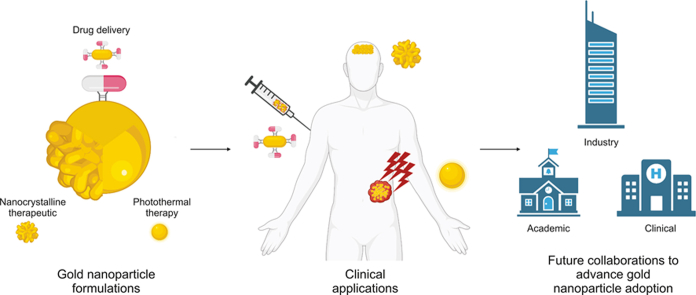
Abstract
Use of gold nanoparticles (GNPs) in medicine is an emerging field of translational research with vast clinical implications and exciting therapeutic potential. However, the safety of using GNPs in human subjects is an important question that remains unanswered. This study reviews over 20 clinical trials focused on GNP safety and aims to summarize all the clinical studies, completed and ongoing, to identify whether GNPs are safe to use in humans as a therapeutic platform. In these studies, GNPs were implemented as drug delivery devices, for photothermal therapy, and utilized for their intrinsic therapeutic effects by various routes of delivery. These studies revealed no major safety concerns with the use of GNPs; however, the number of trials and total patient number remains limited. Multi-dose, multi-center blinded trials are required to deepen our understanding of the use of GNPs in clinical settings to facilitate translation of this novel, multifaceted therapeutic device. Expanding clinical trials will require collaboration between clinicians, scientists, and biotechnology companies.
Keywords: Nanomedicine, Clinical studies, Gold nanoparticles, Safety, Applications
Graphical abstract
Highlights
-
•
Gold nanoparticles (GNPs) are developing in the field of nanomedicine.
-
•
Clinical trials have revealed no major safety concerns with the use of GNPs.
-
•
GNPs have been used as drug delivery systems and in photothermal therapy.
-
•
Gold nanocrystallines are being investigated clinically for therapeutic potential.
-
•
Collaboration among clinician, scientists and industry is essential for translation of GNPs.
1. Introduction
Nanotechnology is a cutting-edge field of research that has opened doors to widespread innovation of diagnostic and therapeutic medicine. Uses of organic nanomaterials such as liposomes [1] and extracellular vesicles [2], as well as inorganic nanomaterials such as gold and silver [3,4] have been explored. Nanotools in medicine provide an accurate, modifiable, versatile, and cost-effective approach to various medical applications. A particular interest developed for gold nanoparticles (GNPs) following the discovery of its remarkable properties which favored its use over other nanoforms [5]. Gold has many advantages over other nanomaterials including ease of synthesis [6,7], tunable size/shape [5,8,9], biocompatibility, low toxicity [10], and surface modifiability [11].
GNPs can be synthesized, using various physical or chemical approaches, into a variety of shapes including nano-spheres, -rods, -shells, and -clusters, which typically range from 1 to 100 nm in size [4]. The unique physical and chemical properties that results from the nanonization of the structures have many medical applications [12]. One unique property of metal nanoparticles is the surface plasmon resonance (SPR) effect which describes the impact of shape, size, surface composition, and temperature on photon excitation and oscillation of valence electrons. The SPR effect enables GNPs to generate an array of different colors and promotes its role in color-based biosensors in diagnostic medicine [13].
The nanonization of GNPs also allows for an extremely high surface area to volume ratio, which affects the degree and location of GNP bioaccumulation, while also increasing the exposure of the nanoparticle and its cargo to target tissue [14]. It is widely believed that nanoparticles accumulate in tumor tissue due to the tumor's characteristically permeable vasculature which allows for passive migration, a process known as the enhanced permeation and retention (EPR) effect [15]. However, a more recent study suggested that the primary mechanism of nanoparticle uptake is an active process [16]. Nonetheless, both the size and surface area to volume ratio, enable tumor selectivity and targeted therapy to lesion sites which has many implications for cancer treatment [15,16]. These features could address the challenges associated with cancer therapy by potentiating better therapeutic efficacy, lower toxicity, and fewer side effects. GNPs may be ideal vehicles to deliver chemotherapeutics and other drugs [17].
To facilitate better uptake of nanosystems in targeted tissue sites, GNP surfaces have been modified to create GNP hybrids. Hybridization of GNPs commonly occurs with the conjugation of biomolecules and molecular ligands such as saccharides, peptides, oligonucleotides, monoclonal antibodies, proteins, and nucleic acids, which facilitate better stability, biocompatibility, and targeting of the system to the resident tissue. Hybrids also enhance the system's interaction with cell surface receptors and intracellular molecules to facilitate receptor-mediated endocytosis. The ability to undergo surface modifications also enables conjugation of GNPs with therapeutic substances such as drugs, DNA, RNA, short interfering RNA (siRNA), proteins, peptides, and antibodies. The surge of nanomedical research over the past decade has demonstrated nanoparticle-based drug delivery as a promising platform to overcoming the pharmacokinetic challenges faced with conventional drug formulations.
All in all, GNPs have unique properties with significant advantages that make them an ideal tool in clinical research. They can be formulated for various routes of administration (i.e., intravascular, topical, oral, etc.), which enhances their clinical versatility and appeal [7]. In animal models, GNPs have been studied in applications for multiple disease states with encouraging results [[18], [19], [20], [21], [22], [23]]. Encouraging pre-clinical results have led to clinical applications of GNPs. To date, more than 20 studies have assessed the use of GNPs as a medical intervention that is administered to humans. As the first step in translational research, these studies have focused on the safety concerns associated with GNP systems. Most of these trials were recently completed, with several still ongoing. The objective of the present study is to review the current evidence on GNP safety in clinical applications and comment on their future use in humans to aid the clinical translation of this promising new nano-application in medicine.
2. GNP clinical trials
2.1. Drug delivery systems (DDS)
Advancements in drug delivery systems are driven by the need to increase drug specificity and efficacy; a difficult task as improvement in one often results in the compromise of the other. Many pre-clinical studies using GNPs as a DDS were successful in tackling this challenge [[24], [25], [26], [27], [28]]. Henceforth, several studies explored clinical translation of this promising application (Table 1) [[29], [30], [31], [32]].
Table 1.
Clinical trials with gold nanoparticles as a drug delivery system.
| Trial Number | Sponsor | Device | Drugs | Disease | Number of enrolments | Number of centers | Status | Starting year | Ending year | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT00356980 | National Institutes of Health Clinical Center, National Cancer Institute |
CYT-6091 | rhTNF | Unspecified adult solid tumor | 60 | 2 | Completed | 2006 | 2009 | [29] |
| NCT03020017 | Northwestern University, National Cancer Institute |
NU-0129 | Bcl2L12 specific RNAi | Glioblastoma | 8 | 1 | Completed | 2017 | 2020 | [30] |
| NCT02837094 | Cardiff University | C19-A3 GNP | Proinsulin peptide | Type I diabetes | 8 | 2 | Completed and data submitted | 2016 | 2020 | [31] |
| DNA_SPN_B001_17 AYUSH, Indian∗ |
Dhanvantari Nano Ayushadi | Nano Swarna Bhasma | Swarna Bhasma | Breast cancer | 3:3 | 2 | Completed | – | – | [32] |
∗ Permission from Indian government agent AYUSH. Bcl2L12: Bcl-2-like protein 12; GNP: gold nanoparticle; rhTNF: recombinant human tumor necrosis factor alpha; RNAi: RNA interference; –: no data.
The first clinical trial to assess the use of GNPs as a DDS was conducted by Libutti et al. [29] in 2010 (NCT00356980). In this study, recombinant human tumor necrosis factor alpha (rhTNF) was formulated as the therapeutic for delivery. Together, rhTNF and thiolated polyethylene glycol (PEG) were bound to a 27 nm colloidal GNP, a system which was coined CYT-6091. This solution was administered intravenously to patients ≥18 years-old with advanced and/or metastatic solid organ cancers. The study exhibited safe, systemic delivery of 1.2 mg of rhTNF with none of the dose limiting toxicities such as hypotension and hepatotoxicity that were previously encountered with native rhTNF. The most common adverse events that occurred with CYT-6091 treatment included lymphopenia, hypoalbuminemia, hypokalemia, hypophosphatemia, hyperbilirubinemia, and increased aminotransferases. Both GNPs and/or the drug loaded on GNPs may cause systemic toxicities and adverse events; however, these consequences were resolved with necessary protocol management, and none were considered dose limiting toxicities. These results suggest that while adverse effects were observed, they were treatable and should not promote doubt in the safety of GNPs. Additionally, CYT-6091 was non-antigenic and was rarely visualized in healthy tissue [29].
In 2018, CYT-6091 was further developed as CYT-21625 to deliver rhTNF and a paclitaxel prodrug to tumor vasculature and cancer cells that express high levels of TNF receptor. Mice with metastatic anaplastic thyroid cancer xenografts were treated weekly with CYT-21625, either CYT-6091 or paclitaxel alone, or saline as controls. Mice treated with CYT-21625 had a significantly lower tumor burden with no toxicities observed. Because of these promising results, translation to clinical trials was recommended [33].
More recently, GNPs were used to promote gene silencing through RNA interference (RNAi). In 2021, Kumthekar et al. [30] completed a clinical trial (NCT03020017) assessing the safety and efficacy of an RNAi mediated nanoparticle formulation, NU-0129, for the treatment of glioblastoma. The study aimed to silence the Bcl-2-like protein 12 (Bcl2L12) oncogene and improve glioblastoma prognosis by limiting tumorigenic Bcl2L12 products. NU-0129 is composed of a spherical nanoparticle gold core covalently conjugated with Bcl2L12-targeting siRNA, and oligoethylene glycol backfill. Eight patients with recurrent glioblastoma, aged 32-66 years-old, were intravenously administered 0.04 mg/kg of NU-0129 and underwent weekly assessment over 21 days for the presence of adverse events. During the follow up period, a total of seven adverse events were observed; however, only two were considered severe: hypophosphatemia and lymphopenia. These were only possibly related to NU-0129 formulation and improved by the end of the follow up period. As a GNP based DDS, NU-0129 treatment was well tolerated and not associated with long term toxicity [30].
While most of the GNP DDS research has targeted cancers, recent expansion in its therapeutic applications has promoted a clinical trial in a non-cancerous disease. In this study, researchers from Cardiff University, United Kingdom, tested a novel GNP formulation for the treatment of type I diabetes. The study evaluated a proinsulin derived peptide coupled to a GNP, termed C19-A3 GNP. A total of 8 patients between the ages of 18 and 40 with clinically diagnosed type I diabetes were administered 50 μL of C19-A3 GNP intramuscularly every 28 days for 8 weeks. Follow up was then completed for an additional 6 weeks after the final treatment (NCT02837094) [31]. There were no systemic hypersensitivities, systemic gold retention, or C19-A3 GNP-related serious adverse events observed throughout the study. All patients experienced a delayed local skin reaction at the injection site which faded over 12–24 months but did not fully disappear. Histopathological results showed gold retention in the dermis with inflammatory infiltrate but no granulomas [31].
It is evident from the DDS studies above that injection is a common route of administration for GNPs. However, GNPs can also be delivered parenterally. In 2020, Khoobchandani et al. [32] published a pilot clinical trial that investigated an oral nano-based drug, Nano Swarna Bhasma (NSB), comprised of GNPs functionalized with phytochemicals in mango peel for the treatment of breast cancer. NSB provides a reproducible and scientifically verifiable formulation of ‘Swarna Bhasma’, an ancient Indian Ayurvedic medicine with established anticancer effects but limited development due to lack of reproducible Ayurvedic formulations [32]. In the study, six patients with invasive breast carcinoma were divided into two groups and received either “Standard of care treatment” consisting of doxorubicin and cyclophosphamide, or “Standard of care treatment'' with NSB. Results from the study indicated that hyperacidity and generalized weakness were present in one patient from each treatment arm. Only one case of severe anemia was reported in the NSB drug adjuvant group, but this was determined to be unrelated to NSB. Overall, the authors believe that NSB demonstrated acceptable safety as an adjuvant therapeutic agent in treating human breast cancer [32].
2.2. Photothermal therapy
Photothermal therapy (PTT) is a technique that uses photon-mediated conversion of light to heat to stimulate a hyperthermic physiological response. The characteristic SPR effect seen with GNPs makes them ideal systems for PTT [5,34]. Classically, PTT has been used to induce cytotoxicity in cancer cells but is expanding its application (Table 2) [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]].
Table 2.
Clinical trials using gold nanoparticles in photothermal therapy.
| Trial Number | Sponsor | Device | Disease | Number of enrolments | Number of centers | Status | Starting year | Ending year | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| NCT01270139 | Ural State Medical University Ural Institute of Cardiology De Haar Research Task Force Ural Federal University Transfiguration Clinic |
Silica-GNP Silica-Iron-GNP |
Atherosclerosis | 60:60:60 | Multi- | Completed | 2007 | – | [35] [36] |
| CAS/OR/01/CMN/103300410C0010-1968/201 ∗ | Nanospectra Biosciences, Inc. | AuroShell | Prostate cancer | 22 | Multi- | Completed | – | – | [37] |
|
NCT02680535 (NBI-PC-002) |
Nanospectra Biosciences, Inc. | AuroShell | Prostate cancer | 16 | Multi- | Completed | 2016 | 2019 | [38] |
| – | Sebacia Inc. | Sebashell | Acne vulgaris | 37 | Multi- | Completed | – | – | [39] |
| YK/92/2016∗∗ | Nanfang University, China | GNRs @ Pd hydrogel eye patch | Dry eye syndrome | 20 | 1 | Completed | – | – | [40] |
| NCT02680535 | Nanospectra Biosciences, Inc. | AuroShell | Prostate cancer | 45 | Multi- | Completed | 2016 | 2019 | [41] |
| NCT04240639 | Nanospectra Biosciences, Inc. | AuroShell | Prostate cancer | 60 | Multi- | Recruiting | 2020 | 2023 | [42] |
| NCT01679470 | Nanospectra Biosciences, Inc. | AuroShell | Lung cancer | 1 | 1 | Terminated | 2012 | 2014 | [43] |
| NCT00848042 | Nanospectra Biosciences, Inc. | AuroShell | Head & neck cancer | 11 | 2 | Completed | 2008 | 2014 | [44] |
| NCT02219074 | Sebacia Inc. | Sebashell | Acne vulgaris | 350 | 2 | Completed | 2014 | 2016 | [45] |
∗ Research authorization number under the supervision of the Federal Commission for Protection against Health Risks (Comision Federal para la Proteccio n contra Riesgos Sanitarios, COFE-PRIS); ∗∗ Research Ethics Committee from Nanfang University, China. GNRs: Gold nanorods; Pd: Pallidium
In 2015, Kharlamov et al. [35] reported safety and feasibility in a clinical trial for atheroprotective management of plaques (NCT01270139). Patients were assigned to receive one of the following treatments: nano-intervention with delivery of silica–GNP in a bioengineered on-artery patch grown with stem cells pre-cultivated in medium with GNP; nano-intervention delivery of silica–iron-bearing GNP using an intracoronary infusion of stem cells and targeted micro-bubbles pre-cultivated in medium with GNP; or stent implantation control (n = 60 cases for each group). At 12 months post-therapy, one case of definite thrombosis was confirmed in the iron-based group and 3 cases of target lesion revascularization were reported in each treatment group [35]. A 5-year follow-on study confirmed lower incidence of major adverse cardiovascular events and target lesion revascularization in both nano treatment arms compared to the stenting control group [36].
In 2016, Stern et al. [37] implemented a gold nanoshell (AuroShell) to treat prostate cancer. AuroShell is a 150 nm silica core-based nanoparticle with a gold shell and thiolated PEG surface. For the study, AuroShell was administered intravenously at 7.5 mL/kg to 22 patients with prostate cancer. Patients were monitored for six months post-intervention wherein a total of 28 adverse events were observed. Ten of these occurred within a single individual, and only two were considered related to the treatment: allergic reaction or a burning sensation of the epigastrium at the time of infusion. Both adverse events subsided with either administration of diphenhydramine and dexamethasone or spontaneously. Overall, no indication of immunological or toxic consequences was observed in this study [37].
An apparent positive safety profile in the initial AuroShell clinical trial led to further clinical evaluation of the gold coated nanoparticle. A study conducted by Rastinehad and colleagues [38] evaluated the thermal ablating abilities of AuroShell in prostate cancers with the inclusion of multiparametric magnetic resonance imaging (MRI)/ultrasound targeted fusion biopsies (NCT02680535) [41]. Results indicated that the treatment protocol was safe. Mild negative effects including transient epigastric pain, penile bleeding, edema, and scar formation were all noted during the 12-month follow up period. However, several of these events were not linked to the AuroShell composition and were deemed mitigable with procedural modification [38]. Verification of the AuroShell safety profile has led to a new clinical study to test three different doses of the GNP formulation (NCT04240639) [42]. Additionally, the safety of AuroShell was proposed to be tested for lung cancers (NCT01679470) [43], and for head and neck cancers (NCT00848042) [44]. However, the results from these studies remain unpublished.
While most clinical studies involving GNPs have focused on fatal diseases, several recent trials have expanded GNP applications to non-life-threatening conditions. Notably, in 2015, Paithankar et al. [39] evaluated the role of GNPs in treating acne. Sebashell, a 150 nm nanoparticle composed of a silica core coated with a gold shell and PEG, was topically administered to 37 male and female subjects, aged 18–40, with a diagnosis of acne vulgaris. Patients were subsequently exposed to low frequency ultrasounds and a near-infrared (NIR) laser. Results showed that ultrasonic application promoted deep tissue penetration of Sebashell nanoparticles, without penetrating surrounding non-follicular tissue. Furthermore, thermal activation induced thermolysis of sebaceous glands without damaging surrounding tissue. Other than the presence of perifollicular edema, no severe adverse events were observed in the study [39]. A new Sebashell clinical trial with 350 patients was completed to further evaluate the GNP formulation, but the results have yet to be published (NCT02219074) [45].
PTT has also been delivered locally through an eye patch. Pang et al. [40] engineered a 20 mm hydrogel eye patch for the treatment of dry eye syndrome. The patch was composed of gelatin incorporated with palladium-coated gold nanorods up to 100 nm long. The activated patch created a gentle heat source at the lacrimal gland to stimulate tear secretion. Twenty healthy patients between 18 and 30 years-old wore eye patches while watching a 3 h video. The patch did not produce adverse effects and did not affect the outermost lipid layer of the tear film which is largely responsible for tear film evaporation in a significant portion of the total dry eye population [40].
2.3. Gold nanocrystalline as a therapeutic agent
Bulk gold is recognized as chemically inert. Contrary, recent research has revealed that gold at the nanoscale can be highly catalytic. This property has been widely used in industrial applications [46] and by biologists [47]. One of the important reactions in the body is the oxidation of nicotinamide adenine dinucleotide hydride (NADH) to nicotinamide adenine dinucleotide (NAD+) [48]. NAD+ and NADH serve as the essential redox couple for adenosine triphosphate (ATP)-generation, oxidative phosphorylation, and glycolysis [49]. Furthermore, NADH oxidation is crucial in the energetically demanding process of myelination in the central nerve system (CNS) [50,51]. This pathway has been tested to promote remyelination in neurogenerative disorders with GNPs, as they facilitate the oxidation of NADH to NAD+.
CNM-Au8 is a novel preparation of clean-surfaced, faceted gold nanocrystals developed by Clene Nanomedicine. CNM-Au8 nanocrystals are approximately 13 nm in diameter and suspended in a drinkable bicarbonate solution. In vitro studies evaluating gold nanocrystal treatment of oligodendrocyte precursor cells resulted in oligodendrocyte maturation and expression of myelin differentiation markers. Further, in response to gold nanocrystals, CNS cells exhibited elevated levels of NAD+, intracellular ATP, and extracellular lactate, along with upregulation of myelin-synthesis related genes. Oral delivery of gold nanocrystals demonstrated robust remyelinating activity in rodent models as well [52].
Following favorable preclinical results, an initial CNM-Au8 clinical trial assessed the safety profile of the preparation in humans (NCT02755870) [53]. CNM-Au8 dosages of 15, 30, 60, or 90 mg were administered orally to 86 healthy subjects who were then monitored for 21 days. While no peer-reviewed publications are available for this study, an additional 9 CNM-Au8 trials have followed, evaluating the role of CNM-Au8 in patients with a variety of neurodegenerative diseases. Safety remains a primary end point for these studies, but a focus on therapeutic effects is now also taking center-stage. Assessment of CNS metabolic effects, pharmacokinetics (PK), and pharmacodynamics (PD) of CNM-Au8 are all factors assessed in ongoing trials (Table 3) [[53], [54], [55], [56], [57], [58], [59], [60], [61], [62]].
Table 3.
Clinical trials with gold nanoparticles as a therapeutic agent.
| Trial Number | Sponsor | Device | Disease | Number of enrolments | Number of centers | Status | Starting year | Ending year | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| NCT02755870 | Clene Nanomedicine | CNM-Au8 | Healthy volunteers | 86 | 1 | Completed | 2015 | 2016 | [53] |
| NCT03843710 (REPAIR-ALS) | Clene Nanomedicine University of Texas Southwestern Medical Center |
CNM-Au8 | Amyotrophic lateral sclerosis | 24 | 1 | Not yet recruiting | 2019 | 2022 | [54] |
| NCT03993171 (REPAIR-MS) | Clene Nanomedicine University of Texas Southwestern Medical Center |
CNM-Au8 | Relapsing multiple sclerosis | 30 | 1 | Recruiting | 2019 | 2021 | [55] |
| NCT03815916 (REPAIR-PD) | Clene Nanomedicine University of Texas Southwestern Medical Center |
CNM-Au8 | Parkinson's disease | 30 | 1 | Recruiting | 2019 | 2021 | [56] |
| NCT04081714 | Clene Nanomedicine Massachusetts General Hospital |
CNM-Au8 | Amyotrophic lateral sclerosis | ≤30 | 1 | Ongoing | 2019 | – | [57] |
| NCT04414345 (HEALEY ALS Regimen C) | Clene Nanomedicine, | CNM-Au8 | Amyotrophic lateral sclerosis | 160 | Multi- | Recruiting | 2020 | 2022 | [58] |
| NCT04297683 (HEALEY ALS) | Clene Nanomedicine Massachusetts General Hospital |
Zilucoplan Verdiperstat CNM-Au8 Pridopidine |
Amyotrophic lateral sclerosis | 640 | Multi- | Recruiting | 2020 | 2022 | [59] |
| NCT04098406 (RESCUE-ALS) | Clene Nanomedicine, Clene Australia Pty Ltd |
CNM-Au8 | Amyotrophic lateral sclerosis | 42 | 2 | Recruiting | 2019 | 2022 | [60] |
| NCT03536559 (VISIONARY-MS) | Clene Nanomedicine, Clene Australia Pty Ltd |
CNM-Au8 | Relapsing multiple sclerosis | 150 | Multi- | Recruiting | 2018 | 2022 | [61] |
| NCT04626921 (VISIONARY-LTE (long term extension)) | Clene Nanomedicine George Clinical |
CNM-Au8 | Relapsing multiple sclerosis | 150 | Multi- | Recruiting | 2020 | 2024 | [62] |
ALS: amyotrophic lateral sclerosis; LTE: long-term extension; MS: multiple sclerosis; PD: Parkinson's disease.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by demyelination. As such, CNM-Au8 is being evaluated as a therapeutic intervention for patients with ALS in the REPAIR-ALS (NCT03843710) [54] clinical study. The trial is currently active as a single-center open label pilot, sequential group, investigator, and patient blinded study at the University of Texas Southwestern Medical Center. The primary endpoint of this study is the ratio of NAD+: NADH, measured non-invasively by 31phosphorous magnetic resonance spectroscopy. The University of Texas Southwestern Medical Center has also devised two other clinical trials, REPAIR-MS (NCT03993171) [55] and REPAIR-PD (NCT03815916) [56], nearly identical to REPAIR-ALS (NCT03843710) [54], other than the diseases of interest being relapsing multiple sclerosis and Parkinson's Disease, respectively.
Other institutions are also exploring the role of CNM-Au8 in patients with ALS. Led by Massachusetts General Hospital, two clinical trials are characterizing the effects of CNM-Au8 (NCT04081714, NCT04414345) [57,58]. In the single-center study (NCT04081714) [57], researchers are assessing the safety, PK, and PD of CNM-Au8 alone. Moreover, to compare CNM-Au8 against other investigational products for ALS, the perpetual multi-center, multi-regimen clinical study (NCT04297683) [59] is evaluating several ALS treatment products. In this trial, Regimen C (NCT04414345) [58] will evaluate CNM-Au8. Expanding beyond the North American border, the RESCUE-ALS clinical trial in Australia similarly aims to characterize the safety, PK and PD of CNM-Au8 in ALS patients. The RESCUE-ALS trial will further identify the role of CNM-Au8 by utilizing electrophysiological measures to detect preservation of motor neuron function (NCT04098406) [60].
As a result of promising CNM-Au8 trials, extension studies are currently being developed. VISIONARY-MS is a study implementing CNM-Au8 as a remyelinating treatment for vision-impairing MS lesions in participants who have relapsing-remitting multiple sclerosis (NCT03536559) [61]. An open-label, long-term extension study for participants who have completed this trial will be continued in the VISIONARY-LTE study (NCT04626921) [62]. With results of many of these trials to be released in the coming years, support for CNM-Au8 efficacy and safety may promote further expansion of GNPs as a conventional medical intervention.
3. Discussion
Over the last two decades, several hundred research articles on GNPs have been published. These studies paved the way of nano fabrication of GNP preparations and pre-testing of GNPs both in vitro (e.g., cell cultures) and in vivo (e.g., animal models). We are pleased to find more than 20 clinical studies, either recently published, or currently registered in the national clinical trial database that are evaluating the safety profile of GNP formulations. More than 700 patients have been tested with the results collected, and more than 1,200 new cases are undergoing with results expected in the coming years.
3.1. Clinical safety and limitations of current clinical trials on GNPs
In the clinical trials summarized above, various compositions of GNP-systems, from nanorods to gold-covered particles, were tested and delivered through various routes of administration (i.e., intravenous, parenteral, intramuscular). They demonstrated good safety profiles with reported adverse events which were either transient, clinically manageable, or deemed unrelated to GNP-based treatment. Overall, GNPs tested in clinical trials thus far do not raise major safety concerns. Recently, multi-dose, multi-center blinded trials have been initiated to further expand the clinical understanding of GNPs.
However, the major limitation of these studies is the sparsity of trials and their relatively small sample sizes. Most of these initial trials also only evaluated one therapeutic dose. Additionally, quality of trials should be improved as some earlier publications were not registered in the national clinical trial database, and some of these studies have not been published.
GNPs, as inorganic nanoparticles, are not degradable. Therefore, long-term toxicity of GNPs will require further investigation and potential cumulative toxicity should be considered when repeated treatments are developed. Preclinical studies with CYT-6091 showed that gold content remained in the liver for up to 120 days [63]. In the clinical study using NU-0129, gold content was identified in two tumors that recurred up to 174 days following treatment and over 40% of initial GNP levels were still detectable in tumors [30]. This suggests that although GNPs undergo hepatic elimination and demonstrate good tumor targeting, future studies will require further investigations into the potential side effects of long-term gold accumulation.
While gold is a common feature, different surface compositions play a primary role in determining type and severity of side effects. Both CYT-6091 and CYT-21625 demonstrated good tumor trafficking which restricted biodistribution and improved the safety of higher doses of rhTNF by utilizing a combination of passive and active mechanisms through the well-known EPR effect and rhTNF's tumor-targeting properties, respectively [[29], [33]]. Future studies might consider utilizing a combination of tumor-targeting ligands on GNP-based DDS as a strategy to improve safety of nanomedicines. Further studies must test each preparation individually to elucidate the toxicological profile of GNPs in humans, as each nanoparticle formulation could promote different pharmacological reactions. Moreover, pharmacokinetic behaviors (i.e., half-life, clearance rate, etc.) and tissue distribution of GNPs in human should be studied in clinical trials. Results from pre-clinical animal studies should be carefully reviewed for guidance to address the differences between humans and animals in terms of pharmacokinetics and tolerance to toxicity.
While this review broadly summarizes the safety profile of current GNP-based interventions, most clinical studies report specific information regarding the dose of drug administered to patients, the respective dose strength of GNPs, the physical properties of the GNPs (i.e., different size, shape, manufactural methods), and the stability of the formulations, which are all factors that may affect the pharmacokinetics and toxicological profile of specific GNP systems. Furthermore, in clinical practice co-medications may affect the pharmacokinetics and toxicity of GNPs. Thus, all these factors should be further tested in future clinical studies.
3.2. Diversified clinical applications of GNPs
The relative safe profiles of GNPs reported in the present review encouraged exploration of more clinical applications of GNP-based technology. Most pre-clinical literature has capitalized on applications of GNPs as a DDS. While various types of cargo (rhTNF, RNAi, proinsulin peptide, etc.) have been tested in humans, many other therapeutic agents can be delivered in a similar fashion. For example, peptide-GNP hybrids can modify immune responses [64] to attenuate Toll-like receptor signaling related to inflammation [65]. A peptide-GNP hybrid that inhibits protein kinase C delta activity showed protective effects on ischemia-reperfusion induced acute lung injury, and it attenuated more severe lung injury at much lower dose in comparison to the drug-peptide delivered by a Tat cell penetration peptide [66]. These GNP-cargo hybrids could be further assessed in clinical trials, which may provide more therapeutic reagents to the bedside.
Another property of GNPs which should be further explored is their SPR effect in photoimaging to address MRI and computer tomography (CT) limitations. The use of GNPs could offer an alternative contrast agent for patients sensitive to the standard iodinated contrasts [67]. This approach should not raise any safety concerns, as in preclinical studies, GNPs' use in imaging systems have demonstrated a favorable safety profile [68,69]. Additionally, given the modifiable size and surface composition of GNPs, clinicians can tailor the circulation time and region of accumulation of the GNPs to promote more precise diagnosis [70]. In relation to cancer treatment, GNPs’ intrinsic ability to promote increased relaxivity for MRI [71,72] supports better contrasted images which can lead to better tumor visualization. The accumulation of GNPs at the tumor site during the imaging process can guide PTT more precisely to the tumor and limit damage to surrounding tissue. Additional cancer fighting properties of GNPs are also present in their intrinsic radiosensitivity abilities as high Z materials. While an abundance of pre-clinical data has shown potential use of GNPs as radiosensitizer, this application has not been translated into clinical setting [73]. This uncertainty further supports the necessity to better understand the safety of GNPs in clinical settings.
The clean-surface, faceted gold nanocrystalline opens a new avenue for the use of GNPs as a primary therapeutic. In addition to oxidation of NADH to NAD+, GNPs have demonstrated peroxidase, oxidase, catalase, and super oxide dismutase-like properties [74,75]. These effects could lead to the use of GNPs in a new wide spectrum of clinical disorders [76,77].
Finally, GNPs and related preparations accommodate several routes of administration (intravenously, intramuscularly, orally, and locally) in several disease states (e.g., type I diabetes, atherosclerosis, acne vulgaris, ALS, relapsing multiple sclerosis and Parkinson's disease). Different routes of administration and clinical applications suggest GNPs can be modified depending on the clinical presentations.
3.3. Collaborations among basic and clinician scientists and industry
For conventional clinical drug studies, the tested therapeutics are usually produced by pharmaceutical companies, with many already clinically available and tested drugs for repurposing. In contrast, clinical grade GNPs are usually prepared by biotechnology companies. The relatively small quantity of GNP preparations leads to higher costs. Since these therapeutics are still under development, most of the clinical trials require collaborations among basic and clinician scientists with sponsorship from biotechnology companies. Promotion of collaborations among academic, medical, and industry personnel/institutions will bring together resources necessary to overcome the technical and financial obstacles in GNP translation.
Facilitating these collaborations can be promoted through external financial support. Government aid in the form of grants for nanotechnological work will foster the translation of GNPs to the clinic. One of the largest campaigns is the National Nanotechnological Initiative (NNI) in the United States which advocates and supports nanotechnology-based approaches. Focused on contributing to the full spectrum of development (i.e., student training, research, design of clinical trials, and clinical application), the NNI requested $1.7 billion in federal support for 2021 [78]. Advertisement and development of programs like the NNI could significantly reduce the financial burden for biotechnological companies, while concurrently attracting more basic scientists and clinicians to investigate GNP based applications.
Government support for GNPs should not be limited to nanotechnology specific grants alone. Broad funding categories which nanotechnology may fall under, i.e., biomanufacturing, offer an alternative avenue of financial subsidization. Further, collaborations with diverse industries that may optimize GNP use, such as artificial intelligence to streamline the manufacturing and application processes, will also have their own independent grants to indirectly support GNP translation. In focusing on the collaborative approach, global partnerships offering diverse specializations will form and can further improve GNP applications. Thus, this strategy will not only offer financial relief, but also optimize GNP translation.
4. Conclusion
All GNP-based clinical trials conducted thus far demonstrated good safety profiles with minimal adverse events. However, the limited quantity of trials, small sample sizes, and lack of understanding of the adverse effects of long-term gold accumulation remain major limitations to the translation of GNP to clinical settings. Furthermore, as GNPs have the advantage of being highly modifiable structures, each formulation must be studied individually in the future to understand the pharmacokinetics, biodistribution, and toxicological profile of the GNP system. The clinical applications of GNPs are expanding as researchers begin to employ different characteristics such as its surface modifiability for hybridizing DDS, the SPR effect for enhancing medical imaging, its radiosensitivity as a high Z material for combatting cancer, and its intrinsic ability to promote oxidization of coenzymes for the treatment of neurodegenerative disorders. A global and collaborative approach is essential to the success of GNPs in clinic. Recent rapid development of clinical trials has demonstrated promising safety and efficacy of GNP-based therapeutics. Encouraging future clinical trials with GNPs as therapeutic devices will lead to novel therapies for patients in need of hope for new treatments.
CRediT author statement
Leeann Yao: Conceptualization, Data collection, Investigation, Writing - Original draft preparation, Reviewing and Editing; Dejan Bojic: Data collection, Investigation, Writing - Original draft preparation, Reviewing and Editing, Figure generation; Mingyao Liu: Conceptualization, Data collection, Investigation, Supervision, Writing - Original draft preparation, Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was supported by grants from Canadian Institutes of Health Research (Grant Nos.: MOP-42546, MOP-119514, PJT-148847).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Chaudhari M.B., Desai P.P., Patel P.A., et al. Solid lipid nanoparticles of amphotericin B (AmbiOnp): In vitro and in vivo assessment towards safe and effective oral treatment module. Drug Deliv. Transl. Res. 2016;6:354–364. doi: 10.1007/s13346-015-0267-6. [DOI] [PubMed] [Google Scholar]
- 2.Campanella C., Caruso Bavisotto C., Logozzi M., et al. On the choice of the extracellular vesicles for therapeutic purposes. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anselmo A.C., Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015;17:1041–1054. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mir M., Ishtiaq S., Rabia S., et al. Nanotechnology: From in vivo imaging system to controlled drug delivery. Nanoscale Res. Lett. 2017;12 doi: 10.1186/s11671-017-2249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh Y.-C., Creran B., Rotello V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4:1871–1880. doi: 10.1039/c1nr11188d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A., Zhang X., Liang X.-J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013;31:593–606. doi: 10.1016/j.biotechadv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Majdalawieh A., Kanan M.C., El-Kadri O., et al. Recent advances in gold and silver nanoparticles: Synthesis and applications. J. Nanosci. Nanotechnol. 2014;14:4757–4780. doi: 10.1166/jnn.2014.9526. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Liu S., Yao T., et al. Controllable synthesis of gold nanoparticles with ultrasmall size and high monodispersity via continuous supplement of precursor. Dalton Trans. 2012;41:11725–11730. doi: 10.1039/c2dt31270k. [DOI] [PubMed] [Google Scholar]
- 9.Grzelczak M., Pérez-Juste J., Mulvaney P., et al. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008;37:1783–1791. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 10.Dykman L.A., Khlebtsov N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Naturae. 2011;3:34–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilton-Ely J.D.E.T. The surface functionalisation of gold nanoparticles with metal complexes. Dalton Trans. 2008:25–29. doi: 10.1039/b714144k. [DOI] [PubMed] [Google Scholar]
- 12.Thakor A.S., Jokerst J., Zavaleta C., et al. Gold nanoparticles: A revival in precious metal administration to patients. Nano Lett. 2011;11:4029–4036. doi: 10.1021/nl202559p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang A., Liu Q., Wen G., et al. The surface-plasmon-resonance effect of nanogold/silver and its analytical applications. TrAC Trends Anal. Chem. 2012;37:32–47. [Google Scholar]
- 14.Yang H., Fung S.-Y., Liu M. Programming the cellular uptake of physiologically stable peptide-gold nanoparticle hybrids with single amino acids. Angew. Chem. Int. Ed. 2011;50:9643–9646. doi: 10.1002/anie.201102911. [DOI] [PubMed] [Google Scholar]
- 15.Singh P., Pandit S., Mokkapati V.R.S.S., et al. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19071979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sindhwani S., Syed A.M., Ngai J., et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M., Liu M. New Avenues for Nanoparticle-Related Therapies. Nanoscale Res. Lett. 2018;13 doi: 10.1186/s11671-018-2548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javed I., Hussain S.Z., Shahzad A., et al. Lecithin-gold hybrid nanocarriers as efficient and pH selective vehicles for oral delivery of diacerein–In-vitro and in-vivo study. Colloids Surf. B. 2016;141:1–9. doi: 10.1016/j.colsurfb.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Dalvi B.R., Siddiqui E.A., Syed A.S., et al. Nevirapine Loaded Core Shell Gold Nanoparticles by Double Emulsion Solvent Evaporation: In vitro and In vivo Evaluation. Curr. Drug Deliv. 2016;13:1071–1083. doi: 10.2174/1567201813666160114093005. [DOI] [PubMed] [Google Scholar]
- 20.Somasuntharam I., Yehl K., Carroll S.L., et al. Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials. 2016;83:12–22. doi: 10.1016/j.biomaterials.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhary K., Moore H., Tandon A., et al. Nanotechnology and adeno-associated virus-based decorin gene therapy ameliorates peritoneal fibrosis. Am. J. Physiol. Ren. Physiol. 2014;307:F777–F782. doi: 10.1152/ajprenal.00653.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W., Zeng W., Sun J., et al. Construction of an Aptamer–SiRNA Chimera-Modified Tissue-Engineered Blood Vessel for Cell-Type-Specific Capture and Delivery. ACS Nano. 2015;9:6069–6076. doi: 10.1021/acsnano.5b01203. [DOI] [PubMed] [Google Scholar]
- 23.Nemati H., Ghahramani M.-H., Faridi-Majidi R., et al. Using siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation in psoriasis. J. Control. Release. 2017;268:259–268. doi: 10.1016/j.jconrel.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Lee K.Y.J., Lee G.Y., Lane L.A., et al. Functionalized, long-circulating, and ultrasmall gold nanocarriers for overcoming the barriers of low nanoparticle delivery efficiency and poor tumor penetration. Bioconj. Chem. 2017;28:244–252. doi: 10.1021/acs.bioconjchem.6b00224. [DOI] [PubMed] [Google Scholar]
- 25.Bhowmik T., Saha P.P., Sarkar A., et al. Evaluation of cytotoxicity of a purified venom protein from Naja kaouthia (NKCT1) using gold nanoparticles for targeted delivery to cancer cell. Chem. Biol. Interact. 2017;261:35–49. doi: 10.1016/j.cbi.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Hua H., Zhang N., Liu D., et al. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo-and chemotherapy. Int. J. Nanomed. 2017;12:7869–7884. doi: 10.2147/IJN.S143977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heo R., Yoon H.Y., Ko H., et al. Gold-installed biostable nanocomplexes for tumor-targeted siRNA delivery in vivo. Chem. Commun. 2015;51:16656–16659. doi: 10.1039/c5cc05639j. [DOI] [PubMed] [Google Scholar]
- 28.Davidi E.S., Dreifuss T., Motiei M., et al. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck. 2018;40:70–78. doi: 10.1002/hed.24935. [DOI] [PubMed] [Google Scholar]
- 29.Libutti S.K., Paciotti G.F., Byrnes A.A., et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumthekar P., Ko C.H., Paunesku T., et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abb3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatovic D., McAteer M.A., Barry J., et al. Safety of the use of gold nanoparticles conjugated with proinsulin peptide and administered by hollow microneedles as an immunotherapy in type 1 diabetes. Immunother. Advanc. 2022;2 doi: 10.1093/immadv/ltac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoobchandani M., Katti K.K., Karikachery A.R., et al. New approaches in breast cancer therapy through green nanotechnology and nano-ayurvedic medicine–pre-clinical and pilot human clinical investigations. Int. J. Nanomed. 2020;15:181–197. doi: 10.2147/IJN.S219042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilubol N., Yuan Z., Paciotti G.F., et al. Novel dual-action targeted nanomedicine in mice with metastatic thyroid cancer and pancreatic neuroendocrine tumors. J. Natl. Cancer Inst. 2018;110:1019–1029. doi: 10.1093/jnci/djy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vines J.B., Yoon J.-H., Ryu N.-E., et al. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019;7 doi: 10.3389/fchem.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharlamov A.N., Tyurnina A.E., Veselova V.S., et al. Silica-gold nanoparticles for atheroprotective management of plaques: Results of the NANOM-FIM trial. Nanoscale. 2015;7:8003–8015. doi: 10.1039/c5nr01050k. [DOI] [PubMed] [Google Scholar]
- 36.Kharlamov A.N., Feinstein J.A., Cramer J.A., et al. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: Long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 2017;13:345–363. doi: 10.2217/fca-2017-0009. [DOI] [PubMed] [Google Scholar]
- 37.Stern J.M., Kibanov Solomonov V.V., Sazykina E., et al. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int. J. Toxicol. 2016;35:38–46. doi: 10.1177/1091581815600170. [DOI] [PubMed] [Google Scholar]
- 38.Rastinehad A.R., Anastos H., Wajswol E., et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. U.S.A. 2019;116:18590–18596. doi: 10.1073/pnas.1906929116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paithankar D., Hwang B.H., Munavalli G., et al. Ultrasonic delivery of silica-gold nanoshells for photothermolysis of sebaceous glands in humans: Nanotechnology from the bench to clinic. J. Control. Release. 2015;206:30–36. doi: 10.1016/j.jconrel.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Pang Y., Wei C., Li R., et al. Photothermal conversion hydrogel based mini-eye patch for relieving dry eye with long-term use of the light-emitting screen. Int. J. Nanomed. 2019;14:5125–5133. doi: 10.2147/IJN.S192407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov MRI/US Fusion Imaging and Biopsy in Combination with Nanoparticle Directed Focal Therapy for Ablation of Prostate Tissue. https://clinicaltrials.gov/ct2/show/NCT02680535
- 42.ClinicalTrials.gov An Extension Study MRI/US Fusion Imaging and Biopsy in Combination with Nanoparticle Directed Focal Therapy for Ablation of Prostate Tissue. https://clinicaltrials.gov/ct2/show/NCT04240639
- 43.ClinicalTrials.gov Efficacy Study of AuroLase Therapy in Subjects with Primary and/or Metastatic Lung Tumors. https://www.clinicaltrials.gov/ct2/show/NCT01679470
- 44.ClinicalTrials.gov Pilot Study of AuroLase(tm) Therapy in Refractory and/or Recurrent Tumors of the Head and Neck. https://clinicaltrials.gov/ct2/show/NCT00848042
- 45.ClinicalTrials.gov Multiple Arm Study of Sebacia Microparticles in the Treatment of Acne Vulgaris. https://clinicaltrials.gov/ct2/show/NCT02219074
- 46.Hayashi T., Tanaka K., Haruta M. Selective vapor-phase epoxidation of propylene over Au/TiO2 catalysts in the presence of oxygen and hydrogen. J. Catal. 1998;178:566–575. [Google Scholar]
- 47.Shen X., Liu W., Gao X., et al. Mechanisms of Oxidase and Superoxide Dismutation-like Activities of Gold, Silver, Platinum, and Palladium, and Their Alloys: A General Way to the Activation of Molecular Oxygen. J. Am. Chem. Soc. 2015;137:15882–15891. doi: 10.1021/jacs.5b10346. [DOI] [PubMed] [Google Scholar]
- 48.Huang X., El-Sayed I.H., Yi X., et al. Gold nanoparticles: Catalyst for the oxidation of NADH to NAD+ J. Photochem. Photobiol. B. 2005;81:76–83. doi: 10.1016/j.jphotobiol.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Cantó C., Menzies K.J., Auwerx J. NAD(+) Metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fünfschilling U., Supplie L.M., Mahad D., et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rone M.B., Cui Q.-L., Fang J., et al. Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival. J. Neurosci. 2016;36:4698–4707. doi: 10.1523/JNEUROSCI.4077-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson A.P., Zhang J.Z., Titus H.E., et al. Nanocatalytic activity of clean-surfaced, faceted nanocrystalline gold enhances remyelination in animal models of multiple sclerosis. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-58709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ClinicalTrials.gov A Phase I SAD and MAD Clinical Trial of CNM-Au8 in Healthy Male and Female Volunteers. https://clinicaltrials.gov/ct2/show/NCT02755870
- 54.ClinicalTrials.gov 31P-MRS Imaging to Assess the Effects of CNM-Au8 on Impaired Neuronal Redox State in Amyotrophic Lateral Sclerosis (REPAIR-ALS) (REPAIR-ALS) https://clinicaltrials.gov/ct2/show/NCT03843710
- 55.ClinicalTrials.gov 31P-MRS Imaging to Assess the Effects of CNM-Au8 on Impaired Neuronal Redox State in Multiple Sclerosis (REPAIR-MS) https://clinicaltrials.gov/ct2/show/NCT03993171
- 56.ClinicalTrials.gov 31P-MRS Imaging to Assess the Effects of CNM-Au8 on Impaired Neuronal Redox State in Parkinson’s Disease (REPAIR-PD) https://clinicaltrials.gov/ct2/show/NCT03815916
- 57.ClinicalTrials.gov Intermediate Expanded Access Protocol for ALS. https://clinicaltrials.gov/ct2/show/NCT04081714
- 58.ClinicalTrials.gov HEALEY ALS Platform Trial - Regimen C CNM-Au8. https://clinicaltrials.gov/ct2/show/NCT04414345
- 59.ClinicalTrials.gov HEALEY ALS Platform Trial - Master Protocol. https://clinicaltrials.gov/ct2/show/NCT04297683
- 60.ClinicalTrials.gov Therapeutic Nanocatalysis to Slow Disease Progression of Amyotrophic Lateral Sclerosis (ALS) (RESCUE-ALS) https://clinicaltrials.gov/ct2/show/NCT04098406
- 61.ClinicalTrials.gov Nanocrystalline Gold to Treat Remyelination Failure in Chronic Optic Neuropathy in Multiple Sclerosis (VISIONARY-MS) https://clinicaltrials.gov/ct2/show/NCT03536559
- 62.ClinicalTrials.gov A Multi-Center, Open-Label Long-Term Extension Study of CNM-Au8 in Patients with Stable Relapsing Multiple Sclerosis (VISIONMS-LTE) https://clinicaltrials.gov/ct2/show/NCT04626921
- 63.Goel R., Shah N., Visaria R., et al. Biodistribution of TNF-α-coated gold nanoparticles in an in vivo model system. Nanomedicine (Lond). 2009;4:401–410. doi: 10.2217/nnm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang H., Zhou Y., Fung S.-Y., et al. Amino acid structure determines the immune responses generated by peptide-gold nanoparticle hybrids, Part. Part. Syst. Charact. 2013;30:1039–1043. [Google Scholar]
- 65.Yang H., Fung S.-Y., Xu S., et al. Amino acid-dependent attenuation of tolllike receptor signaling by peptide-gold nanoparticle hybrids. ACS Nano. 2015;9:6774–6784. doi: 10.1021/nn505634h. [DOI] [PubMed] [Google Scholar]
- 66.Lee D., Zhao J., Yang H., et al. Effective delivery of a rationally designed intracellular peptide drug with gold nanoparticle-peptide hybrids. Nanoscale. 2015;7:12356–12360. doi: 10.1039/c5nr02377g. [DOI] [PubMed] [Google Scholar]
- 67.Cormode D.P., Naha P.C., Fayad Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging. 2014;9:37–52. doi: 10.1002/cmmi.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai Q.-Y., Kim S.H., Choi K.S., et al. Colloidal gold nanoparticles as a blood-pool contrast agent for X-ray computed tomography in mice. Investig. Radiol. 2007;42:797–806. doi: 10.1097/RLI.0b013e31811ecdcd. [DOI] [PubMed] [Google Scholar]
- 69.Kim D., Park S., Lee J.H., et al. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2007;129:7661–7665. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- 70.Dong Y.C., Hajfathalian M., Maidment P.S.N., et al. Effect of gold nanoparticle size on their properties as contrast agents for computed tomography. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moriggi L., Cannizzo C., Dumas E., et al. Gold nanoparticles functionalized with gadolinium chelates as high-relaxivity MRI contrast agents. J. Am. Chem. Soc. 2009;131:10828–10829. doi: 10.1021/ja904094t. [DOI] [PubMed] [Google Scholar]
- 72.Shahid M. Water soluble gold nanoparticles based high relaxivity MRI contrast agents. Mater. Res. Express. 2019;6 [Google Scholar]
- 73.Penninckx S., Heuskin A.-C., Michiels C., et al. Gold nanoparticles as a potent radiosensitizer: A transdisciplinary approach from physics to patient. Cancers. 2020;12 doi: 10.3390/cancers12082021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He W., Zhou Y.-T., Wamer W.G., et al. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials. 2013;34:765–773. doi: 10.1016/j.biomaterials.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Lin Y., Ren J., Qu X. Nano-gold as artificial enzymes: Hidden talents. Adv. Mater. 2014;26:4200–4217. doi: 10.1002/adma.201400238. [DOI] [PubMed] [Google Scholar]
- 76.Khan A.A., Rahmani A.H., Aldebasi Y.H., et al. Biochemical and pathological studies on peroxidases–an updated review. Glob. J. Health Sci. 2014;6:87–98. doi: 10.5539/gjhs.v6n5p87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Góth L., Rass P., Páy A. Catalase enzyme mutations and their association with diseases. Mol. Diagn. 2004;8:141–149. doi: 10.1007/BF03260057. [DOI] [PubMed] [Google Scholar]
- 78.National Nanotechnology Initiative National Nanotechnology Initiative Supplement to the President’s 2021 Budget. https://www.nano.gov/sites/default/files/pub_resource/NNI-FY21-Budget-Supplement.pdf