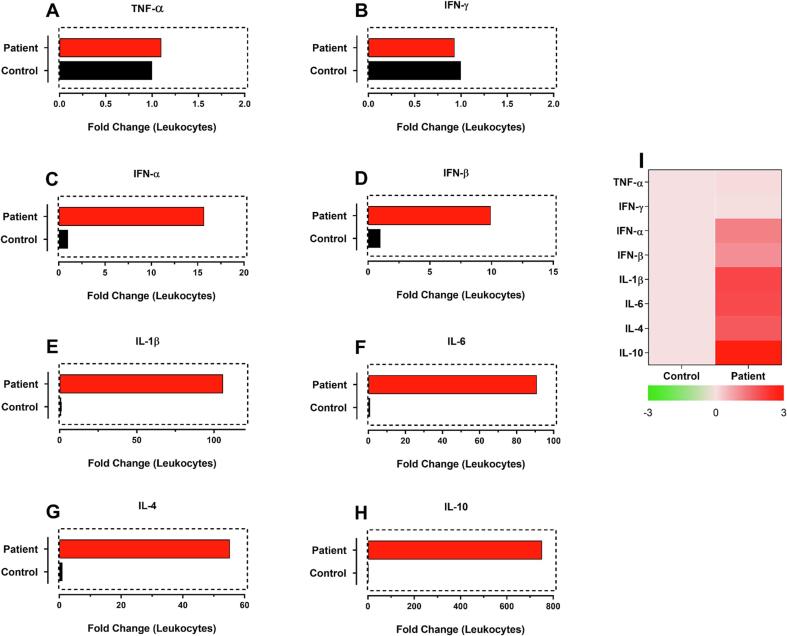
Ten days after IE (Immunization error) we evaluated the gene expression of genes related to cellular and humoral response in the patient's leukocytes, and the results are shown as fold change (Fig. 1). It was observed that there was not a significant expression of both TNF-α (Fig. 1A) and interferon (IFN)-γ genes (Fig. 1B), but there was a significant increase in type 1 IFNs (α and β; Fig. 1C and 1D), of 15 and 10-fold, respectively. We also observed an increase in the expression of pyrogenic cytokine IL-1β (100-fold) (Fig. 1E) and a strong expression of cytokines related to the Th2 profile of the immune response [IL-6 (Fig. 1F) and IL-4 (Fig. 1G)]. Finally, when compared with the other analyzed cytokines, the highest observed gene expression was that of the anti-inflammatory cytokine IL-10 (Fig. 1H; 751.55-fold). These results correlate with a strong induction of antibody production and a well-controlled inflammatory state even after receiving an excessive dose of the vaccine.
Fig. 1.
Cytokine gene expression data obtained after 10 days of immunization with Pfizer-BioNTech COVID-19 vaccine overdose. Gene expression is shown as fold change for TNF-α, IFN-γ, IFN-α, IFN-β, IL-1β, IL-6, IL-4 and IL-10. Analyzes were performed by relative gene expression using a control sample from an unvaccinated infant.
Evaluation of cytokine gene expression after 43 days of immunization error
After 43 days of vaccination (Fig. 2), we observed an increase in IFN-γ (Fig. 2B) expression. Interestingly, compared with the evaluation conducted 10 days after vaccination, gene expression of type 1 interferons (Fig. 2C-2D) was even higher than at that time, further increasing the antiviral status of the cells. Cytokine IL-1β expression level (Fig. 2E) was reduced but remained above control, and the IL-6 and IL-4 gene expression (Fig. 2F-2G) also decreased, as well as the anti-inflammatory cytokine IL-10 (Fig. 2H). These results show that the inflammation status was reduced as IFN-γ expression increased compared to the analysis carried out on day 10 after vaccination and that the patient's cells were found in an antiviral state greater than those observed earlier.
Fig. 2.
Analysis of gene expression of cytokines related to the immune response after 43 days of vaccination with an overdose of Pfizer-BioNtec COVID-19 vaccine. Gene expression is shown as fold change for TNF-α, IFN-γ, IFN-α, IFN-β, IL-1β, IL-6, IL-4 and IL-10. Analyzes were performed by relative gene expression using a control sample from an unvaccinated infant.
Evaluation of cytokine gene expression after 90 days of immunization error
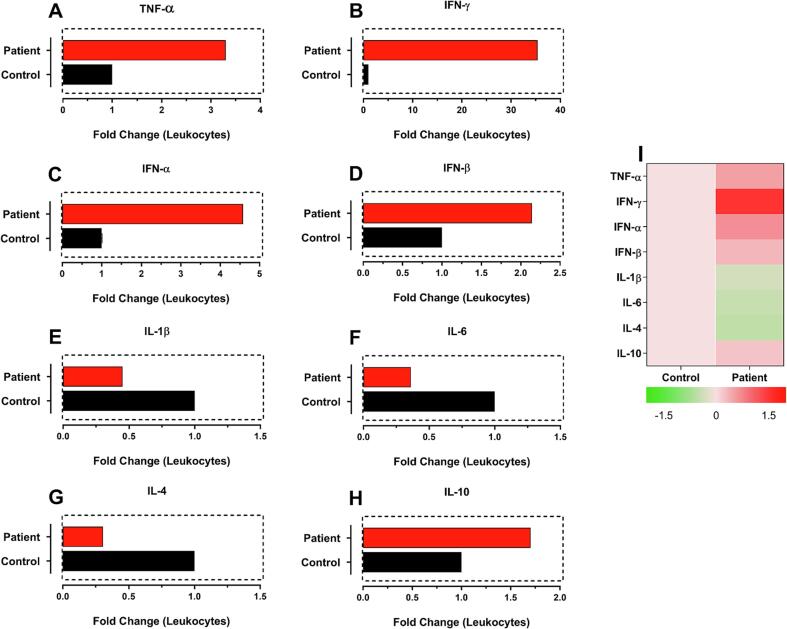
After 90 days of vaccination (Fig. 3), we observed that type 1 interferons (Fig. 3C-3D) and IL-1β (Fig. 3E) reduced their gene expression levels compared with previous assayed times, but, interestingly, there was a marked increase in the gene expression of TNF-α (Fig. 3A) and IFN-γ (Fig. 3B). The increase in these cytokine levels correlated with the consolidation of the cellular immune response, with a predominance of the Th1 profile and a complete inhibition of the Th2 profile since the IL-6 (Fig. 3F) and IL-4 (Fig. 3G) cytokines were down-regulated compared to the non-vaccinated control. In addition, the anti-inflammatory cytokine IL-10 expression (Fig. 3H) was still increased, but less expressed than in previous assayed times (10 and 43 days after vaccination), showing that the immune system was on the way to be restored to normal parameters.
Fig. 3.
Analysis of gene expression of cytokines related to the immune response after 90 days of vaccination with an overdose of Pfizer-BioNtec COVID-19 vaccine. Gene expression is shown as fold change for TNF-α, IFN-γ, IFN-α, IFN-β, IL-1β, IL-6, IL-4 and IL-10. Analyzes were performed by relative gene expression using a control sample from an unvaccinated infant.
Evaluation of neutralizing antibodies against SARS-CoV-2 variants after 90 days of vaccination with an excessive dose of the Pfizer-BioNtec vaccine
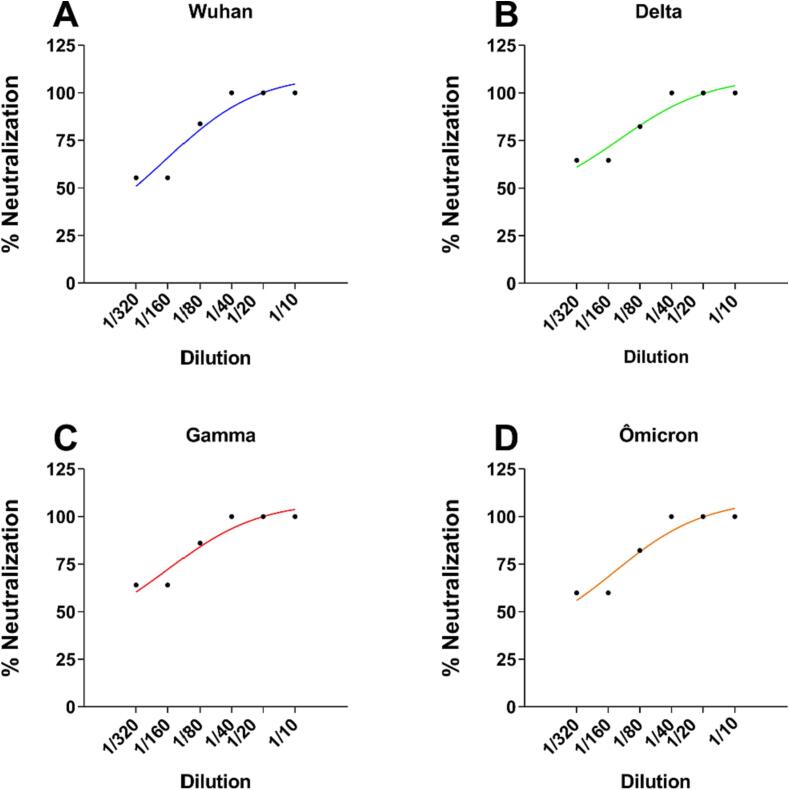
Plaque reduction neutralization test (PRNT) was used to detect anti-SARS-CoV-2 antibodies induced after the immunization error with the Pfizer vaccine (Fig. 4). We tested four variants of the virus, namely, Wuhan, Gamma, Delta, and Ômicron. After 90 days of receiving the excessive dose of the COVID-19 Pfizer vaccine, the child serum contained high titers of neutralizing antibodies, as titers of up to 1:40 neutralized 100 % of the viral infection in Vero E6 cells for all strains. These results show that vaccination was very effective in producing long-term high leveled antibodies against the main variants of SARS-CoV-2, which may have been directly influenced by the dosage received by the infant.
Fig. 4.
Plaque reduction neutralization Test 50 (PRNT50) from serum sample collected after 90 days of Pfizer-BioNTec overdose vaccination. Neutralization of SARS-CoV-2 variants: (A) Wuhan, (B) Delta, (C) Gamma and (D) Ômicron. Serum dilutions from 1:10 to 1:320 were evaluated to detect 50 % of virus neutralization in Vero E6 cells.
Materials and methods
Obtaining leukocytes from whole blood
The blood sample was collected in a 4.0 mL tube containing ethylenediaminetetraacetic acid (EDTA) to obtain the leukocytes, and the red blood cells were lysed using ammonium chloride lysis solution (ACK) pH: 7.2 and washed in 1X-Phosphate buffered saline (PBS), pH: 7.2.
RNA extraction and RT-qPCR
RNA extraction from leukocytes was performed using an RNA extraction kit (Promega), according to the manufacturer's recommendations. The samples were suspended in 50 μL of RNAse-free deionized water and RNA concentration quantified in a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). cDNA was obtained using a high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA, USA) and the relative expression of TNF-α, IFN-γ, IFN-α, IFN-β, IL-1β, IL-6, IL-4 and IL-10 RNAs were analyzed using a SYBR Green Step One Plus® system (Applied Biosystems, USA). The control sample was obtained from a child not vaccinated against SARS-CoV-2. Primer sequences for specific genes are provided in Table 2. The mRNA expression levels were normalized relative to the β-actin (Actb) levels using the comparative 2−ΔΔCt method.
Table 2.
Primer sequences used on the evaluation of cytokine gene expression on samples of the child immunized with an overdose of COVID-19 Pfizer vaccine.
| Gene name | ||
|---|---|---|
| Actb | TCCTCTCCCAAGTCCACACAGG | GGGCACGAAGGCTCATCATTC |
| TNF-α | ATGAGCACTGAAAGCATGATCC | GAGGGCTGATTAGAGAGAGGTC |
| IFN-γ | TCGGTAACTGACTTGAATGTCCA | TCGCTTCCCTGTTTTAGCTGC |
| IFN-α | GATGGCAACCAGTTCCAGAA | AAAGAGGTTGAAGATCTGCT |
| IFN-β | CTGTAAGTCTGTTAATGAAG | TTGTGCTTCTCCACTACAGC |
| IL-1β | CAGCTACGAATCTCCGACCAC | GGCAGGGAACCAGCATCTTC |
| IL-6 | CCAGGAGCCCAGCTATGAA | CCCAGGGAGAAGGCAACTG |
| IL-4 | CCAACTGCTTCCCCCTCTG | TCTGTTACGGTCAACTCGGTG |
| IL-10 | TCAAGGCGCATGTGAACTCC | GATGTCAAACTCACTCATGGCT |
Plaque reduction neutralization assay
The neutralizing activity was evaluated by plaque reduction neutralization assays with a 50 % cut-off (PRNT50). The Vero E6 cells were plated in a 48-well plate in DMEM with 10 % FBS and 1 % antibiotics and cultivated at 37 °C and 5 % CO2. In a BSL3 facility, the serum samples were heat-inactivated at 56 °C for 1 h and 2-fold serially diluted, from 1:10 to 1:320, in DMEM cell culture medium and incubated with 10 [2] PFU of different SARS-CoV-2 variants (Wuhan, Gamma, Delta and Ômicron), and reduction in plaque numbers were compared to a control where only cell culture medium was used. The virus and serum dilutions were incubated for 1 h at 37 °C to allow the binding between antibodies to the virus. Afterwards, the cell culture supernatant was removed, the antibody-virus complex was inoculated, in duplicate, to cell monolayers and the plates were incubated for 1 h under gentle rocking at 37 °C for virus adsorption. Next, the antibody-virus complex was removed, the cell monolayer was washed with warm culture medium and prewarmed overlay medium was added to each well and plates were incubated for 4 days (Wuhan), 5 days (Gamma and Delta variants) and 7 days (Ômicron) at 37 °C and 5 % CO2. Before plaque counting, cells were fixed for 2 h with a 4 % formaldehyde solution and stained with 0.2 % Crystal Violet (Sigma-Aldrich, St. Louis, Missouri, USA) solution for 1 h. The neutralization activity was calculated by reduction in plaque formation observed in every sample-virus mixture and compared to the positive control. The area under the curve (AUC) was calculated using Prism v.9 (GraphPad, San Diego, CA, USA).
Discussion
COVID-19 is caused by SARS-CoV-2, a virus composed of four predominant protein structures: spike (S protein), nucleocapsid (N protein), envelope (E protein), and membrane (M protein) [7], [8]. SARS-CoV-2 infects cells through the spike protein binding to the cellular angiotensin-converting enzyme 2 (ACE2) receptor [9]. As a result, vaccine production has been focusing on the viral S protein, as these vaccines induce antibodies that will block the main target of viral entry into cells [10]. The mRNA-based vaccine from Pfizer-BioNTec can induce the expression of S protein and thus elicit neutralizing antibodies against viral infection [11].
Clinical trials evaluating the Pfizer BNT162b1 vaccine showed that it induced the proliferation of a robust population of specific CD4 + T cells that consisted of predominantly T-helper-1 (TH1) expressing cytokines, such as IFN-γ, IL-2, or TNF-α, as opposed to T-helper-2 (TH2) cytokines like IL-4, IL-5, or IL-13. These results indicate a bias toward a TH1-mediated immune response [12], [13]. However, a separate study involving the mRNA-1273 vaccine indicated that the TH1 response is dose-dependent, with higher response levels observed in the 100 µg dose group compared to both the control and the 10 µg dose groups. Conversely, TH2 responses remained low or undetectable in both the 10 µg and 100 µg dose groups. Furthermore, the presence of CD40L, a cell-surface marker abundantly expressed following CD4 + T-cell activation, and IL-21, produced by CD4 + T follicular helper (TfH) cells, was detected in both groups. CD40L plays a crucial role in B-cell activation and efficient isotype switching, while TfH cells are essential for the generation of long-term B-cell memory [14]. Thus, although detection levels of TH2 responses were lower than TH1-mediated immune responses, the detection of these markers indicate the presence of a humoral immune response.
According to the World Health Organization (WHO), there have been numerous immunization errors in children during COVID-19 vaccination campaigns. Common mistakes include using adult doses in children, inappropriate age range, underdosing, overdose, use of unapproved vaccines, improper scheduling between doses, preparation errors, and administering the COVID-19 vaccine instead of other vaccines of the vaccination schedule. Non-compliance with the necessary storage conditions and use of expired doses were also identified. These errors have been reported using the Pfizer-BioNTec, Moderna, AstraZeneca, Janssen, Sinovac, Novavax, and other vaccines against COVID-19 [15].
In this case report, we describe a vaccination error involving a 6-month-old female infant submitted to vaccination against COVID-19 with the equivalent of 6 adult doses of Pfizer-BioNTec vaccine. After the immunization error was reported, the child was closely evaluated for any clinical manifestation of severe adverse effects, and the characteristics of the immune response induced by this vaccination error were studied. Although the infant received a dose 60-fold higher than that recommended, the clinical follow-up showed very little changes in the clinical manifestations and the laboratory examination. After almost one year of follow-up, the child’s development has been completely normal for age, and she had no COVID-19 infection.
During her clinical follow-up, an investigation on the impact of this extremely high dose of vaccine on the immunity against SARS-CoV-2 was evaluated through gene expression of cytokines related to the induction of innate and adaptive immune response. Total cellular mRNAs were obtained at three time points (10, 43, and 90 days after vaccination) during medical follow-up, after parental authorization. We observed that on days 10 and 43 after administering the COVID-19 vaccine, as expected, the child showed strong induction of the innate immune response as evidenced by a high expression of type 1 interferon genes (IFN-1) genes, which are responsible for controlling viral replication and essential for the excellent prognosis following COVID-19 infections [16], [17], [18]. Furthermore, it is known that recognition of uridine-containing RNA, present in mRNA vaccines, is associated with increased expression of pro-inflammatory cytokines, particularly type 1 IFNs [19], [20]. Another pro-inflammatory cytokine that showed increased levels of gene expression on days 10 and 43 after vaccination, especially on day 10, was IL-1β. This massive increase on day 10 may be related to lipid nanoparticles containing ionizable lipids (iLNPs), which allow efficient delivery of intact mRNA to the cell cytoplasm by the mRNA vaccines [21], [22] and then stimulate the translation of the encoded S protein resulting in the expression of this cytokine via the innate immune system. This high gene expression has been demonstrated in peripheral blood mononuclear cells (PBMCs) of humans and mice exposed to mRNA-iLNPs via toll-like-receptors (TLRs) and cytoplasmic detection of pathogen-associated molecular patterns (PAMPs) [23]. Interestingly, after 90 days of vaccination, despite a considerable reduction in type 1 IFNs and IL-1β gene expression, these gene expression remained increased compared to the non-vaccinated control. This finding might be associated with the high dose of vaccine administered to this child as it might be the slight increase in glutamic-oxaloacetic transaminase (data not shown), probably related to liver and muscle tissue toxicity [24], [25]. Studies show that the intramuscular administration of mRNA-iLNPs, in addition to being absorbed at the administration site, can also be absorbed, to a limited extent, via lymph nodes and be detected in other tissues such as the liver, lung, spleen and non-draining lymph nodes. With this excessive dose administration, it is plausible to hypothesize that the increase of this liver enzyme was related to the muscle inflammation at the vaccination site [26], [27], [28]. In addition, the patient had a fever during clinical follow-up, which is directly related to the increased expression of inflammatory genes such as IL-1β, IL-6, and TNF-α, which are significant inducers of a febrile state [29].
The adaptive immune responses, directed by T and B lymphocytes, were investigated through the expression of IFN-γ, TNF-α, IL-4, and IL-6 genes. Initially, a strong induction of the Th2 profile was observed after ten days of vaccination, characterized by the increase in the expression of the IL-4 and IL-6 genes, evidence that TCD4+ lymphocytes, secreting IL-4 and IL-6, are helping in the process of development and differentiation of B lymphocytes into immunoglobulin-secreting plasmocytes [29], [30]. It is believed that early in life, CD4 + T cells are biased to differentiate into cells of the Th2 profile. This trend has been demonstrated in newborn mice, but the evidence for Th2 bias in human newborns is less clear [31], [32]. However, this hypothesis may be one of the factors behind the explanation of why this profile was so evident ten days after vaccination. At 43- and 90-days post-vaccination, there was a shift from Th2 to Th1 profile, characterized by TCD4+ lymphocytes producing IFN-γ and TNF-α, and thus, stimulating the development of cytotoxic CD8+ T cells [33], consolidating the Th1 profile after 90 days of vaccination. In agreement with these findings, the Pfizer vaccine BNT16b2, when administered at a concentration of 30 µg separated by 21 days, was able to produce high titers of neutralizing antibodies against SARS-CoV-2, in addition to a robust antigen-specific response by TCD4+ lymphocytes, TCD8+ in the Th1 profile, with transient mild to moderate reactogenicity with 95 % efficacy in participants without previous SARS-CoV-2 infection [34].
Finally, when IL-10 cytokine gene was evaluated, it was observed a significant increase in the expression of this gene 10 days after vaccination and a constant reduction on days 43 and 90 post-vaccination. This high expression of IL-10 may have been essential for the patient to avoid the development of serious adverse effects after administration of an excessive dose of the Pfizer-BioNTec vaccine since this cytokine acts as an anti-inflammatory and immunosuppressive agent [35], [36]. Corroborating to this hypothesis, IL-10 gene expression after 10 days of the vaccination error was increased above the level of inflammatory cytokines, and on days 43 and 90, its gene expression level gradually decreased. Early IL-10 increased levels prevent hyperinflammation and tissue damage during severe SARS-CoV-2 infections [37], [38]. However, an increase in IL-10 and several other inflammatory cytokines has been associated with disease severity, and when this happens it may suggest that this cytokine is failing to adequately suppress inflammation, which was not observed in the patient who received the excessive vaccine dosage [39]. We should note that the immune response elicited here was induced by a vaccine and not by SARS-CoV-2 infection and the cytokine profile observed here is expected when there is a correct stimulation of the immune system by a foreign antigen.
It is well known that COVID-19 patients have a prothrombotic or thrombophilic state, with elevations in the levels of several biomarkers of thrombosis, including high levels of fibrinogen and D-dimer, which are associated with disease severity and prognosis. In addition, spike protein-induced coagulopathy may also provide a potential explanation for rare episodes of thrombosis reported post-vaccination. Thrombosis and/or thrombocytopenia can be seen after vaccination with any COVID-19 vaccine, since the spike protein activates macrophages and elicits inflammation. In this case, increased D-dimer was not associated with either thrombocytopenia or a decrease in fibrinogen levels, suggesting that there was not a presence of enhanced-fibrinolytic-type disseminated intravascular coagulation or a high risk of bleeding. However, due to the possible association of spike protein and this thrombophilic state, the clinical follow-up included the laboratory evaluation of D-dimers and, in a few days after the vaccination error, the D-dimer levels returned to a normal level.
Other observed clinical findings were fever and local inflammation, the most common adverse events associated with vaccines. These symptoms were not intense and rapidly subsided.
Although the infant received an overdose of the Pfizer-BioNTec vaccine, she presented with an expected clinical picture and an immune response profile favorable to developing immunity against SARS-CoV-2. In addition, an appropriate medical follow-up implemented rapidly after the immunization error was essential for the patient's and parents' well-being.
It is important to describe and analyze the immunization errors in order to prevent the occurrence of future errors, to protect the exposed individual, and to generate a better knowledge of the vaccine and the possible consequences of an inadequate dosage. In this sense, this case report shows that although adverse events may occur with COVID-19 vaccines, the Pfizer vaccine is very immunogenic and safe to be administered at an early age. The intensity of the immune response may not be the same as the normal dose, but it is also important to consider that the excess of antigen may impair the quality of the immune response. Although there were some minor adverse effects, the results obtained with this case of vaccination error indicate that this infant developed a robust and efficient immune response against SARS-CoV-2 infections.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Global manual on surveillance of adverse events following immunization. (Updated 2016). WHO. WHO Library Cataloguing-in-Publication Data https://www.who.int/publications/i/item/10665206144.
- 2.Caselli Pacheco F., et al. Análise do Sistema de Informação da Vigilância de Eventos Adversos Pós-Vacinação no Brasil, 2014 a 2016 Artigo Original. Rev Panam Salud Publica. 2018;42 doi: 10.26633/RPSP.2018.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hibbs B.F., Moro P.L., Lewis P., Miller E.R., Shimabukuro T.T. Vaccination errors reported to the Vaccine Adverse Event Reporting System, (VAERS) United States, 2000–2013. Vaccine. 2015;33:3171–3178. doi: 10.1016/j.vaccine.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Erro de Imunização: um evento adverso evitável. https://portaldeboaspraticas.iff.fiocruz.br/atencao-crianca/erro-de-imunizacao-um-evento-adverso-evitavel/.
- 5.MINISTÉRIO DA SAÚDE. Secretaria de Vigilância em Saúde Doença pelo Novo Coronavírus – COVID-19. BOLETIM EPIDEMIOLÓGICO ESPECIAL. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/covid-19/2022/boletim-epidemiologico-no-122-boletim-coe-coronavirus.
- 6.Hudson A., Montelpare W.J. Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging. Int J Environ Res Publ Health. 2021;18(15):8054. doi: 10.3390/ijerph18158054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muralidar S., Ambi S.V., Sekaran S., Krishnan U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie. 2020;179:85–100. doi: 10.1016/j.biochi.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen S.A., Kellogg C., Equils O. Neutralizing and cross-reacting antibodies: implications for immunotherapy and SARS-CoV-2 vaccine development. Hum Vaccines Immunotherapeut. 2021;17:1–4. doi: 10.1080/21645515.2020.1787074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R., Qin C. Expression pattern and function of SARS-CoV-2 receptor ACE2. Biosaf Health. 2021;3:312–318. doi: 10.1016/j.bsheal.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matchett W.E., et al. Cutting Edge: Nucleocapsid Vaccine Elicits Spike-Independent SARS-CoV-2 Protective Immunity. J Immunol. 2021;207:376–379. doi: 10.4049/jimmunol.2100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pormohammad A., et al. Efficacy and safety of covid-19 vaccines: A systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel) 2021;9:467. doi: 10.3390/vaccines9050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin U. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020 586:7830 586, 594–599 (2020). [DOI] [PubMed]
- 13.Vogel AB. et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021 592:7853 592, 283–289 (2021). [DOI] [PubMed]
- 14.Corbett K.S., et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. New Engl J Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO statement regarding COVID-19 immunization errors in children. https://www.who.int/news/item/30-08-2022-statement-covid-19-immunization-errors-children.
- 16.Paces J., Strizova Z., Smrz D., Cerny J. COVID-19 and the Immune System. Physiol Res. 2020;69:379. doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos A.F., Póvoa P., Paixão P., Mendonça A., Taborda-Barata L. Changes in Glycolytic Pathway in SARS-COV 2 Infection and Their Importance in Understanding the Severity of COVID-19. Front Chem. 2021;9:752. doi: 10.3389/fchem.2021.685196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Karikó K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142–e. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullis P.R., Hope M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol Therapy. 2017;25:1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tahtinen S. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol 2022 23:4 23, 532–542 (2022). [DOI] [PubMed]
- 23.Li C. et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol 2022 23:4 23, 543–555 (2022). [DOI] [PMC free article] [PubMed]
- 24.Zanardo V., Bondio M., Perini G., Temporin G.F. Serum Glutamic-Oxaloacetic Transaminase and Glutamic-Pyruvic Transaminase Activity in Premature and Full-Term Asphyxiated Newborns. Neonatology. 1985;47:61–69. doi: 10.1159/000242092. [DOI] [PubMed] [Google Scholar]
- 25.Handbook of Liver Disease. (2018) doi:10.1016/C2015-0-04097-7.
- 26.CHMP. COVID-19 Vaccine Moderna, INN-COVID-19 mRNA Vaccine (nucleoside modified). (2021).
- 27.CHMP. Committee for Medicinal Products for Human Use (CHMP) Assessment report Comirnaty Common name: COVID-19 mRNA vaccine (nucleoside-modified).
- 28.Hassett K.J., et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol Ther Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.papel das interleucinas MOURA, H. V. de, POMERANTZEFF, P. M. A. & GOMES, W. J. Síndrome da resposta inflamatória sistêmica na circulação extracorpórea. Braz J Cardiovasc Surg. 2001;16:376–387. [Google Scholar]
- 30.Nakayama T. et al. Th2 Cells in Health and Disease. https://doi.org/10.1146/annurev-immunol-051116-052350 35, 53–84 (2017). [DOI] [PubMed]
- 31.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004 4:7 4, 553–564 (2004). [DOI] [PubMed]
- 32.Olin A., et al. Stereotypic Immune System Development in Newborn Children. Cell. 2018;174:1277–1292.e14. doi: 10.1016/j.cell.2018.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniuchi I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. https://doi.org/10.1146/annurev-immunol-042617-053411 36, 579–601 (2018). [DOI] [PubMed]
- 34.AB, V. et al. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. (2020) doi:10.1101/2020.09.08.280818.
- 35.Saraiva M., Vieira P., O’Garra A. Biology and therapeutic potential of interleukin-10. J Exper Med. 2020;217 doi: 10.1084/jem.20190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore K.W., De Waal Malefyt R., Coffman R.L., O’Garra A. Interleukin-10 and the Interleukin-10. Receptor. 2003;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y., et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu L., Zhang H., Dauphars D.J., He Y.W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021;42:3–5. doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han H., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.