Summary
Background
COVID-19 and antimicrobial resistance (AMR) are two intersecting public health crises. Antimicrobial overuse in patients with COVID-19 threatens to worsen AMR. Guidelines are fundamental in encouraging antimicrobial stewardship. We sought to assess the quality of antibiotic prescribing guidelines and recommendations in the context of COVID-19, and whether they incorporate principles of antimicrobial stewardship.
Methods
We performed a systematic survey which included a search using the concepts “antibiotic/antimicrobial” up to November 15, 2022 of the eCOVID-19 living map of recommendations (RecMap) which aggregates guidelines across a range of international sources and all languages. Guidelines providing explicit recommendations regarding antibacterial use in COVID-19 were eligible for inclusion. Guideline and recommendation quality were assessed using the AGREE II and AGREE-REX instruments, respectively. We extracted guideline characteristics including panel representation and the presence or absence of explicit statements related to antimicrobial stewardship (i.e., judicious antibiotic use, antimicrobial resistance or adverse effects as a consequence of antibiotic use). We used logistic regression to evaluate the relationship between guideline characteristics including quality and incorporation of antimicrobial stewardship principles. Protocol registration (OSF): https://osf.io/4pgtc.
Findings
Twenty-eight guidelines with 63 antibiotic prescribing recommendations were included. Recommendations focused on antibiotic initiation (n = 52, 83%) and less commonly antibiotic selection (n = 13, 21%), and duration of therapy (n = 15, 24%). Guideline and recommendation quality varied widely. Twenty (71%) guidelines incorporated at least one concept relating to antimicrobial stewardship. Including infectious diseases expertise on the guideline panel (OR 9.44, 97.5% CI: 1.09–81.59) and AGREE-REX score (OR 3.26, 97.5% CI: 1.14–9.31 per 10% increase in overall score) were associated with a higher odds of guidelines addressing antimicrobial stewardship.
Interpretation
There is an opportunity to improve antibiotic prescribing guidelines in terms of both quality and incorporation of antimicrobial stewardship principles. These findings can help guideline developers better address antibiotic stewardship in future recommendations beyond COVID-19.
Funding
This project was funded by Michael G. DeGroote Cochrane Canada and McMaster GRADE centres.
Keywords: COVID-19, Practice guidelines, Antibiotic prescribing, Antimicrobial stewardship, Antimicrobial resistance
Research in context.
Evidence before this study
Guideline recommendation quality and incorporation of antimicrobial stewardship principles may help support more optimal antimicrobial prescribing. We searched the eCOVID-19 living map of recommendations for practice guidelines addressing antibiotic prescribing in COVID-19 up to November 15, 2022. Guidelines were screened by two reviewers and guideline characteristics were extracted. Guideline and recommendation quality were assessed using AGREE II and AGREE REX instruments. Twenty-eight guidelines with 63 antibiotic prescribing recommendations were included.
Added value of this study
Our systematic survey of antibiotic prescribing recommendations in COVID-19 identified a wide range in guideline and recommendation quality. There was inconsistency in the extent to which guidelines incorporated antimicrobial stewardship principles (i.e., judicious prescribing, risk of AMR, risk of adverse events), with 71% of guidelines addressing at least one of these concepts and only 14% addressing all three. We found that higher guideline and recommendation quality score was associated with greater odds of incorporating antimicrobial stewardship considerations for a number of guideline (rigor of development, clarity of presentation) and recommendation (evidence, applicability, and purpose) quality domains. Further, including an infectious diseases expert and pharmacist on the guideline panel was also associated with a greater odds of incorporating considerations related to antimicrobial stewardship and resistance.
Implications of all the available evidence
These findings can help guideline developers better address antibiotic stewardship in future recommendations beyond COVID-19.
Introduction
COVID-19 and antimicrobial resistance (AMR) are two intersecting public health emergencies.1,2 With over 6 million cumulative reported deaths and over 700 million reported cases as of September 2023, the COVID-19 pandemic has overwhelmed public health and healthcare systems.3 While recent public health efforts have largely focused on mitigating the impact of COVID-19 on human health, another more insidious pandemic threatens similar societal harm.
Bacterial AMR is directly attributable to an estimated 1.27 million deaths each year, making it one of the leading causes of global mortality.2 Despite the viral aetiology and low bacterial co-infection rate in COVID-19,4,5 the proportion of patients with COVID-19 receiving an antibiotic may be as high as 75%6 which may further contribute to the threat of antimicrobial resistance. Antibiotic use during the pandemic was highly heterogeneous across settings, risking exacerbating global disparities in the prevalence of AMR. Antibiotic overuse is common due to initial uncertainty in aetiology while awaiting test results and concerns about possible co-infection even once SARS-CoV-2 is identified.7 Further, efforts to address COVID-19 have disrupted antimicrobial stewardship programs due to redeployed staffing and competing tasks such as addressing drug shortages, acquiring therapeutic agents, and developing COVID-19 guidelines.8 A recent systematic review performed by our team indicates that as many as 60% of patients that have bacterial infections and COVID-19 harbour an antibiotic resistant organism9 and data evaluating AMR during the pandemic compared to pre-COVID-19 have shown an increase in AMR in a number of microorganism species.10,11
Clinical practice guidelines are a key antimicrobial stewardship strategy and play an important role in supporting appropriate antibiotic prescribing, yet wide variability in practice patterns persist.6 Guideline recommendation quality, consistency, and incorporation of antimicrobial stewardship principles may help support more optimal prescribing. We aimed to assess the quality of antibiotic prescribing guidelines and recommendations in the context of COVID-19, and whether these guidelines incorporate principles of antimicrobial stewardship.
Methods
Study design
The methodology follows recommendations from the methodological guide for systematic reviews of clinical practice guidelines by Johnston et al.12 The protocol was registered on Open Science Framework (OSF): https://osf.io/4pgtc.
Eligibility
Guidelines providing explicit recommendations regarding antibacterial use for prevention or management of infection related to COVID-19 from any jurisdiction, in any language, were eligible for inclusion.
Data source
We searched the eCOVID-19 living map of recommendations (RecMap) for eligible guidelines. COVID-19 RecMap aggregates globally published clinical, public health, and health policy guidelines to support contextualised decision making.13,14
Search strategy
COVID-19 RecMap was searched using the terms ‘antibiotic’, ‘antibacterial’, ‘antimicrobial’, and ‘anti-infective’ as well as additional search for specific antibacterial agents (i.e., azithromycin, doxycycline) that may be have been prescribed for patients with COVID-19. Searches were performed from August 24 2022 to November 15 2022.
Guideline selection process
Screening was performed by two independent reviewers (BL and VL) to assess guidelines for eligibility. The full-text and any supplementary/accompanying material for each search result was screened by each reviewer.
Data collection process
One independent reviewer (BL) extracted data from eligible guidelines which was checked independently by a second reviewer (VL). A spreadsheet was used for data extraction which was piloted and refined based on initial extraction of eligible guidelines. Data extracted included guideline name, date, location, organisation, panel composition, patient population, and aspect of antibiotic prescribing addressed. Aspects of antibiotic prescribing included a) antibiotic initiation (e.g., empiric use of antibiotics, diagnostic recommendations, timeliness of initiation), b) antibiotic selection (e.g., empiric choice of agent based on local resistance rates or targeted based on culture and susceptibility results, use of institutional or local protocols to support, and c) duration of therapy (e.g., fixed duration of therapy or tailored to diagnostic and microbiological findings).
Outcome
We evaluated whether guidelines incorporated at least one element of antimicrobial stewardship, defined as either a) statement regarding careful/judicious antibiotic use, b) statement regarding risk of contributing to antimicrobial resistance, and/or c) statement on adverse effects associated with antibiotic use.
Quality appraisal
Two instruments were used to evaluate the quality of included guidelines: AGREE II (to assess quality at the guideline level) and AGREE-REX (to assess quality at the recommendation level). The Appraisal of Guidelines for REsearch & Evaluation (AGREE II) Instrument aims to assess guideline quality in terms of methodological rigour and transparency.15 Three main goals of AGREE II include to 1) assess guideline quality, 2) provide methodological outline for guideline development and, 3) inform which information should be included in guidelines and how it should be reported. Each guideline in RecMap has previously been appraised across 6 items by two reviewers using the online AGREE II tool and this score was incorporated into this study and was not re-scored for this study. The AGREE-REX (Recommendation EXcellence) is a newly developed tool to accompany AGREE II aimed at assessing the quality of guideline recommendations across three domains: 1) credibility, 2) values and preferences, and 3) implementibility.16 The tool includes 9 items and considers the target users of the guideline, context in which it will be implemented, the patient population, and any other relevant stakeholders. Three independent reviewers appraised eligible antibiotic prescribing recommendations using the AGREE-REX tool following recommendations from the AGREE REX checklist.17 Multiple recommendations for the same guideline were graded with a single score. The scoring team deemed items 4 (Values and Preferences of Target Users), 5 (Values and Preferences of Patient/Population), and 9 (Local Application and Adoption) to be less relevant to the use of antibiotics in COVID-19 and as such guideline recommendations may have intentionally not addressed these aspects thoroughly. We agreed to modify the AGREE-REX tool so that items 4, 5 and 9 were scored at a minimum of ‘neutral’ (4 out of 7).
Statistical analysis
A descriptive approach was used to illustrate the characteristics of antibiotic recommendations in the context of COVID-19. Guidelines were categorised as low quality if they score <60% in two or more AGREE II domains and/or <50% in domain 3 (rigor of development), moderate quality if they score ≥60% in 3 domains except domain 3, high quality if they score ≥60% in at least 3 domains including domain 3.15,18 Recommendations were categorised as high quality if the overall AGREE-REX score was above 70%, moderate quality if 30–70%, and low quality if less than 30%.17
To evaluate the association between guideline/recommendation characteristics (e.g., guideline year, incorporation of expertise on guideline panel, and AGREE II and AGREE-REX scores per 10% increase) and inclusion of at least one antimicrobial stewardship concept, we performed univariable generalised linear model (GLM) logistic regression to estimate odds ratio (OR) and 97.5% confidence interval. Analyses were carried out in using R for statistics (Vienna, Austria) version 4.2.2.
Ethics
As this study is a systematic review, ethical review was not required.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, interpretation, or writing of the report. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Results
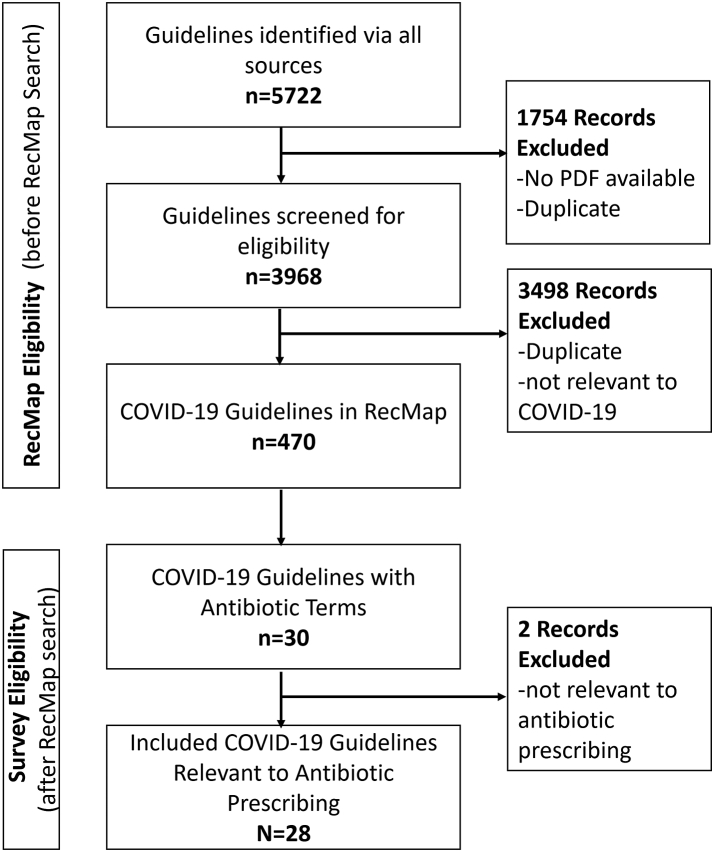
Of 470 guidelines in the eCOVID19 RecMap database as of November 2022, 30 were screened and 28 guidelines with 63 recommendations were eligible for inclusion (Fig. 1).19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46
Fig. 1.
PRISMA flow diagram.
Characteristics of guidelines
The most common regions/countries represented were global (n = 5, 18%), Europe (n = 3, 11%), United States (n = 3, 11%), Americas (n = 2, 7%), Brazil (n = 2, 7%), and one guideline (n = 1, 4%) from each of the following: Australia; Canada (Ontario); China; Czech Republic; France; Germany; Italy; Mexico; Pakistan; Portugal; Spain; The Netherlands; and United Kingdom. Including international guidelines, low-and middle-income countries were represented in 15 guidelines (54%). Years of publication were 2020 (n = 7, 25%), 2021 (n = 9, 32%), and 2022 (n = 12, 43%).
Of the 28 eligible guidelines, 20 (71%) indicated there was infectious diseases specialist representation, 9 (32%) stated that there was public health expertise on the panel, and 9 (32%) indicated a pharmacist representative participated on the panel (Supplementary Table S1).
Guidelines exhibited a wide range in quality as shown by their AGREE II scores. According to the scoring criteria listed above, most guidelines were categorised as low quality (n = 14, 50%), followed by high quality (n = 12, 43%), and moderate quality (n = 2, 7%) (Supplementary Table S2).
Characteristics of recommendations
Of the 63 recommendations that pertain to antibiotic use, 49 (78%) focused on general antibiotic use, whereas 14 (22%) specifically addressed azithromycin use in the context of COVID-19. Healthcare setting varied across recommendations; all patients (n = 22, 35%); hospitalised (n = 19, 30%); ICU only (n = 13, 21%); outpatient (n = 5, 8%); inpatient non-ICU (n = 3, 5%), and outpatient and inpatient non-ICU (n = 1, 2%). Guidelines focused on adult patients (n = 40, 63%), both adults and children or not specified (n = 22, 35%), or children only (n = 1, 2%).
Recommendations were most likely to address antibiotic initiation (n = 52, 83%) and less commonly antibiotic selection (n = 13, 21%), and duration of therapy (n = 15, 24%). The full list of guidelines and recommendations is available in Supplementary Table S1.
Of recommendations focusing on empiric antibiotic use in COVID-19, 6/43 (14%) suggest empiric antibiotic therapy regardless of evidence of bacterial infection. All but one of these recommendations apply to critically ill patients with COVID-19. A single guideline recommended “usual antimicrobial treatment” for pneumonia for all patients with COVID-19 (e.g., with and without radiographic abnormalities, in all levels of severity). Five of the above six recommendations suggest bacteriological microbiological sampling in order to rule out co-infection. Similarly, four of the six guidelines recommend de-escalating or discontinuing antimicrobial on the basis of additional (e.g., microbiological) findings.
Certainty of evidence for recommendations pertaining to all antibiotics was generally very low (n = 11), followed by low (n = 4) or moderate (n = 3). Recommendation strength for those pertaining to all antibiotics was split between strong (n = 14) and conditional (n = 12). However for recommendations pertaining to azithromycin certainty of evidence ranged from high (n = 3), moderate (n = 4), low (n = 3), to very low (n = 3). The strength of recommendation was higher for azithromycin than those focusing on general antibiotic prescribing, with 9 recommendations considered strong and 3 recommendations conditional.
Similar to AGREE II score, there was a wide range in AGREE-REX score between guidelines across items, domains, and overall. Based on the overall AGREE-REX score, most guidelines recommendations were deemed moderate quality (n = 19, 70%), followed by high quality (n = 7, 26%), whereas only one guideline met the criteria for classification as low quality recommendations (n = 1, 4%) Supplementary Table S2.
Guideline incorporation of antimicrobial stewardship concepts
Of the 28 guidelines, twenty (71%) incorporated at least one antimicrobial stewardship concept. Ten (36%) contained explicit statements regarding judicious antibiotic use (i.e., regarding the need for appropriate, careful prescribing, or explicitly mentioning antimicrobial stewardship). Twelve (43%) incorporated explicit statements on antimicrobial resistance as a potential outcome (e.g., indiscriminate of antibiotics drive selective pressure for antimicrobial resistance). Fifteen (54%) mentioned other harms of antibiotics as part of the guideline (e.g., adverse effects, C. difficile infection). Four (14%) guidelines incorporated all three concepts, explicitly mentioning antimicrobial stewardship, antimicrobial resistance, and other antimicrobial-associated harms (Panel 1 for examples).
Panel 1. Example guideline statements addressing antibiotic stewardship in the setting of COVID-19.
| Concept addressed | Example statement |
|---|---|
| Judicious prescribing | “… Based on the currently available evidence and antibiotic stewardship principles, the committee recommends restrictive use of antibacterial drugs in patients with community-acquired respiratory infection and proven or high likelihood of COVID-19.” Guideline 15 |
| Antimicrobial resistance | “..indiscriminate and perilous use of antibiotics in many patients with COVID-19 without bacterial infections…increasing the selective pressure for antimicrobial resistance both in patients and in the environment.” Guideline 1 |
| Other antimicrobial harms | “Adverse events and secondary infections were slightly increased in groups treated with azithromycin compared to placebo...” Guideline 19 “… this recommendation is intended to mitigate the unintended consequences of side effects and resistance.” Guideline 7 |
We found several notable predictors of whether guidelines addressed antimicrobial stewardship considerations. Panel representation was associated with a higher odds of incorporating antimicrobial stewardship in the guideline (infectious diseases expert OR 9.44, 97.5% CI: 1.09–81.59; public health expert OR 4.67, 97.5% CI: 0.34–63.17; pharmacist OR >1000, 97.5% CI: 0 to infinity). Higher guideline quality overall was associated with an OR of 8.56 (97.5% CI: 0.64–115.11) for incorporating antimicrobial stewardship considerations. Specific domains of AGREE II score were associated a statistically significantly higher odds of including antimicrobial stewardship considerations in the guidelines. Most notably, guidelines with higher AGREE II scores for domain 4 (Clarity of Presentation OR 3.45, 97.5% CI: 1.15–10.39) and domain 3 (Rigor of Development OR 1.67, 97.5% CI: 1.02–2.72) had a higher odds of addressing antimicrobial stewardship. The odds of incorporating antimicrobial stewardship principles for each year of the pandemic compared to the 2020 was as follows: 2021 OR 1.50 (97.5% CI: 0.16–15.46) and 2022 OR 3.75 (97.5% CI: 0.33–42.95) (Table 1).
Table 1.
Association between guideline characteristics and incorporation of antimicrobial stewardship considerations (univariate model).
| Characteristic | Addresses Antimicrobial Stewardship (n = 20) | Does not address Antimicrobial Stewardship (n = 8) | Odds ratio for addressing Antimicrobial Stewardship | 97.5% confidence interval |
|---|---|---|---|---|
| Guideline-level characteristics | ||||
| Year of publication | ||||
| 2020 | 4 | 3 | Reference | – |
| 2021 | 6 | 3 | 1.50 | 0.15–15.46 |
| 2022 | 10 | 2 | 3.75 | 0.33–42.95 |
| Country Income | ||||
| HIC | 18 | 5 | Reference | – |
| LMIC | 2 | 3 | 0.19 | 0.02–1.92 |
| ID expert on panel | ||||
| No | 3 | 5 | Reference | – |
| Yes | 17 | 3 | 9.44 | 1.09–81.59 |
| Public health expert on panel | ||||
| No | 12 | 7 | Reference | |
| Yes | 8 | 1 | 4.67 | 0.34–63.17 |
| Pharmacy expert on panel | ||||
| No | 11 | 8 | Reference | – |
| Yes | 9 | 0 | >1000 | 0 to infinity |
| Any expert on panel | ||||
| No | 3 | 5 | Reference | |
| Yes | 17 | 3 | 9.44 | 1.09–81.59 |
| AGREE II score (Guideline Level) | ||||
| 1. Scope and purpose | Per 10% increase | 1.76 | 0.73–4.29 | |
| 2. Stakeholder involvement | 1.53 | 0.91–2.57 | ||
| 3. Rigor of development | 1.67 | 1.02–2.72 | ||
| 4. Clarity of presentation | 3.45 | 1.15–10.39 | ||
| 5. Applicability | 2.14 | 0.73–6.33 | ||
| 6. Editorial independence | 1.01 | 0.71–1.44 | ||
| AGREE II quality | ||||
| Low or moderate | 9 | 7 | Reference | – |
| High | 11 | 1 | 8.56 | 0.64–115.11 |
| AGREE REX score (Recommendation Level) | ||||
| 1. Evidence | Per 10% increase | 1.51 | 1.01–2.25 | |
| 2. Applicability to target users | 2.25 | 1.08–4.69 | ||
| 3. Applicability to patients/populations | 2.16 | 1.01–4.65 | ||
| 4. Values of users | 10.82 | 0.96–122.52 | ||
| 5. Values of patients | >1000 | 0 to infinity | ||
| 6. Values of policy makers | 4.08 | 1.25–13.28 | ||
| 7. Values of guideline developers | 1.90 | 1.01–3.57 | ||
| 8. Purpose | 1.82 | 0.93–3.59 | ||
| 9. Local adaptation and adoption | 6.37 | 0.75–54.00 | ||
| Overall score | 3.26 | 1.14–9.31 | ||
| AGREE REX quality | ||||
| Low or moderate | 12 | 8 | Reference | – |
| High | 8 | 0 | >1000 | 0 to infinity |
At the guideline recommendation level, recommendation quality was associated with incorporation stewardship considerations across a number of AGREE-REX domains. Recommendations scoring higher in the domain 1. Evidence, 2. Applicability to target users, 3. Applicability to patients and populations, 6. Values and preferences of policy makers, 7. Values and preferences of guideline developers, were all associated with a higher odds of addressing antimicrobial stewardship. Similarly, overall AGREE-REX score was associated with a higher odds of addressing antimicrobial stewardship (OR 3.26, 97.5% CI: 1.14–9.31, per 10% increase in score) (Table 1).
Discussion
Our systematic survey of antibiotic prescribing recommendations in COVID-19 identified a wide range in guideline and recommendation quality. There was inconsistency in the extent to which guidelines incorporated antimicrobial stewardship considerations (i.e., judicious prescribing, risk of AMR, risk of adverse events), with 71% of guidelines addressing at least one of these concepts and only 14% addressing all three. We found that higher guideline and recommendation quality score is associated with greater odds of incorporating antimicrobial stewardship considerations for a number of guideline (rigor of development, clarity of presentation) and recommendation (evidence, applicability, and purpose) quality domains. Further, including an infectious diseases expert and pharmacist on the guideline panel was also associated with a greater odds of incorporating considerations related to antimicrobial stewardship and resistance.
Our survey found consistency in recommendations to avoid empiric antibiotic prescribing in most outpatient and non-critically ill inpatient COVID-19 populations who do not exhibit signs or symptoms of bacterial infection. However, some guidelines suggested empiric antibiotic use in critically ill patients given their severity of illness and the urgency for immediate therapy prior to identifying infectious etiology. This suggests guideline authors have taken into account patient severity in the risk-benefit assessment for empiric antibiotic therapy. However high antibiotic use reported in patients with COVID-19 who are not critically ill and those in community settings, suggests that further emphasis of the potential harms of antibiotic use in guideline recommendations may be needed, particularly for less sick patients.
Our findings echo those of a previous systematic survey evaluating the extent to which non-COVID infectious disease guidelines, specifically tuberculosis, gonorrhea, and respiratory tract infections, consider antimicrobial resistance. The authors identified that only 35% of guideline recommendations considered AMR as an untoward outcome of antimicrobial therapy.47 While our systematic survey focused on guidelines as a whole, rather than individual recommendations, we found a similar, albeit slightly higher, prevalence of 46% of guidelines addressing AMR as an outcome. Both systematic surveys also found a wide range in AGREE II score with a substantial proportion of low quality guidelines, suggesting there is an opportunity to improve the rigor and robustness of antimicrobial prescribing recommendations.
Strengths of this systematic survey include our use of COVID-19 RecMap as a pre-existing tool to streamline the guideline and recommendation identification process. Some key limitations exist. Data regarding COVID-19 management were rapidly evolving compared with other more established conditions (e.g., influenza), as such it should be expected that quality and certainty of evidence will also evolve over time. For example, the prevalence of concomitant bacterial infection in COVID-19 may have changed with loosening non-pharmaceutical public health interventions.48 The rapidly changing nature of COVID-19 may limit the generalisability of these findings to other infectious diseases, but the importance of antimicrobial stewardship considerations likely holds true regardless of the novelty of the disease. While important in principle, it is not yet clear if addressing antimicrobial stewardship and antimicrobial resistance as part of a guideline recommendation influences prescribing practice. While clinical practice guidelines are perceived as a foundational aspect of antimicrobial stewardship efforts,49 and their implementation is associated with more appropriate prescribing,50 there is a lack of data on whether the structure and language of such recommendations can influence practice. Similarly, the link between guideline and recommendation quality and impact on clinical practice is not well established.
This systematic survey identifies important considerations that apply to infectious diseases guidelines more generally beyond COVID-19. There is an opportunity for guidelines to further emphasise the potential risks of antibiotic harms to provide more balanced recommendations. Inclusion of infectious diseases experts (e.g., infectious diseases physicians, antimicrobial stewardship pharmacists) on guideline panels may help to encourage such statements. From an implementation science perspective, however, the mere existence of high quality guidelines incorporating antimicrobial stewardship principles may not necessarily lead to improved antimicrobial prescribing. Efforts should also be made to ensure recommendations are easy to use and incorporated into the day to day practice and workflow of prescribers.
Based on the findings of the study, we developed a brief checklist of 15 considerations to help increase the quality of antimicrobial prescribing recommendations (Fig. 2). This checklist is adapted from previous work to provide a comprehensive list of 146 items to facilitate high quality guideline development and implementation.51
Fig. 2.
Checklist for guidelines relating to antimicrobial prescribing and/or antimicrobial stewardship∗. ∗adapted from: Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, Ventresca M, Brignardello-Petersen R, Laisaar KT, Kowalski S, Baldeh T. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014; 186 (3):E123-42.
There is an opportunity to improve antibiotic prescribing guidelines in terms of both quality and incorporation of antimicrobial stewardship principles. These findings provide considerations for the development of future antibiotic prescribing recommendations beyond COVID-19.
Contributors
Bradley J. Langford and Valerie Leung contributed to study conceptualisation, protocol development, screening, extraction, scoring, analysis and interpretation, and manuscript writing and editing. Jennifer Lo contributed to screening, extraction, scoring, analysis and interpretation, and manuscript writing and editing. Elie Akl, Robby Nieuwlaat, Valerie Leung, Nick Daneman, Kevin L. Schwartz, Kevin A. Brown contributed to study conceptualisation, protocol development, analysis and interpretation, and manuscript writing and editing. Tamara Lotfi contributed to data curation, analysis and interpretation and manuscript writing and editing. Holger J. Schunemann contributed to study conceptualisation, protocol development, analysis and interpretation, and manuscript writing and editing and supervision. Valerie Leung has verified the underlying data. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Data sharing statement
Data collection form and extracted data can be made available upon request to the authors.
Declaration of interests
The authors have no relevant conflicts of interest to declare.
Acknowledgements
This project was funded by Michael G. DeGroote Cochrane Canada and McMaster GRADE centres. COVID-19 RecMap was funded by the Canadian Institutes of Health Research (CIHR). The authors wish to thank the eCOVID19 RecMap team.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102257.
Appendix A. Supplementary data
References
- 1.Nieuwlaat R., Mbuagbaw L., Mertz D., et al. Coronavirus disease 2019 and antimicrobial resistance: parallel and interacting health emergencies. Clin Infect Dis. 2021;72:1657–1659. doi: 10.1093/cid/ciaa773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray C.J., Ikuta K.S., Sharara F., et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399 doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Our World In Data . world; 2023. Cumulative confirmed COVID-19 cases and deaths.https://ourworldindata.org/grapher/cumulative-deaths-and-cases-covid-19 [Google Scholar]
- 4.Langford B.J., So M., Leung V., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a rapid living review: 2021 update with meta-regression. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021241098
- 5.Langford B.J., So M., Leung V., et al. Predictors and microbiology of respiratory and bloodstream bacterial infection in patients with COVID-19: living rapid review update and meta-regression. Clin Microbiol Infection. 2021;28 doi: 10.1016/j.cmi.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langford B.J., So M., Raybardhan S., et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infection. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huttner B.D., Catho G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don't neglect antimicrobial stewardship principles! Clin Microbiol Infection. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung V., Quirk J., Muir S., Daneman N., Schwartz K.L., Langford B.J. A cross-sectional study of hospital antimicrobial stewardship programmes in the COVID-19 era. JAC Antimicrob Resist. 2022;5:dlac134. doi: 10.1093/jacamr/dlac134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langford B.J., So M., Simeonova M., et al. Antimicrobial resistance in patients with COVID-19: a systematic review and meta-analysis. Lancet Microbe. 2023;4:e179–e191. doi: 10.1016/S2666-5247(22)00355-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford B.J., Soucy J.-P.R., Leung V., et al. Antibiotic resistance associated with the COVID-19 pandemic: a systematic review and meta-analysis. Clin Microbiol Infection. 2022;29 doi: 10.1016/j.cmi.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Centers for Disease Control and Prevention . National Center for Emerging and Zoonotic Infectious Diseases; 2022. COVID-19: U.S. Impact on antimicrobial resistance, special Report 2022. [DOI] [Google Scholar]
- 12.Johnston A., Kelly S.E., Hsieh S.-C., Skidmore B., Wells G.A. Systematic reviews of clinical practice guidelines: a methodological guide. J Clin Epidemiol. 2019;108:64–76. doi: 10.1016/j.jclinepi.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 13.COVID19 recommendations and gateway to contextualization. 2022. https://covid19.recmap.org/ [Google Scholar]
- 14.Lotfi T., Stevens A., Akl E.A., et al. Getting trustworthy guidelines into the hands of decision-makers and supporting their consideration of contextual factors for implementation globally: recommendation mapping of COVID-19 guidelines. J Clin Epidemiol. 2021;135:182–186. doi: 10.1016/j.jclinepi.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The AGREE Enterprise. AGREE: advancing the science of practice guidelines. 2022. https://www.agreetrust.org/ [Google Scholar]
- 16.Brouwers M.C., Spithoff K., Kerkvliet K., et al. Development and validation of a tool to assess the quality of clinical practice guideline recommendations. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appraisal of Guidelines Research and Evaluation (AGREE) 2019. AGREE-REX: recommendation EXcellence reporting checklist.www.agreetrust.org [Google Scholar]
- 18.Bargeri S., Iannicelli V., Castellini G., Cinquini M., Gianola S. AGREE II appraisals of clinical practice guidelines in rehabilitation showed poor reporting and moderate variability in quality ratings when users apply different cuff-offs: a methodological study. J Clin Epidemiol. 2021;139:222–231. doi: 10.1016/j.jclinepi.2021.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Bassetti M., Giacobbe D.R., Bruzzi P., et al. Clinical management of adult patients with COVID-19 outside intensive care units: guidelines from the Italian society of anti-infective therapy (SITA) and the Italian Society of Pulmonology (SIP) Infect Dis Ther. 2021;10:1837–1885. doi: 10.1007/s40121-021-00487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan American Health Organization . 2021. Guidelines for care of critically ill adult patients with COVID-19 in the Americas. Summary, version 3.https://iris.paho.org/handle/10665.2/53895 [Google Scholar]
- 21.Peyronnet V., Sibiude J., Huissoud C., et al. [Infection with SARS-CoV-2 in pregnancy. Update of Information and proposed care. CNGOF] Gynecol Obstet Fertil Senol. 2020;48:858–870. doi: 10.1016/j.gofs.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu E., Smyth R.L., Luo Z., et al. Rapid advice guidelines for management of children with COVID-19. Ann Transl Med. 2020;8:617. doi: 10.21037/atm-20-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal-Cortés P., Díaz Santos E., Aguilar Alonso E., et al. Recommendations for the management of critically ill patients with COVID-19 in Intensive Care Units. Med Intensiva. 2022;46:81–89. doi: 10.1016/j.medine.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Simone B., Chouillard E., Sartelli M., et al. The management of surgical patients in the emergency setting during COVID-19 pandemic: the WSES position paper. World J Emerg Surg. 2021;16:14. doi: 10.1186/s13017-021-00349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institutes of Health . 2022. COVID-19 treatment guidelines.https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/ [PubMed] [Google Scholar]
- 26.Waghmare A., Abidi M.Z., Boeckh M., et al. Guidelines for COVID-19 management in hematopoietic cell transplantation and cellular therapy recipients. Biol Blood Marrow Transplant. 2020;26:1983–1994. doi: 10.1016/j.bbmt.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendes J.J., Paiva J.A., Gonzalez F., et al. Update of the recommendations of the sociedade portuguesa de Cuidados intensivos and the infection and sepsis group for the approach to COVID-19 in intensive care medicine. Rev Bras Ter Intensiva. 2022;33:487–536. doi: 10.5935/0103-507X.0103-507X-rbti-20210080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadeem S., Aamdani S.S., Ayub B., et al. Development of evidence-based COVID-19 management guidelines for local context: the methodological challenges. Global Health. 2022;2022:1–30. doi: 10.1155/2022/4240378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Álvarez J.L., García-Vigil J.L. Guidelines for clinical management of SARS-CoV-2 infection. GMM. 2021;156:6012. doi: 10.24875/GMM.M21000461. [DOI] [PubMed] [Google Scholar]
- 30.National Institute for Health and Care Excellence . 2022. COVID-19 rapid guideline: managing COVID-19.https://www.nice.org.uk/guidance/ng191/resources/covid19-rapid-guideline-managing-covid19-pdf-51035553326 [PubMed] [Google Scholar]
- 31.Shetty V.U., Brotherton B.J., Achilleos A., et al. Pragmatic recommendations for therapeutics of hospitalized COVID-19 patients in low- and middle-income countries. Am J Trop Med Hyg. 2020;104:48–59. doi: 10.4269/ajtmh.20-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halpin D.M.G., Criner G.J., Papi A., et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203:24–36. doi: 10.1164/rccm.202009-3533SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sieswerda E., de Boer M.G.J., Bonten M.M.J., et al. Recommendations for antibacterial therapy in adults with COVID-19–an evidence based guideline. Clin Microbiol Infect. 2021;27:61–66. doi: 10.1016/j.cmi.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falavigna M., Colpani V., Stein C., et al. Guidelines for the pharmacological treatment of COVID-19. The task force/consensus guideline of the Brazilian association of intensive care medicine, the Brazilian society of infectious diseases and the Brazilian society of pulmonology and tisiology. Rev Bras Ter Intensiva. 2020;32 doi: 10.5935/0103-507X.20200039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ontario COVID-19 Science Advisory Table . 2022. Clinical practice guideline summary: recommended drugs and biologics in adult patients with COVID-19.https://covid19-sciencetable.ca/sciencebrief/clinical-practice-guideline-summary-recommended-drugs-and-biologics-in-adult-patients-with-covid-19-version-11-0/ [Google Scholar]
- 36.Brazilian Association of Emergency Medicine (ABRAMEDE) 2022. Brazilian Medical Association (AMB), Brazilian Society of Angiology and Vascular Surgery (SBACV), Brazilian Society of Geriatrics and Gerontology (SBGG), Brazilian Society of Infectious Diseases (SBI), Brazilian society of Family and Community Medicine (SBFMC), and Brazilian Thoracic Society (SBPT). Brazilian guidelines for the treatment of outpatients with suspected or confirmed COVID-19. A joint guideline of the Brazilian Association of Emergency Medicine (ABRAMEDE), Brazilian Medical Association (AMB), Brazilian Society of Angiology and Vascular Surgery (SBACV), Brazilian Society of Geriatrics and Gerontology (SBGG), Brazilian Society of Infectious Diseases (SBI), Brazilian Society of Family and Community Medicine (SBFMC), and Brazilian Thoracic Society (SBPT)https://www.bjid.org.br/en-brazilian-guidelines-for-treatment-outpatients-articulo-S1413867022000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malin J.J., Spinner C.D., Janssens U., et al. Key summary of German national treatment guidance for hospitalized COVID-19 patients: key pharmacologic recommendations from a national German living guideline using an Evidence to Decision Framework (last updated 17.05.2021) Infection. 2022;50:93–106. doi: 10.1007/s15010-021-01645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartoletti M., Azap O., Barac A., et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infection. 2022;28:222–238. doi: 10.1016/j.cmi.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalmers J.D., Crichton M.L., Goeminne P.C., et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J. 2021;57 doi: 10.1183/13993003.00048-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Australian COVID-19 Clinical Evidence Taskforce . 2022. Australian guidelines for the clinical care of people with COVID-19.https://snlg.iss.it/wp-content/uploads/2020/11/LG-australian-Full-guidelines-COVID.pdf [Google Scholar]
- 41.Pan American Health Organization . 2021. Guidelines for prophylaxis and management of patients with mild and moderate COVID-19 in Latin America and the caribbean.https://iris.paho.org/handle/10665.2/55068 [Google Scholar]
- 42.Alunno A., Najm A., Machado P.M., et al. 2021 update of the EULAR points to consider on the use of immunomodulatory therapies in COVID-19. Ann Rheum Dis. 2022;81:34–40. doi: 10.1136/annrheumdis-2021-221366. [DOI] [PubMed] [Google Scholar]
- 43.Infectious Diseases Society of America . 2022. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19.https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ [Google Scholar]
- 44.Ko H.-K., Yu W.-K., Pan S.-W., et al. Consensus statement and recommendations on the treatment of COVID-19: 2021 update. J Chin Med Assoc. 2021 doi: 10.1097/JCMA.0000000000000617. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 45.Czech Health Research Council (AZV ČR) 2022. Prevention and treatment of COVID-19 [Prevence a léčba COVID-19]https://kdp.uzis.cz/index.php?pg=kdp&id=52 [Google Scholar]
- 46.Clinical management of COVID-19: living guideline. World Health Organization; Geneva: 2022. http://www.ncbi.nlm.nih.gov/books/NBK582435/ [PubMed] [Google Scholar]
- 47.Stalteri Mastrangelo R., Santesso N., Bognanni A., et al. Consideration of antimicrobial resistance and contextual factors in infectious disease guidelines: a systematic survey. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-046097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ankert J., Hagel S., Schwarz C., et al. Streptococcus pneumoniae re-emerges as a cause of community-acquired pneumonia, including frequent co-infection with SARS-CoV-2, in Germany, 2021. ERJ Open Res. 2023;9 doi: 10.1183/23120541.00703-2022. 00703–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kruger S.Z., Bronskill S.E., Jeffs L., Steinberg M., Morris A.M., Bell C.M. Evaluating and prioritizing antimicrobial stewardship programs for nursing homes: a modified Delphi panel. Infect Control Hosp Epidemiol. 2020;41:1028–1034. doi: 10.1017/ice.2020.214. [DOI] [PubMed] [Google Scholar]
- 50.Mol P.G.M., Wieringa J.E., NannanPanday P.V., et al. Improving compliance with hospital antibiotic guidelines: a time-series intervention analysis. J Antimicrob Chemother. 2005;55:550–557. doi: 10.1093/jac/dki037. [DOI] [PubMed] [Google Scholar]
- 51.Schünemann H.J., Wiercioch W., Etxeandia I., et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. Can Med Assoc J. 2014;186:E123–E142. doi: 10.1503/cmaj.131237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.