Abstract
Introduction
Negative symptoms and cognitive impairment are common residual symptoms of schizophrenia that seriously affect the quality of life and social function of patients. The intervention of residual symptoms is an important part of schizophrenia rehabilitation. Traditional Chinese exercise has been applied as a supplementary rehabilitation method for schizophrenia. However, research on its use and pertinence in the rehabilitation of residual symptoms remains lacking. In this study, we will verify the intervention effect of a new method, namely, shen-based qigong exercise, on the residual symptoms of schizophrenia, in the hopes of finding a safe and effective rehabilitation method for the residual symptoms of schizophrenia.
Methods
This is a single-centre randomised controlled trial. A total of 60 schizophrenics who meet the criteria will be randomly divided into the control and intervention groups in accordance with the ratio of 1:1. Conventional drug treatment will remain unchanged in both groups. In this case, the control group will be given daily rehabilitation, whereas the intervention group will be given daily rehabilitation and shen-based qigong exercise intervention. The intervention period will be 12 weeks. The primary outcome will be negative symptoms assessed by the Scale for the Assessment of Negative Symptoms. The secondary outcome will be the global cognitive function assessed by the Repeatable Battery for the Assessment of Neuropsychological Status and event-related potential P300. Other outcomes will include specific cognitive domain (i.e. working memory), quality of life and social function. The results will be measured within 1 week before and after the intervention.
Discussion
The results of this study will likely help find an economical and convenient rehabilitation method for the residual symptoms of schizophrenia and, at the same time, may promote the popularisation and application of traditional Chinese exercises and traditional Chinese medicine theories in the treatment of mental diseases.
Trial registration
ClinicalTrials.gov registry number: NCT05310955.
Keywords: Schizophrenia, Qigong, Rehabilitation, Randomised controlled trial, Clinical trial protocol
Abbreviations
- TCM
traditional Chinese medicine
- TCE
traditional Chinese exercise
- RCT
randomised controlled trial
- SANS
Scale for the Assessment of Negative Symptoms
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
- ERP P300
event-related potential P300
- N-back
N-back task
- SF-36
36-item Short Form Health Survey
- SAFE
Social-Adaptive Functioning Evaluation
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, fifth edition
1. Introduction
Schizophrenia is a common severe mental disease with serious harm and high disability rates [1]. Surveys showed that schizophrenia has the worldwide prevalence of 0.5%–1.0% and the lifetime prevalence rate of approximately 0.7% in China, where it is the most important cause of mental disability [2,3]. The prognosis of schizophrenia is poor. Many patients with schizophrenia have long-term residual symptoms, which are mainly negative and cognitive symptoms [[4], [5], [6]]. Of all schizophreniacs, 49% have affective blunting, 42% present conceptual disorganisation and 40% experience social withdrawal [7]. Moreover, schizophrenics exhibit extensively low cognitive ability for a long time, including a comprehensive decline in attention, memory, language, motor operation, processing speed, visual space ability, executive function and social cognitive ability [[8], [9], [10]]. A study reported that 50% of patients with first-episode schizophrenia still have negative symptoms after 5 years of remission [11], and another study revealed that the cognitive impairment of schizophrenics can last for 15 years [12]. As a result of long-term residual negative symptoms and cognitive impairment, the daily life and work of patients are seriously affected. These effects become a obstacle to their return to society and exert a heavy burden on the patients’ families and society [13]. Although drug therapy remains the main intervention mode for schizophrenia, the effect of existing antipsychotics on negative symptoms and cognitive impairment is limited [14]. Therefore, finding safe and effective intervention methods for residual symptoms is an important issue to be solved in the rehabilitation of schizophrenia.
Traditional Chinese medicine (TCM) has attracted considerable attention in recent years given its unique theoretical understanding of mental illness and various treatment methods [15]. Traditional Chinese exercise (TCE) is an aspect of TCM rehabilitation methods. TCE is a kind of mind–body and aerobic exercise with moderate intensity and gentle rhythm, which is also called “qigong” in Chinese. It mainly includes tai chi, baduanjin, yijinjing, wuqinxi and liuzijue, which all emphasise ‘body–spirit syncretism’. At present, many studies, mainly focusing on tai chi and baduanjin, have applied TCE in the rehabilitation of schizophrenia.
Meta-analyses have shown that tai chi has a potential positive effect on improving the negative symptoms of schizophrenia [16,17]. A randomised controlled trial (RCT) revealed that in schizophrenics, tai chi has a favourable effect on improving psychiatric symptoms and motor function and enhancing memory and attention [18]. Another RCT showed that tai chi training is more beneficial than the standard hospitalisation intervention in the prevention of the deterioration of motor coordination and interpersonal function of inpatients with schizophrenia [19]. In terms of baduanjin, Li et al. conducted a study and found that compared with brisk walking, baduanjin training can improve the logical memory function of patients with schizophrenia [20]. Other studies also confirmed that 12-week baduanjin training is helpful for reducing negative symptoms and promoting the recovery of social function and has a positive effect on the quality of life of schizophrenics [[21], [22], [23]].
In conclusion, tai chi and baduanjin have been gradually promoted in the clinic as supplementary rehabilitation methods given their rehabilitative effect on schizophrenia. A recent study applied yijinjing for first time in the treatment of schizophrenia and found that yijinjing can also improve the negative symptoms and cognitive function of patients with chronic schizophrenia [24]. However, other TCEs, such as wuqinxi and liuzijue, have not yet been applied in the treatment of schizophrenia, and the research on yijinjing as an intervention method is just beginning. Moreover, the research on the effect of TCEs on the global cognitive function of schizophrenics is still lacking, and their improvement effect on long-term negative symptoms remains to be further verified [25,26]. At the same time, previous studies only selected a single exercise, and research on the selection of exercise actions with specific efficacy for targeted intervention based on the characteristics of schizophrenia does not exist. Therefore, we hypothesised that if we can pointedly select the movements in a variety of TCEs with the effect of improving mental cognition, we may be able to improve the residual symptoms of schizophrenia.
Therefore, based on the TCM theory of ‘heart dominating mind’ and ‘five shen-zang’, we target the TCM dialectics of the residual negative symptoms and cognitive impairment of schizophrenia and select movements in various TCEs that improve mental cognition from the perspective of heart, spleen and kidney for rearrangement. We refer to this new form as ‘shen-based qigong exercise’. The purpose of this study is to observe the rehabilitation effect of this new exercise on the residual symptoms of schizophrenia and to evaluate the intervention effect of 12-week shen-based qigong exercise on the negative symptoms, cognitive function, quality of life and social function of schizophrenics. We assume that the 12-week shen-based qigong exercise can provide improvement and benefit to patients with schizophrenia.
2. Methods
2.1. Design and setting
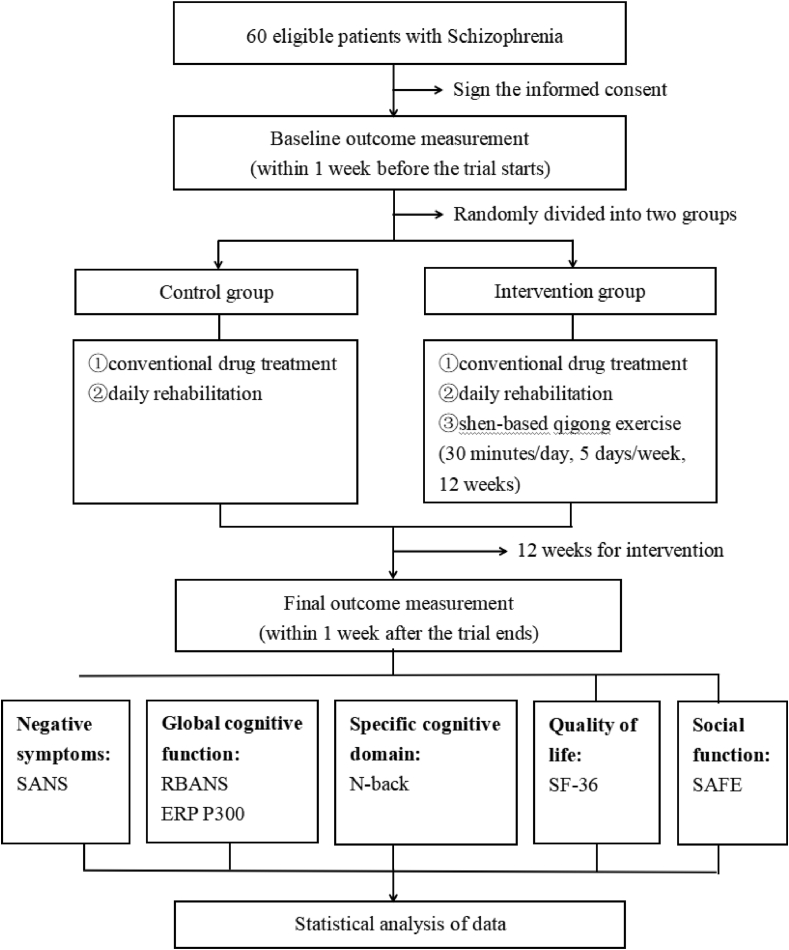
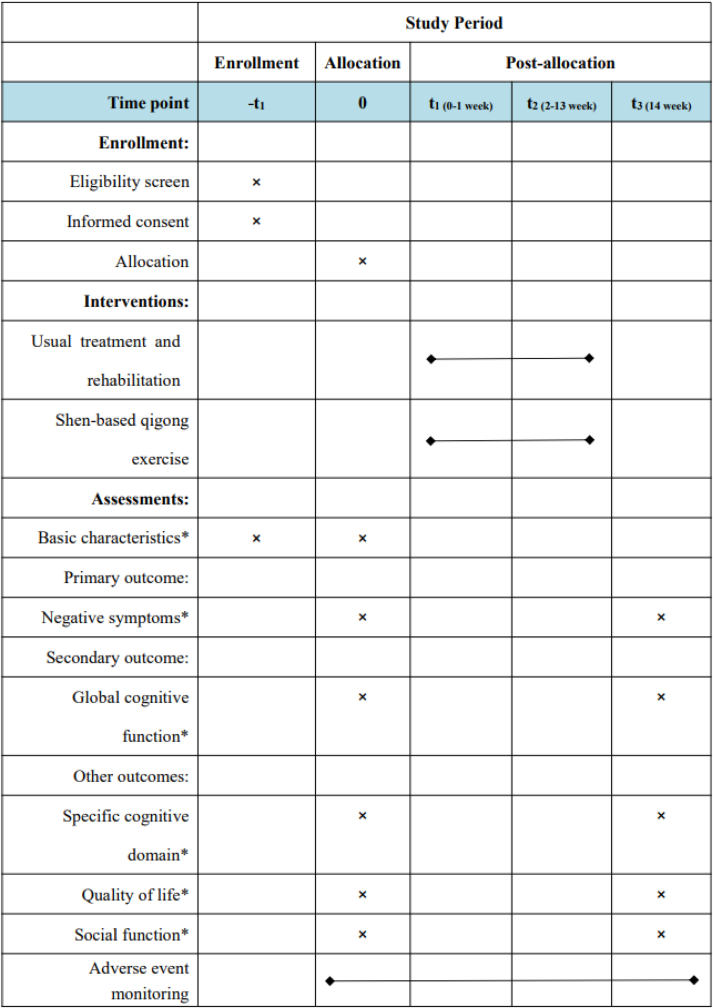
This is a single-centre RCT conducted in Shanghai Mental Health Centre. A total of 60 patients with schizophrenia who meet the criteria will be randomly divided into the control and intervention groups in accordance with the ratio of 1:1. Conventional drug treatment will remain unchanged in both groups. In this case, the control group will receive daily rehabilitation, whereas the intervention group will receive daily rehabilitation and shen-based qigong exercise. The intervention period will be 12 weeks. The primary outcome will be negative symptoms assessed by the Scale for the Assessment of Negative Symptoms (SANS). The secondary outcome will be the global cognitive function evaluated by the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and event-related potential P300 (ERP P300). Other outcomes will include specific cognitive domain (i.e. working memory) assessed with the N-back task (N-back), quality of life assessed with the 36-item Short Form Health Survey (SF-36) and social function assessed with Social-Adaptive Functioning Evaluation (SAFE). The results will be measured within 1 week before and after the intervention. The flow chart of the study is shown in Fig. 1. This protocol follows the SPIRIT 2013 checklist (Additional file 1) and SPIRIT figure (Fig. 2).
Fig. 1.
Study flow chart. ERP P300, event-related potential P300; N-back, N-back task; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SANS, Scale for the Assessment of Negative Symptoms; SF-36, 36-item Short Form Health Survey; SAFE, Social-Adaptive Functioning Evaluation.
Fig. 2.
SPIRIT figure showing time points for enrollment, interventions and assessment. *Basic characteristics include demographic information such as gender, age, years of schooling and medication. *Negative symptoms will be assessed by the Scale for the Assessment of Negative Symptoms. *Global cognitive function will be assessed by the Repeatable Battery for the Assessment of Neuropsychological Status and event-related potential P300. *Specific cognitive domain (i.e. working memory) will be assessed by the N-back task. *Quality of life will be assessed by 36-item Short Form Health Survey. *Social function will be assessed by Social-Adaptive Functioning Evaluation.
2.2. Patient and public involvement
No patient and/or public was involved in the design, or conduct, or reporting, or dissemination plans of this research.
2.3. Sample size
This study is a superiority trial. SANS is the commonly used negative symptom evaluation scale. We use the calculating formula for sample size of superiority test in accordance with the previous SANS score (10.02 ± 1.66; 7.20 ± 0.11) in related literature [27]. By setting alpha = 0.05, power = 90% and superiority margin = 1, we obtain the minimum sample size of 15 for each group. Under the assumption of a drop-out rate of 20% and in consideration of clinical operability, each group will comprise 18 participants. Given that the duration of this study is long and the drop-out rate may be high, a sample size of 30 per group is planned for inclusion for total of 60 participants.
2.4. Participants
2.4.1. Inclusion criteria
Participants will be included if they (1) are Han Chinese; (2) are at least 18 years of age; (3) have received at least 6 years of schooling, are capable of filling in the questionnaire independently and have a sufficient audio-visual level for completing the necessary examination; (4) satisfy the diagnostic criteria for schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5); (5) reside in the rehabilitation ward and have not experienced relapse within the past 6 months; (6) have residual negative symptoms with at least one item ≥2 on the negative subscale of PANSS (N1–N7) [7]; (7) take second-generation antipsychotics; (8) have no training history of TCEs and (9) have agreed to participate in the study and are willing to give written informed consent.
2.4.2. Exclusion criteria
Patients will be excluded if they (1) have severe physical diseases, such as cardiovascular, lung, liver, kidney and haematopoietic diseases; (2) satisfy the diagnostic criteria for other mental disorders based on DSM-5; (3) have alcohol or substance abuse/dependence; (4) have mental retardation (Wechsler Adult Intelligence Scale <70) and/or severe cognitive impairment (Mini-Mental State Examination <24); (5) have visual and/or hearing problems and are unable to complete the relevant tests; (6) have received electroconvulsive or repetitive transcranial magnetic stimulation therapy within the past 3 months; (7) currently enrolled in other clinical studies or have participated within the past 3 months; (8) participated in regular exercise training in the past 6 months and (9) failed to sign or refuse to sign informed consent.
2.5. Recruitment and screening
Participants will be recruited from the inpatient wards of Shanghai Mental Health Centre. This centre has a large number of patients that can guarantee the recruitment of a sufficient number of participants. Recruitment began in August 2022. The recruitment and screening process is as follows: Firstly, the primary researchers will inform the recruiting researchers of the purpose and inclusion and exclusion criteria of this study. Then, the recruiting researchers will present information about the study, including the purpose, content, possible benefits or risks, to potential subjects diagnosed with schizophrenia. If the subjects have interest in participating, the recruiting researchers will hold a detailed interview with them to confirm whether they meet the screening criteria. The content of the interview is about the inclusion and exclusion criteria which is already listed above. Finally, eligible subjects or their families will sign the written informed consent to ensure their rights and interests.
2.6. Randomisation and blinding
A random number table generated by SPSS V.25.0 will be used to randomly divide the subjects into the control and intervention groups at the ratio of 1:1. In this study, the evaluators and data analysts will be blinded. An independent research coordinator will place the random numbers in opaque envelopes to achieve allocation concealment. The evaluators will not know the grouping of the subjects, and the subjects will be asked not to mention their treatment condition to the evaluators during the assessment.
2.7. Intervention
All the participants will receive conventional drug treatment and daily rehabilitation interventions, including Naikan, Morita, group art and group painting therapies. The control group will not receive any other interventions and will retain only their existing daily habits, without any additional food restrictions. Subjects whose drugs are changed or who participate in other interventions at the same time will be considered as dropped or eliminated. The intervention group will receive an additional 12-week shen-based qigong exercise practice (Fig. 3) and will be taught by a professional TCE teacher uniformly within 1 week before the formal intervention starts. The intervention will be conducted once a day for 30 min at 10:30–11:00, 5 days per week. The participants will be gathered in a quiet and spacious rehabilitation hall, and the researchers will supervise the whole exercise. Exercise load will be monitored by using the Borg CR10 scale at the level of 4–6, at which the participant will feel somewhat severe but not very severe fatigue. If one's Borg self-rated score is less than 4 or greater than 6, he/she will be instructed to increase/decrease exercise intensity by adjusting their range of motion, speed and breathing method.
Fig. 3.
Movements of shen-based qigong exercise. The presenter in the figure is LIAN Anbei, one of the authors of this study.
The 30-min shen-based qigong exercise intervention includes (1) a 3-min warm-up period with the main contents of joint activities, including head movement, chest expansion, arm vibration, body rotation, wrist and ankle movement and leg pressing followed by calm breathing and mental focusing; (2) a 20-min shen-based qigong exercise that includes the following eight movements: starting posture, ‘HE exercise’ in liuzijue, ‘raising a single arm to regulate the spleen and stomach’, ‘looking back to treat five strains and seven impairments’, ‘shaking head and buttocks to expel heart-fire’ in baduanjin, ‘plucking fruit like a monkey’ in wuqinxi, ‘Wei Tuo presenting the pestle’ in yijinjing and ending posture and (3) a 7-min cool-down period with the main contents of muscle stretching and relaxation, including leg stretching, upper back stretching, waist stretching, neck stretching and shoulder stretching while paying attention to breath adjustment and mind relaxation.
2.8. Outcome measurement
2.8.1. Primary outcome
Negative symptoms will be evaluated by using SANS, a standardised scale for measuring negative symptoms in clinic. It contains five subscales: affective blunting, alogia, avolition/apathy, anhedonia/asociality and attention. A total of 24 items are scored at six levels for each item. SANS has been proven to show consistent inter-rater reliability and moderate temporal stability [28].
2.8.2. Secondary outcome
Secondary outcome will be the global cognitive function measured using RBANS and ERP P300. The RBANS consists of five cognitive domain indexes: (A) immediate memory (including two subtests—list learning and story memory), (B) visuospatial/constructional abilities (including two subtests—figure copy and line orientation), (C) language (including two subtests—picture naming and semantic fluency), (D) attention (including two subtests—digit span and coding) and (E) delayed memory (including four subtests—list recall, list recognition, story recall and figure recall) [29]. P300 will be recorded by using a 64-channel electroencephalogram recording and analysis system (eego™ mylab, ANT Neuro, Germany). Participants will sit in a shielded room and remain relaxed and concentrate as much as possible. The classic auditory ‘Oddball’ paradigm will be adopted with the standard tones of 1000 Hz and 100 ms accounting for 80% of the stimuli and the target tones of 2000 Hz and 100 ms, which are pseudo-random in the standard tone series, accounting for 20% of the stimuli [30]. A total of 252 stimuli will be provided at the sound pressure level of 75 dB. The participants will be instructed to respond only to the target stimulus and press the button as quickly and accurately as possible. The positions of the electrode will follow the international 10/20-electrode placement standard. The collected signals will be finally analysed by using the EEGLAB Toolkit.
2.8.3. Other outcomes
The specific cognitive domain (i.e. working memory) will be assessed by using the numeric N-back task. During the task, numbers from 0 to 9 will be displayed as stimuli on the screen. The task will consist of a 0-back condition and a 2-back condition. In the 0-back trials, the participants will be told to match the current number with the number 9, and in the 2-back trials, the current number will be matched with the number displayed two trials earlier. Each block will comprise three targets and nine nontargets for a total of 12 stimuli with a 1000 ms interstimulus interval. A total of six 0-back blocks and six 2-back blocks will appear in semirandom order (0–2–0–0–2–2–2–0–0–2–2–0) [31]. The accuracy rate (the percentage of correct responses) of the participants will be recorded as the index for evaluating working memory.
Quality of life: SF-36 will be used to assess the health-related quality of life. SF-36 is one of the most commonly used standardised measurement tools for quality of life worldwide [32]. It comprises eight subscales with a total of 36 items involving physical and mental health. The rough score of each subscale will be converted into a standard score of 0–100. A high score is indicative of the good quality of life in this aspect.
Social function will be evaluated by using SAFE. SAFE has a total of 19 items that are divided into basic life skills, advanced life skills, social skills and communication skills; each item will be given a score from 0 to 4 with high scores indicating serious damage [33]. The retest reliability of the Chinese version of SAFE is 0.96–0.99 with the interrater consistency of 0.77 and the internal consistency of 0.77–0.95, so it can be used to evaluate the social adaptation function of patients with mental disorders over 18 years old [34].
2.9. Monitoring of compliance and quality
All the participants will be educated on compliance to understand fully the significance of this study and actively cooperate with the investigation in accordance with the requirements of this study protocol. For the participants of the control group, compliance will be judged in accordance to whether they change to conventional drugs or whether they participate in other similar exercises during the trial. Exercise recording cards will be used to monitor the compliance of the participants of the intervention group, and two research assistants will record each exercise session. The standard of achieving a valid intervention is defined as that the actual exercise times of the participant that account for more than 85% of the estimated exercise times. All the researchers will receive uniform training. A dedicated team will be established to monitor the progress and quality of the study, and the research team will be gathered regularly to address potential problems in time. Furthermore, the following will be ensured: that each participant signs the informed consent form; that the clinical facilities meet requirements and that the researchers follow the study protocol and record data accurately and completely.
2.10. Safety
Any adverse events occurring in the trial will be reported to the primary researcher in time to determine whether the events are related to the trial. The assessment of adverse events, including classification, grade, relationship to treatment, management and outcome, will be recorded in the case report form. In case of serious adverse events, the doctors in Shanghai Mental Health Centre will be told to take action in a timely manner, and the Institutional Review Board will get informed at the same time. All adverse events will be followed up until remission or stabilisation. For the cases that drop out due to other reasons, researchers will make efforts to identify the reasons for quitting and record the time that the participants quit the study.
2.11. Data management
All the assessment data and medical records of the participants in the trial will be stored on an encrypted mobile hard disc, and paper files will be stored in a locked filing cabinet, which will be kept by a designated person to ensure safety. At the same time, the research-related data will be accessible only to and consulted by authorised researchers.
2.12. Statistical analysis
SPSS V.25.0 software will be used for statistical analysis, which will be performed by an independent statistician who is not participating in outcome evaluation. Kolmogorov–Smirnov test will be used for the normality test, and normal data will be expressed as mean ± SD, whereas non-normal data will be expressed as median ± interquartile range. Enumeration data will be expressed as a relative ratio. Missing data will be analysed by using the mixed effect model method. For the comparison of baseline differences between the two groups, one-way ANOVA or Kruskal–Wallis H test will be used for continuous variables, and χ2 test will be used for categorical variables. ANOVA for repeated measurement or nonparametric test will be used to compare the changes in indexes before and after intervention and to assess the intervention-related effects (time × group) of two groups. P value less than 0.05 will be considered statistically significant.
2.13. Ethics and dissemination
This trial will be performed in accordance with the Declaration of Helsinki. This study has been approved by the Institutional Review Board of Shanghai cval No. 2021-59), and this trial has already been registered at ClinicalTrials.gov (registry number: NCT05310955). Before participating in the trial, every subject will sign a written informed consent voluntarily and be informed of the contents, potential risks and possible benefits of the study. During the whole trial period, all the participants can voluntarily choose whether to continue at any time. The research results will be published in peer-reviewed journals or disseminated through academic conferences.
3. Discussion
For schizophrenia, the symptoms that still exist after clinical remission are defined as residual symptoms. These symptoms often lead to the chronic course of schizophrenia [7]. Residual negative symptoms have been proven to be an important predictor of disability after schizophrenia [35], and long-standing cognitive impairment is closely related to the outcome of schizophrenia and is almost unaffected by drug treatment [9].
Aerobic exercise is considered as an effective intervention method for schizophrenia and has comprehensive benefits in improving clinical symptoms (especially negative symptoms), cognitive function and social function [14,36,37]. Meditation-based mind–body therapies also show effects on cognitive function and negative symptoms of schizophrenia [25,38,39]. As a kind of mind–body exercise, TCE is a combination of aerobic exercise and meditation. Although the action mechanism of TCE has not been elucidated, its advantages may be related to this kind of exercise property. TCE is based on the TCM theory of ‘body–spirit syncretism’. During exercise, it combines stretching body movements with breathing, and at the same time emphasises the concentration of the mind, which can integrate body, breath and mind adjustment and promote the balance and rehabilitation of the body and mind. In contrast to conventional aerobic exercises, such as jogging or swimming, TCE emphasises meditation and breathing, which can relax and adjust mood. Meanwhile, in contrast to static exercises, such as meditation or mindfulness, TCE can strengthen the body and mind through active movement. In addition, it helps relax muscles and tendons and promote blood circulation, which are the healthcare effects of TCM. All these effects may be the advantages of TCE in mental diseases.
On the basis of original TCEs, shen-based qigong exercise has strong pertinence to the residual symptoms of schizophrenia. It is based on two primary TCM theories regarding mental cognition. In TCM, people's mental and cognitive activities are collectively referred to as ‘shen’, and ‘heart dominating mind’ is the main theory, which holds that the heart is the inner dominator of people's mental activities. Based on the ‘heart dominating mind’ theory, the ‘five shen-zang’ theory provides an expanded explanation of the cognitive process, emphasising that the five viscera cooperate with each other to complete the whole process of mental cognition [40]. The theory of ‘five shen-zang’ states that the common residual symptoms of schizophrenia can be considered as the lesions of heart, spleen and kidney: negative symptoms, such as affective flattening, poverty of thought and volition weakening, can be attributed to spleen and kidney dysfunction; attention disorder can be attributed to heart and kidney dysfunction and memory impairment can be attributed to the decline or disharmony of spleen and kidney functions [41]. Therefore, we select and reorganise movements with heart-, spleen- and kidney-regulating effects from liuzijue, baduanjin, wuqinxi and yijinjing to design a highly targeted qigong exercise for improving the negative symptoms and cognitive impairment of schizophrenia. This exercise combines the advantages of various TCEs and is thus innovative. Moreover, it involves concise movements that are easy to learn and is not limited by time and place.
In terms of cognitive function assessment tools, we will use RBANS and ERP P300 to assess global cognitive function. RBANS has been proven to be an effective cognitive assessment tool for schizophrenics and has good reliability and validity in the Chinese population [42,43]. ERP technology can reflect the neuroelectrophysiological changes in the brain during the cognitive process and is widely used in studies on neuropsychiatric disorders. ERP P300 is an endogenous cognitive component that is related to advanced psychological activities, such as attention, memory, feeling, learning and reasoning and its latent period and amplitude can reflect the level of cognitive function in different aspects [44]. Furthermore, we select the N-back task as the evaluation tool of the specific cognitive domain (working memory). Working memory is the foundation of almost all complex cognitive activities [45]. Working memory deficit is also the core manifestation of cognitive dysfunction in schizophrenics [46]. The N-back task paradigm is currently regarded as the most classic paradigm of working memory research and includes the processes of cognitive task execution [47]. Therefore, we have selected N-back measured working memory as the observation indicator of specific cognitive domain combined with global cognitive function to evaluate the changes in cognitive function with increased comprehensiveness and meticulousness.
4. Conclusion
In summary, this article proposes a clinical trial protocol that aims to observe the intervention effect of a new method, namely, shen-based qigong exercise, on the residual negative symptoms, cognitive function, quality of life and social function of patients with schizophrenia. The results of this study will likely help find an economical and convenient rehabilitation method for the residual symptoms of schizophrenia and, at the same time, may promote the popularisation and application of TCEs and TCM theories in the treatment of mental diseases.
Ethics statement
This study has been approved by the Institutional Review Board of Shanghai Mental Health Centre (Approval No. 2021-59). Each participant will obtain informed consent before any procedures are implemented. The research results will be published in peer-reviewed journals.
Author contributions
Anbei Lian: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft.
Qing Fan: Conceptualization, Project administration, Supervision, Writing – review & editing.
Wenzheng Wang: Conceptualization, Data curation, Project administration.
Qinxin Liu: Investigation, Writing – original draft.
Jiacheng Shi: Investigation, Writing – original draft.
Min Zhuang: Investigation, Writing – original draft.
Yujie Li: Investigation, Writing – original draft.
Xiaodan Liu: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82072551, 81902307, and 82172551); Key Characteristic Specialized Subject of Shanghai Mental Health Centre (No. 2017-TSXK-08); Shanghai's Three-Year Action Plan for Further Accelerating the Inheritance, Innovation and Development of Traditional Chinese Medicine (2021–2023) (No. ZY(2021–2023)-0105); Key Laboratory of Psychotic Disorders (No. 13dz2260500). The funding organizations have no role in the design of the study and will not have any role during the execution, analyses, interpretation of the data, or decision to submit results.
The authors would like to thank all the doctors and nurses for their assistance in the recruiting process and all the patients for their participation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2023.101214.
Contributor Information
Anbei Lian, Email: amber_lian@foxmail.com.
Qing Fan, Email: fanqing_98@vip.sina.com.
Wenzheng Wang, Email: fffty@163.com.
Qinxin Liu, Email: liuqinxin97@outlook.com.
Jiacheng Shi, Email: sjcfd917@163.com.
Min Zhuang, Email: 491357350@qq.com.
Yujie Li, Email: 15221230270@163.com.
Xiaodan Liu, Email: 0000002635@shutcm.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu S., Zhao L., Fan Y., et al. Interaction between TNF-α and oxidative stress status in first-episode drug-naïve schizophrenia. Psychoneuroendocrinology. 2020;114 doi: 10.1016/j.psyneuen.2020.104595. [DOI] [PubMed] [Google Scholar]
- 2.Smigielski L., Jagannath V., Rössler W., et al. Epigenetic mechanisms in schizophrenia and other psychotic disorders: a systematic review of empirical human findings. Mol. Psychiatr. 2020;25:1718–1748. doi: 10.1038/s41380-019-0601-3. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y., Wang Y., Wang H., et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatr. 2019;6:211–224. doi: 10.1016/S2215-0366(18)30511-X. [DOI] [PubMed] [Google Scholar]
- 4.Millan M.J., Fone K., Steckler T., et al. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 2014;24:645–692. doi: 10.1016/j.euroneuro.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Kahn R.S., Sommer I.E., Murray R.M., et al. Schizophr. Nat. Rev. Dis. Prim. 2015;1 doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- 6.Bobes J., Arango C., Garcia-Garcia M., et al. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J. Clin. Psychiatry. 2010;71:280–286. doi: 10.4088/JCP.08m04250yel. [DOI] [PubMed] [Google Scholar]
- 7.Schennach R., Riedel M., Obermeier M., et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur. Arch. Psychiatr. Clin. Neurosci. 2015;265:107–116. doi: 10.1007/s00406-014-0528-2. [DOI] [PubMed] [Google Scholar]
- 8.Yeo R.A., Ryman S.G., van den Heuvel M.P., et al. Graph metrics of structural brain networks in individuals with schizophrenia and healthy controls: group differences, relationships with intelligence, and genetics. J. Int. Neuropsychol. Soc. 2016;22:240–249. doi: 10.1017/S1355617715000867. [DOI] [PubMed] [Google Scholar]
- 9.Kahn R.S., Keefe R.S. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatr. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 10.Wu C., Dagg P., Molgat C. Measuring stability of cognitive impairment in inpatients with schizophrenia with alternate forms of the Montreal Cognitive Assessment during acute hospitalization. Psychiatr. Res. 2017;258:299–304. doi: 10.1016/j.psychres.2017.08.065. [DOI] [PubMed] [Google Scholar]
- 11.An D.H.W., Leber A., Häfner H. Negative symptoms and their association with depressive symptoms in the long-term course of schizophrenia. Eur. Arch. Psychiatr. Clin. Neurosci. 2016;266:387–396. doi: 10.1007/s00406-016-0697-2. [DOI] [PubMed] [Google Scholar]
- 12.Austin S.F., Lysaker P.H., Jansen J.E., et al. Metacognitive capacity and negative symptoms in first episode psychosis: evidence of a prospective relationship over a 3-year follow-up. J. Exp. Psychopathol. 2019;10 [Google Scholar]
- 13.Penadés R., Catalán R., Puig O., et al. Executive function needs to be targeted to improve social functioning with Cognitive Remediation Therapy (CRT) in schizophrenia. Psychiatr. Res. 2010;177:41–45. doi: 10.1016/j.psychres.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Firth J., Stubbs B., Rosenbaum S., et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 2017;43:546–556. doi: 10.1093/schbul/sbw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng H., Adams C.E. Traditional Chinese medicine for schizophrenia: a survey of randomized trials. Asia Pac. Psychiatr. 2017;9 doi: 10.1111/appy.12265. [DOI] [PubMed] [Google Scholar]
- 16.Zheng W., Li Q., Lin J., et al. Tai chi for schizophrenia: a systematic review. Shanghai Arch. Psychiatr. 2016;28:185–194. doi: 10.11919/j.issn.1002-0829.216051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Kan L., Tang X., et al. Effects of Tai Chi on negative symptoms and activity participation in patients with schizophrenia: a meta-analysis. Chin. J. Evidence-Based Med. 2017;17:206–212. Chinese. [Google Scholar]
- 18.Ho R.T.H., Fong T.C.T., Wan A.H.Y., et al. A randomized controlled trial on the psychophysiological effects of physical exercise and Tai-chi in patients with chronic schizophrenia. Schizophr. Res. 2016;171:42–49. doi: 10.1016/j.schres.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Ho R.T.H., Au Yeung FSW., Lo P.H.Y., et al. Tai-chi for residential patients with schizophrenia on movement coordination, negative symptoms, and functioning: a pilot randomized controlled trial. Evid. base Compl.Alt. 2012;2012:1–10. doi: 10.1155/2012/923925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M., Fang J., Gao Y., et al. Baduanjin mind-body exercise improves logical memory in long-term hospitalized patients with schizophrenia: a randomized controlled trial. Asian J. Psychiatr. 2020;51 doi: 10.1016/j.ajp.2020.102046. [DOI] [PubMed] [Google Scholar]
- 21.Guan F., Zheng H., Cheng N. Evaluation of application effect of Baduanjin exercise in patients with chronic schizophrenia. Chin. Youjiang Med. J. 2017;45:716–719. Chinese. [Google Scholar]
- 22.Du M., Leng H., Zhang G., et al. Study on the application effect of baduanjin in the patients with schizophrenia and influence for life quality. Chin. Foreign Med. Res. 2020;18:167–169. Chinese. [Google Scholar]
- 23.Gao D., Liu Y., Zhang Z., et al. Effect of Baduanjin exercise on mental rehabilitation of patients with schizophrenia. Med. J. Chin. People's Armed Police Force. 2011;22:1061–1063. Chinese. [Google Scholar]
- 24.Gao H., Luo C., Tu S.J., et al. The effect of yijinjing on the cognitive function of patients with chronic schizophrenia. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.739364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabe M., Sentissi O., Kaiser S. Meditation-based mind-body therapies for negative symptoms of schizophrenia: systematic review of randomized controlled trials and meta-analysis. Schizophr. Res. 2019;212:15–25. doi: 10.1016/j.schres.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Vogel J.S., van der Gaag M., Slofstra C., et al. The effect of mind-body and aerobic exercise on negative symptoms in schizophrenia: a meta-analysis. Psychiatr. Res. 2019;279:295–305. doi: 10.1016/j.psychres.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q. Observation on rehabilitation effect of Taiji Quan therapy for patients with schizophrenia. J. Nurs. Rehabil. 2011;10:97–99. Chinese. [Google Scholar]
- 28.Kumari S., Malik M., Florival C., et al. An assessment of five (PANSS, SAPS, SANS, NSA-16, CGI-SCH) commonly used symptoms rating scales in schizophrenia and comparison to newer scales (CAINS, BNSS) J. Addiction Res. Ther. 2017;8 doi: 10.4172/2155-6105.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randolph C., Tierney M.C., Mohr E., et al. The repeatable Battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino K.Y., Takeuchi S., Jodo E., et al. Tripartite relationship among P300, clinical features and brain structure in neuroleptic-naive schizophrenia. Psychiatr. Clin. Neurosci. 2005;59:410–417. doi: 10.1111/j.1440-1819.2005.01393.x. [DOI] [PubMed] [Google Scholar]
- 31.Wu S., Wang H., Chen C., et al. Task performance modulates functional connectivity involving the dorsolateral prefrontal cortex in patients with schizophrenia. Front. Psychol. 2017;8:56. doi: 10.3389/fpsyg.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C., He Y. Introduction of the 36-item Short form health survey (SF-36) J. Int. Psychiatr. 2002:116–119. Chinese. [Google Scholar]
- 33.Harvey P.D., Davidson M., Mueser K.T., et al. Social-Adaptive Functioning Evaluation (SAFE): a rating scale for geriatric psychiatric patients. Schizophr. Bull. 1997;23:131–145. doi: 10.1093/schbul/23.1.131. [DOI] [PubMed] [Google Scholar]
- 34.Yao G., Qian Y., Geng T. Reliability and validity of social-adaptive functioning evaluation in China. Chin. Ment. Health J. 2011;25:200–204. Chinese. [Google Scholar]
- 35.Alptekin K., Erkoç S., Göğüş A.K., et al. Disability in schizophrenia: clinical correlates and prediction over 1-year follow-up. Psychiatr. Res. 2005;135:103–111. doi: 10.1016/j.psychres.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Kimhy D., Tay C., Vakhrusheva J., et al. Enhancement of aerobic fitness improves social functioning in individuals with schizophrenia. Eur. Arch. Psychiatr. Clin. Neurosci. 2021;271:367–376. doi: 10.1007/s00406-020-01220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girdler S.J., Confino J.E., Woesner M.E. Exercise as a treatment for schizophrenia: a review. Psychopharmacol. Bull. 2019;49:56–69. doi: 10.64719/pb.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen H., Zhang L., Li Y., et al. Mindfulness-based intervention improves residual negative symptoms and cognitive impairment in schizophrenia: a randomized controlled follow-up study. Psychol. Med. 2021:1–10. doi: 10.1017/S0033291721002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vancampfort D., Stubbs B., Van Damme T., et al. The efficacy of meditation-based mind-body interventions for mental disorders: a meta-review of 17 meta-analyses of randomized controlled trials. J. Psychiatr. Res. 2021;134:181–191. doi: 10.1016/j.jpsychires.2020.12.048. [DOI] [PubMed] [Google Scholar]
- 40.Yin D., Jia H. Discussion on academic thought of JIA Hong-xiao on the syndrome differentiation and treatment of mental diseases based on the psychiatric symptoms and ‘Wu shen zang’ of traditional Chinese medicine. Chin. J. Tradit. Chin. Med. Pharm. 2017;4 Chinese. [Google Scholar]
- 41.Yin D., Jia H. Discussion on the function of building a new method of syndrome differentiation and treatment by thinking of imagine—the function of syndrome differentiation and treatment by Wu shen zang. Mod. Tradit. Chin. Med. Mater. Med. World J. Sci. Technol. 2018;20:863–868. Chinese. [Google Scholar]
- 42.Zheng W., Jiang W., Zhang X., et al. Use of the RBANS to evaluate cognition in patients with schizophrenia and metabolic syndrome: a meta-analysis of case-control studies. Psychiatr. Q. (N. Y.) 2021 doi: 10.1007/s11126-021-09889-9. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Y., Wu W., Wang J., et al. Reliability and validity of the repeatable Battery for the assessment of neuropsychological status in community-dwelling elderly. Arch. Med. Sci. 2011;7:850–857. doi: 10.5114/aoms.2011.25561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dejanović M., Ivetić V., Nestorović V., et al. The value of P300 event related potentials in the assessment of cognitive function in subclinical hypothyroidism. Minerva Endocrinol. 2017;42:15–23. doi: 10.23736/S0391-1977.16.02327-0. [DOI] [PubMed] [Google Scholar]
- 45.Jaeggi S.M., Buschkuehl M., Shah P., et al. The role of individual differences in cognitive training and transfer. Mem. Cognit. 2014;42:464–480. doi: 10.3758/s13421-013-0364-z. [DOI] [PubMed] [Google Scholar]
- 46.Lett T.A., Voineskos A.N., Kennedy J.L., et al. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol. Psychiatr. 2014;75:361–370. doi: 10.1016/j.biopsych.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 47.Nikolin S., Tan Y.Y., Schwaab A., et al. An investigation of working memory deficits in depression using the n-back task: a systematic review and meta-analysis. J. Affect. Disord. 2021;284:1–8. doi: 10.1016/j.jad.2021.01.084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.