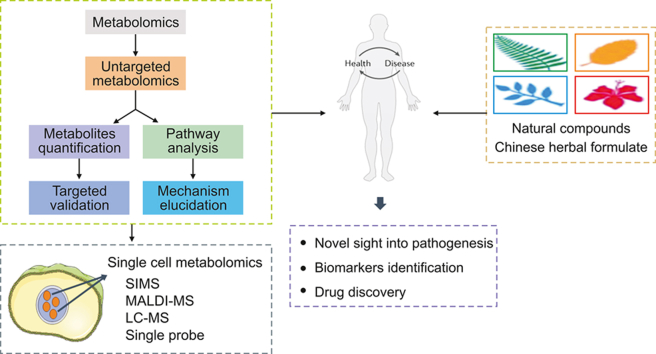
Abstract
Ischemic stroke (IS) is a multifactorial and heterogeneous disease. Despite years of studies, effective strategies for the diagnosis, management and treatment of stroke are still lacking in clinical practice. Metabolomics is a growing field in systems biology. It is starting to show promise in the identification of biomarkers and in the use of pharmacometabolomics to help patients with certain disorders choose their course of treatment. The development of metabolomics has enabled further and more biological applications. Particularly, metabolomics is increasingly being used to diagnose diseases, discover new drug targets, elucidate mechanisms, and monitor therapeutic outcomes and its potential effect on precision medicine. In this review, we reviewed some recent advances in the study of metabolomics as well as how metabolomics might be used to identify novel biomarkers and understand the mechanisms of IS. Then, the use of metabolomics approaches to investigate the molecular processes and active ingredients of Chinese herbal formulations with anti-IS capabilities is summarized. We finally summarized recent developments in single cell metabolomics for exploring the metabolic profiles of single cells. Although the field is relatively young, the development of single cell metabolomics promises to provide a powerful tool for unraveling the pathogenesis of IS.
Keywords: Metabolomics, Ischemic stroke, Biomarker, Precision medicine, Single cell metabolomics
Graphical abstract
Highlights
-
•
Metabolomics approach is used to identify IS biomarkers and reveal the underlying pathophysiological processes.
-
•
Metabolomics approach explores the molecular mechanism of Chinese herbal medicine for IS.
-
•
Metabolomics plays a crucial function in characterizing disease phenotypes and identifying individualized metabolic signatures.
-
•
Single cell metabolomics, a future tool to reveal IS pathogenesis and cellular metabolism.
1. Introduction
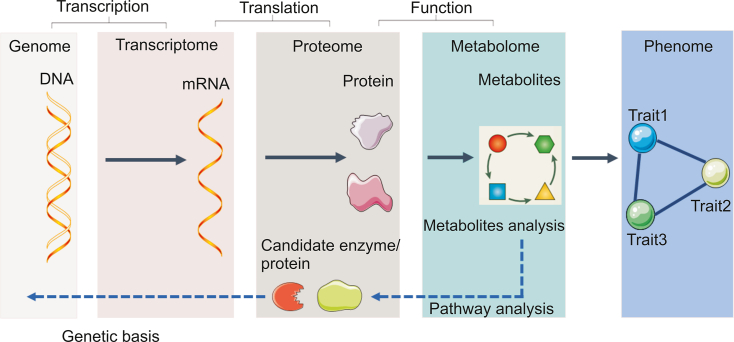
Metabolites stand for both the upstream input of the environment and the downstream output of the genome. Thus, metabolomics, the study of metabolites and metabolism, is the center of systems biology research and enables researchers to study how genes and the environment interact (Fig. 1). Due to the development of analytical techniques and bioinformatics, metabolomics has become a new diagnostic tool in clinical and biological research. It is a relatively young omics approach and may be the most promising omics strategy because it directly reflects cellular phenotypes and aids in understanding the downstream effects of the central dogma (Box 1). Given these characteristics and the ease with which biological samples such as cells, tissues and blood may be studied by metabolomic analysis, metabolomics has great potential for the discovery of biomarkers [1]. Metabolomics has several limitations, most of which relate to sample consistency, confounding factors, and the detection of low-abundance metabolites. Despite the limitations of metabolomics, new metabolic insights and advances in biological sample preparation and instrumentation that allow increasingly easy metabolomic analysis are leading to changes in the way that drugs are discovered, developed and treated [2,3]. Metabolomics makes it possible to analyze individual metabolic phenotypes, which is believed to contribute to the development of precision medicine.
Fig. 1.
A systems biology approach with a focus on metabolomics.
Box 1. Features of metabolomics.
-
▪
Metabolism and metabolic phenotypes indicate what is currently happening compared to genes and genetic risk.
-
▪
Metabolomics is the study of small-molecule metabolites and provides a functional fingerprint of the body's physiological and pathological conditions.
-
▪
For metabolomics studies, pattern recognition and metabolite identification are two helpful methods.
-
▪
Combined with drug metabolism, metabolomics is used for personalized medicine.
Alt-text: Box 1
Stroke is an acute cerebrovascular disease and a common cause of death in the elderly, affecting more than 15 million people worldwide [[4], [5], [6]]. Stroke is the second most common cause of death [7]. Ischemic stroke (IS) is a major disease type of stroke [8]. Among elderly stroke patients, 88.27% had an IS [9]. At present, clinical treatment strategies for IS remain a great challenge [10]. Only two available trials including intravenous tissue plasminogen activator and mechanical thrombectomy have been the currently authorized therapeutic options [11,12]. However, these two treatments both have the limitation of narrow therapeutic window and several safety concerns [13]. Therefore, the development of novel strategies is essential to save people with IS.
Ischemic brain injury is extremely complicated and involves a series of pathological processes [14]. Metabolite disturbances are believed to be important factors in IS. The development of metabolomics makes it possible to discover these potential biomarkers with diagnostic and prognostic relevance. Recently, some metabolomics studies have also revealed the pathological process of IS. Because IS has a complicated pathogenic mechanism, it is difficult to develop an effective treatment method based on the strategy of “one drug, one target,” which leads to a subpar therapeutic outcome [15]. Chinese herbal medicine (CHM) has been used for thousands of years in China to treat patients for stroke. In recent years, the metabolomics approach has been gradually applied to research on CHM, and attempts have been made to emphasize the critical role that metabolomics plays in resolving the numerous problems that CHM faces. It may scientifically express the meaning of syndromes and the efficacy of CHM treatment [16,17]. The development of metabolomics provides a favorable opportunity for CHM research, clarifies the molecular targets and network regulations of the active compounds and CHM formulae, and helps people comprehend the fundamentals of CHM formulae to treating ischemic brain injury.
In this paper, we first look at the analytical platforms and recent advances in metabolomics, and discuss the advantages and disadvantages of each metabolomics approach. We review the application of metabolomics in IS research and analyze the main research information of metabolomics. Our goal is to find metabolic biomarkers that require further investigation to forecast the risk of IS, establish a clinical diagnosis, and recognize complications. Second, by analyzing the abnormal metabolic pathways in IS, we may gain a better understanding of the molecular processes underlying IS, which help develop new therapeutic strategies for IS disease. Additionally, we summarize representative natural ingredients and CHM formulae for the treatment of ischemic brain damage. Then, we discuss metabolomics-based research in personalized medicine and discuss the role of metabolomics in precision medicine and biomarker discovery. Finally, a higher resolution, single-cell level metabolomics analysis is introduced. It is evident that the field of metabolomics is advancing toward single cell analysis and discovery, which is undoubtedly one of the most powerful tools we presently have at our disposal. This will allow us to perform a more detailed and specific analysis of IS.
2. Search syntax and inclusion criteria
Relevant published studies were identified for the years 2005–2023 by means of Pubmed and Web of Science search in terms of the following general string with title: ‘‘Metabolomics’’ or ‘‘Metabolomics and Cerebral ischemia’’ or ‘‘Metabolomics and IS’’ or ‘‘Metabolomics and biomarker’’ or ‘‘Metabolomics and Traditional Chinese Medicine’’ or “Single-cell metabolomics”, and ‘‘IS’’ or ‘‘Cerebral ischemia’’. The pre-set criteria for inclusion were (i) CHM or natural plants (e.g. Traditional Chinese Medicine formulas, herbal extracts): or individual compounds used for the prevention and treatment of IS; (ii) Only single-cell metabolomics studies were searched; (iii) Using the method of metabolomics research rather than using other methods; (iv) The study attempted to elucidate the underlying mechanism of CHM; (v) Only publication in English was included.
3. Analytical platforms in metabolomics
Modern analytical chemistry techniques and advanced computational tools are employed in metabolomics to characterize complicated biological products. The two analytical techniques that are utilized most frequently in metabolomics research are by far nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) [18]. Currently, hundreds of metabolites are detected and quantified simultaneously in a single sample, depending on the analysis platform employed, particularly, the widely used chromatography-MS systems, such as liquid chromatography-MS (LC-MS) and gas chromatography-MS (GC-MS) (Table 1) [[19], [20], [21], [22], [23], [24], [25]].
Table 1.
Comparison of metabolomics approaches.
| Technology | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| NMR spectroscopy |
|
|
[[19], [20], [21]] |
|
|
||
|
|
||
| GC-MS |
|
|
[22,23] |
|
|
||
|
|
||
| LC-MS |
|
|
[24,25] |
|
|
||
|
|
NMR: nuclear magnetic resonance; GC-MS: gas chromatography-mass spectrometry; LC-MS: liquid chromatography-mass spectrometry.
3.1. NMR spectroscopy
NMR spectroscopy is used for quantitative analysis because its signal intensity is proportional to the number of 1H, 13C, 15N, and 31P in a single nucleus [26]. In the field of NMR research, quantitative nuclear magnetic resonance (qNMR) is an important subfield focused on the precise and reproducible measurement of molecular concentrations [27]. Utilizing qNMR, active chemicals in complicated matrices have been extensively investigated and measured (e.g., body fluids, natural product extracts) [28,29]. In metabolomics, one of the main reasons for using 1H NMR spectroscopy is that it is fast and the resulting spectra are not greatly affected by saline biological fluids. However, due to its low sensitivity, NMR is unable to measure low-abundance metabolites. Since there is no proper separation method, the signals from thousands of metabolites overlap, making precise structure identification challenging and time-consuming [30]. However, technological advances have reduced these shortcomings and improved the sensitivity and resolution of NMR methods [31]. For example, when compared to one-dimensional NMR spectra, two-dimensional NMR spectra allow for superior compound identification since crowding and overlap are reduced, and cross peaks exclusive to pairs of spin coupled nuclei are used to identify specific molecules [32]. Identifying compounds is more challenging when there is a chance that the peaks in a NMR spectrum may shift due to changes in sample temperature or pH value. Heteronuclear single quantum coherence (HSQC) methods have improved spectral resolution and metabolite identification. By exploiting the larger 13C chemical shift dispersion on one axis of the two-dimensional spectrum, HSQC spectroscopy could offer better resolution and greater metabolite selectivity.
3.2. MS-based spectroscopy
3.2.1. GC-MS
In contrast to NMR spectroscopy, MS provides a highly sensitive and selective way to quantify hundreds to thousands of metabolites based on their mass/charge ratio and signal strength. Although direct injection of biological samples is feasible, most MS platforms combine separation modes such as GC or LC [33]. The analysis of naturally volatile or derivatized metabolites is done using GC-MS, and GC coupled to MS may be used for improved sensitivity and resolution [34]. Because the 13C-labeled metabolites are heavier than those containing the 12C isotope, GC-MS is also used to track them [35]. The advantages of GC compared to LC are that it has a strong chromatographic form with only insignificant changes during the analysis, and that since the analytes are volatile, they may be conveniently added to the mass spectrometer.
However, many metabolites are not volatile and need to be derivatized first. For example, fatty acids require conversion to methyl esters, amino acids, and sugars, which can be analyzed by adding one or more trimethylsilyl groups [36]. In addition, derivatization itself has the potential to destroy metabolic information. This process also makes it difficult to determine the source of the metabolites.
3.2.2. LC-MS
An alternative strategy to GC-MS is required to investigate intricate macromolecules while ensuring that metabolites are ionized without being destroyed. Due to its ability to detect and quantify a large number of metabolites without the need for derivatization, LC-MS has been widely employed for metabolite studies [37]. Metabolite identification and chromatographic reproducibility are the two main obstacles hindering the development of LC-MS metabolomics techniques. Additionally, determining the structure of a metabolite becomes more challenging if multiple isomers are present, or “adducts” are created when molecules combine with other molecules. As analytical platforms and methods continue to improve and refine, some researchers have removed the chromatography step and used direct injection instead. To improve the ability to identify metabolites, high-resolution instruments or triple quadrupole instruments are used to detect metabolites through their unique fragmentation patterns. Although matrix effects affect the accuracy of LC-MS results, this restriction may be circumvented by utilizing a variety of sample extraction processes and advanced tandem MS technique.
4. Pathophysiological signatures of IS
The pathophysiological effects of IS are caused by multiple complex molecular and cellular interactions that ultimately lead to brain damage and neurological dysfunction. The underlying pathophysiological processes of IS include genetic alterations, oxidative and nitrative stress, inflammatory, ferroptosis, and disturbance of the microbiota.
4.1. Genetic signature of IS
The development of cerebrovascular disease mainly involves genetic variables. IS is a multifactorial polygenic disease in which the role of environmental influences has been well studied, while the role of genetic factors needs to be further elucidated [38]. With the advancement of whole-genome sequencing technology, it has been revealed that the bulk of transcripts are non-coding RNAs and that only approximately 2% of the human genome gets transcribed into proteins [39]. Non-coding RNAs are discovered to perform housekeeping tasks in a variety of biological processes by participating in the transcriptional and post-transcriptional regulation of gene expression [40]. Among them, long non-coding RNAs (lncRNAs) are of special importance in IS biology [41]. Akella et al. [42] showed that numerous lncRNAs are abnormally expressed in the brain and blood after IS. Numerous of these lncRNAs were discovered to alter biochemical pathways, and these changes in expression displayed distinctive temporal and cell-type-dependent patterns. Therefore, it is impossible to overlook the involvement of lncRNA in pathogenesis following IS. LncRNA is expected to develop into a new model and target for regulating IS, as it is a significant endogenous regulatory mechanism (Fig. 2).
Fig. 2.
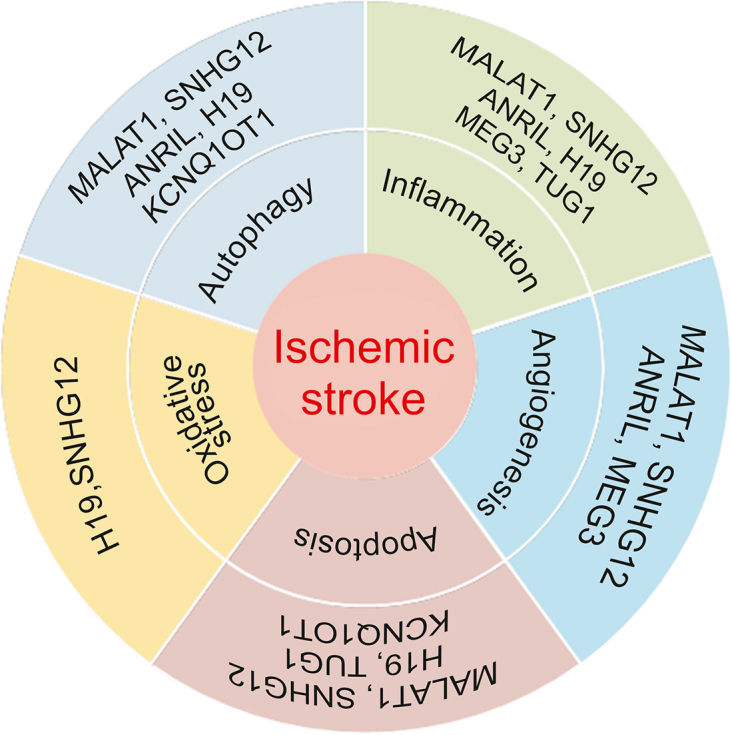
The role of lncRNAs in the pathophysiology of ischemic stroke (IS). MALAT1: metastasis-associated lung adenocarcinoma tran 1; SNHG12: small nucleolar RNA host gene 12; ANRIL: antisense non-coding RNA in the INK4 locus; MEG3: maternally expressed gene 3; TUG1: taurine upregulated 1; KCNQ10T1: potassium voltage-gated channel subfamily Q member 1 opposite strand 1.
4.2. Oxidative and nitrative stress signatures of IS
Free radicals have a crucial part in the damage caused by brain ischemia-reperfusion, including reactive oxygen species (ROS) and reactive nitrogen species (RNS) [43]. Acute IS is known to increase the production of free radicals, and both ROS and RNS molecules are confirmed to play a key role in tissue destruction [44,45]. Free radical production during ischemia is caused by many processes, including N-methyl-d-aspartate-mediated excitotoxicity, increased Ca2+ influx, mitochondrial failure, and activation of neuronal nitric oxide synthase [46,47]. Overproduction of ROS damages cellular macromolecules and contributes to the signaling mechanisms that lead to apoptotic cell death [48]. Reactive radical overproduction has negative consequences that damage brain cells, but at homeostatic levels, these radicals are necessary signaling molecules that support proper neuronal activity [49]. Normally, free radical scavengers and antioxidant enzymes prevent or mitigate the consequences of free radical damage [50]. Redox homeostasis is kept in check by antioxidant and detoxifying enzyme activity. These enzymes include glutathione reductase, glutathione peroxidase, superoxide dismutase (SOD), and glutathione-S-transferase, which are being studied for their potential roles in IS [51]. The SOD enzymes, such as extracellular SOD and manganese SOD, aid in the recovery of the brain after ischemia-reperfusion injury [52].
4.3. Inflammatory signatures of IS
Immunological and inflammatory cells play a major role in IS. Following cerebral ischemia, there is a significant inflammatory response that involves the activation of local inflammatory cells, the production of inflammatory mediators, and the recruitment and migration of leukocytes across the blood-brain barrier (BBB) [53]. These cytokines are key players in the development of the immuno-inflammatory response induced by a stroke, which affects the severity and course of the disease [54]. Cytokines with pro-inflammatory effects are involved in many processes in cerebral ischemia, and they directly activate endothelial cells, neurons and glial cells. For instance, endothelial nitric oxide synthase injury increases the level of ROS because nitric oxide (NO) synthesis is diminished [55]. Increased ROS production causes pro-inflammatory genes to become active. Upon being activated, inflammatory cells, including microglia and astrocytes, release cytokines and chemokines, as well as matrix metalloproteinases (MMPs), NO, and even more ROS [56]. Overall, these processes exacerbate cellular damage and further disrupt BBB, such as endothelial dysfunction due to endothelial cell apoptosis [55,57].
As early as 6 h into the process of ischemic brain damage, infiltrating neutrophils may appear in the ischemic penumbra region and create neutrophil extracellular traps [58]. At 48 and 96 h following an IS, neutrophil infiltration increased further. Pro-inflammatory N1 neutrophils in ischemic brains promote the production of inducible nitric oxide synthase (iNOS), MMP-9 and myeloperoxidase (MPO), exacerbating oxidative stress-related brain damage. At the same time, neutrophil polarization into the N2 type provides neuroprotective benefits [59]. The microenvironment through which neutrophils penetrate may explain their multiple functions. Oxidative/nitrogen stress and its associated redox signaling cascades in the microenvironment may play a crucial role in neutrophil polarization, particularly during ischemic injury. Therefore, inhibiting inflammatory factor-mediated neuronal cell death and enhancing the therapeutic result of IS may be achieved by targeting inflammatory cells and their surroundings.
4.4. Ferroptosis signatures of IS
The buildup of excessive intracellular iron ions, lipid peroxides and associated metabolites, as well as the peroxidation of polyunsaturated fatty acids, are the hallmarks of ferroptosis, a distinct kind of programmed cell death [60]. Recently, it has been shown that individuals with ischemic cerebrovascular diseases have a remarkably increased amount of iron deposition in the brain tissue, and it is believed that this iron deposition may serve as an imaging marker of ischemic areas [61]. According to epidemiological studies of population samples, iron deficiency anemia and IS have a high correlation [62]. In addition, it has been demonstrated that IS patients have considerably higher serum iron ions, and that hepcidin plays a critical role in iron overload following cerebral ischemia [63]. A middle cerebral artery occlusion model was utilized by Tuo et al. [64], who discovered considerable iron buildup in the ischemic half-dark zone of brain tissue and iron death in neurons. The results indicated that silencing Tau protein ameliorates this injury and may be involved in regulating the mechanism of ischemia-reperfusion-induced iron death. Ferroptosis and IS have received comparatively little scientific attention, and consequently the next line of inquiry for researchers should focus on the precise mechanism and therapeutic approach of ferroptosis in ischemia injury to brain tissue.
4.5. Gut microbiota signatures of IS
Recent studies have suggested that compromised gut microbiota may be a risk factor for IS and have an impact on the prognosis following IS [65]. The gut microbiota is a microorganism that is dispersed throughout the digestive system and is primarily composed of Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria and Verrucomicrobia [66]. In particularly, the Firmicutes/Bacteroidetes ratio was used as a predictor of health and disease [67]. The IS alters the gut microflora's makeup. On the contrary, the gut microbiota influences the development of IS and regulates its outcome. The neuronal networks connecting the brain to the gut form a complex gut-brain or brain-gut axis with considerable bilateral connectivity. Growing evidence suggests that gut microbiota may be crucial to the development and management of IS [68,69]. Three days after ischemia-reperfusion in mice, higher levels of Bacteroidetes were found, which was considered a marker of ecological dysbiosis after IS [70]. On the other hand, a clinical study that collected stool samples two days after admission and examined the results showed that patients with acute IS and transient ischemic attacks had lower levels of Bacteroidetes [71]. Relatively low concentrations of Oscillospira, Streptococcus, Lactobacillus, and Faecalibacterium were seen in monkeys following local cerebral ischemia [72]. The principal sources of butyrate in host organisms are known to be Faecalibacterium and Oscillospira species [73,74]. Butyric acid, which reduces the production of pro-inflammatory cytokines and protects the integrity of the intestinal barrier, has been suggested as a potential treatment for brain disorders [75]. It should be stressed that Lactobacillus is an essential host probiotic that has been shown to improve mood, cognition, and reduce inflammation associated with aging [72,76,77]. Along with persistent systemic and brain inflammation, frequent stroke consequences include dementia and depression [[78], [79], [80]]. Future clinical trials should examine the potential benefits of lactobacillus supplementation in patients with stroke. These findings suggest that in addition to gut microbiota dysbiosis, persistent systemic inflammation may emerge following brain infarction. Additionally, correlation studies demonstrated a strong relationship between excessive Bacteroidetes growth and elevated plasma lipopolysaccharide or inflammatory cytokine levels [72]. As a result, pathological alterations in the brain are strongly correlated with the production of proinflammatory cytokines from the gut into the blood circulation [77]. Therapeutic targets for IS may include the post-stroke gut microbiota and persistent systemic inflammation.
5. Metabolomics studies in IS
The metabolome of an organism is regulated by internal processes and the environment, and offers detailed information about the state of the organism. In this sense, metabolomics not only makes it feasible to find disease biomarkers in the form of endogenous (gene-derived) and exogenous (environment-derived) metabolites, but also provides novel insights into the underlying causes of disease [81,82]. With these novel metabolic insights and the expanding availability of metabolomics research, there is little question that metabolomics will play a significant role in IS treatment strategies in the future.
Cerebral ischemia leads to local and systemic metabolic changes, such as changes in cellular energy metabolism pathways. Metabolomics studies are used to identify these metabolic changes to determine whether metabolites are used as biomarkers to conduct IS diagnosis and assess IS risk. Metabolites are made up of small molecules such as nucleotides, amino acids and lipids [83]. Metabolites are the result of genetic, protein and environmental changes [84]. Therefore, profiling of the metabolome might provide system-level information.
Metabolites associated with IS, such as amino acids, lipids, polyamines, and nucleotides, have been explored to understand the disease mechanisms of cerebral ischemia [85]. Guo et al. [86] used LC-MS studies to find that serum concentrations of 37 metabolites were substantially different from those in a control group. These metabolites include sphingolipids, lysophosphatidylchoine, stearic acid, phosphatidylchoines, organic acids, amino acids, and carnitine derivatives, and pathway enrichment analysis has shown that key metabolites were involved in the biosynthesis of unsaturated fatty acids, ɑ-linolenic acid metabolism, fatty acid metabolism, ketone body synthesis and degradation, and the interconversion of pentose and glucuronide. Similarly, Wu et al. [87] employed LC-MS to find that there were reduced quantities of phosphatidylchoines, phosphatidylethanolamines (PEs), lysoPEs, and sphingosine-1-phosphate in the blood samples, showing perturbations in lipid metabolic pathways. In addition, blood concentrations of lactate, glucose, glucose 6-phosphate, succinate, malate, and citrate increased compared to control. Since these metabolites are associated with energy metabolism, this suggests that the glucose and anaerobic glycolytic pathways were disturbed [88]. Luo et al. [89] found that the metabolites of lactate, creatine, glycine, alanine, leucine, and lysine in brain tissue, cerebrospinal fluid, and plasma of rats were changes, indicating disturbances in amino acid metabolism. Metabolic alterations associated with IS were summarized in Table 2 [[90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104]].
Table 2.
Metabolic alteration association with IS.
| Change of metabolites | Metabolites type | Analytical platforms | Statistical analysis | Different stage of IS | Major findings based on metabolomics analysis | Refs. |
|---|---|---|---|---|---|---|
| ↑Lactate, pyruvate, glycolate, formate; ↓Valine, Glutamine, lipid CH2CH2C=C, methanol, VLDL/LDL CH3 |
Amino acids, lipids, other metabolites | 1H NMR | OPLS-DA | Acute IS | The research demonstrated that a metabolomics approach might be useful for the effective diagnosis of cerebral infarction. | [90] |
| ↑S-adenosylhomocysteine, cysteine, oxidized glutathione, hydroxyeicosatetraenoic acid, hydroxyoctadecadienoic acid; ↓Tetrahydrofolate, adenosine, aldosterone, deoxocathasterone, betanin, sucrose 6-phosphate, folic acid |
Lipids, organic acids, amino acids, other metabolites | UPLC/MS | PLS-DA, PLS-KNN | Acute IS | This study proposed a metabolomics approach to study potential biomarkers and metabolic pathways associated with cerebral infarction and developed a predictive model of cerebral infarction for rapid diagnosis. | [91] |
| ↑Glucose, N-carbamoyl-beta-alanine; ↓Asparagine, carnitine, cis-/trans-hydroxyproline, creatinine, cysteamine, glycine, histidine, isoleucine, leucine, lysine, methionine, proline, threonine, uridine, 5′-adenosylhomocysteine |
LC-MS/MS | PCA, PLS-DA | Acute IS | Branched-chain amino acids are reduced in ischemic stroke, and the degree of reduction is associated with worse neurological outcomes. | [92] | |
| ↑Phenylalanine, proline, tyrosine, sphingosine 1-phosphate, hypoxanthine, kynurenine, N-acetylneuraminic acid, uric acid, palmitoylcarnitine; ↓Lysophosphatidylcholines (14:0, 16:0, 18:0, 18:1, 18:2, 20:1), isoleucine, tryptophan, valine |
Amino acids and other metabolites | UPLC/MS | PCA, PLS-DA, OPLS-DA | Acute IS | Metabolomic studies provided a new strategy for stratifying stroke patients with cognitive impairment using serum-based metabolite markers, which might have important implications for understanding pathological mechanisms. | [93] |
| ↑Aspartic acid; ↓Phenylalanine, proline, pyroglutamate, serine, stearic acid, valine, isoleucine, linoleic, oleic, palmitic, creatinine |
Amino acids | GC-MS | PLS-DA | Chronic IS | Metabolomics approach provided a novel metabolic insight about PSD and the metabolites for classifying PSD. | [94] |
| ↑Palmitic acid, 5-hydroxyhexanoic acid, sucrose, lactic acid, azelaic acid, glyceric acid, pseudouridine; ↓Hippuric acid, 3-hydroxyisobutyric acid, b-aminoisobutyric acid, leucine, phenylalanine, indoxyl sulfate, ribose, hypoxanthine, pyroglutamic acid |
Organic acids, amino acids, other metabolites | GC-MS | PLS-DA | Chronic IS | A urine-based biomarker might be useful in the diagnosis of PSD. | [95] |
| ↑Citrulline, 3-hydroxyisovalerylcarnitine (C5-OH), C5-OH/C0, alanine | Amino acids and lipids | LC-MS/MS | Significance analysis of microarrays, student's t-test | Acute IS | This study proved the targeted metabolomics analysis was a promising tool for rapid and timely stroke differentiation. | [96] |
| ↑Leucine, valine, isoleucine | Amino acids | LC-MS/MS | Multivariable Cox regression model | Chronic IS | Higher concentrations of branched-chain amino acids are associated with an increased risk of cardiovascular disease, particularly stroke. | [97] |
| ↑Phenylalanine, formate; ↓Lactate, glutamate, acetamide, hydroxybutyrate, sarcosine |
Organic acids and amino acids | 1H NMR | OPLS-DA | Chronic IS | The metabolomics approach of NMR was applied to explore differential metabolites in the urine of PSD patients and identify potential biomarkers for PSD diagnosis. | [98] |
| ↑Acylcarnitines with short chains (C2−C7) and those with medium chains (C8−C14) | Lipids | LC-MS/MS | Multivariable Cox regression model | Chronic IS | The metabolic product profile characterized by an increase in acylcarnitine concentration is independently associated with the risk of cardiovascular disease and stroke. | [99] |
| ↑Glutamate; ↓Glutamine |
Amino acids | LC-MS/MS | Multivariable Cox regression model | Chronic IS | Glutamine-to-glutamate ratio was associated with decreased risk of IS. | [100] |
| ↑Ceramides (16:0, 22:0, 24:0, and 24:1) | Lipids | LC-MS/MS | Multivariable Cox regression model | Chronic IS | This study indicated that a positive association between plasma ceramide concentrations and incident CVD. | [101] |
| ↑TG (52:5), TG (54:5), TG (54:4), TG (54:3), DG (38:6), LysoPC (20:5), LysoPC (20:4), LysoPC (22:6), LysoPC (24:0), TG (56:5); ↓GluCer (38:2); PE (35:2); fatty acid (16:1) |
Lipids and fatty acids | LC-MS | PCA | Chronic IS | Lipomics methods search for biomarkers for disease diagnosis and provide special insights into stroke metabolism induced by small vessel diseases. | [102] |
| ↑Homocysteine sulfinic acid; ↓N6-acetyl-l-lysine, ubiquinone, 2-oxoglutarate, 5-aminopentanoate, cavaverine, l-lysine, l-valine, nicotinamide, S-(2-methylpropionyl)-dihydrolipoamide-E, cadaverine |
Amino acids, lipids and other metabolites | UPLC/MS | OPLS-DA | Chronic IS | Lysine catabolites in patients with thrombotic stroke serve as neoplasm markers for non-invasive detection of early thrombotic stroke. | [103] |
| ↑Galactose, lysophosphatidylethanolamines (18:2, 20:2, 20:4, 20:5), acetylcarnitine, betaine, carnitine, L-isoleucyl-l-proline, mannos; ↓Proline, serine, threonine, tricarballylic acid, and trihydroxy palmitic acid, alanine, aspartate, glycine, isoleucine, lysine, lysophosphatidylcholine (16:0), ornithine, phosphatidic acid (18:3/0:0), phosphatidylcholine (1:0/16:0, 5:0/5:0), ornithine |
Amino acids and other metabolites | GC-MS, LC-MS | PCA, PLS-DA, Mann-Whitney U test | Acute IS | In this study, metabolomics was utilized to characterize the metabolic features of the serum of patients with AIS to identify novel sensitive biomarkers for diagnosis and progression. | [104] |
NMR: nuclear magnetic resonance; UPLC/MS: ultraperformance liquid chromatography/mass spectrometry; GC-MS: gas chromatography-mass spectrometry; LC-MS: liquid chromatography-mass spectrometry; PLS: partial least squares; PLS-DA: partial least squares discriminant analysis; OPLS-DA: orthogonal partial least squares discriminant analysis; PCA: principal component analysis; IS: ischemic stroke; PSD: post-stroke depression; CVD: cardiovascular disease.
6. Metabolomics in the treatment of IS with CHM
The amount of metabolomics data has exploded dramatically in recent years, which has had an impact on all areas of life sciences, including the discovery and development of drugs [105]. There is currently mounting evidence that CHM may regulate multiple targets with fewer side effects and lower toxicity, and that it is garnering more attention in the face of complex disease causes [106]. For the creation of new drugs, CHM may be an inspiration. Numerous international investigations have recently been conducted to decipher the mechanism of action of CHM. Many metabolomic-based approaches have been used in these investigations to promote the exploration of CHM. Furthermore, metabolomics in conjunction with pharmacology is a reliable method for the study of novel biologically active substances and targets, as well as the presence of interactions [107]. Metabolomics is expected to significantly advance CHM research and contribute to the modernization of CHM by expanding the use of modern means.
6.1. Extraction components from CHM for IS
CHM originates from natural sources and is used for clinical treatment in China. Chinese medicine plays a crucial role in the discovery and development of new drugs, such as, the discovery of artemisinin and its remarkable contribution in the fight against malaria. It includes effective extract ingredients and Chinese herbal formulas and preparations. Researchers find that some CHM extractions, such as sesamin, tanshinone IIA, salvianolic acid A, salvianolic acid B, 6-paradol, baicalin, astragaloside IV, totarol, ginkgetin aglycone, naringin and 6′-O-galloylpaeoniflorin, have a potent neuroprotection effect against IS. As we all know, CHM extractions are frequently used in clinical for therapy, and most of their applications have been shown to be effective against IS. In addition to reducing oxidative stress, inflammation, and neuronal death, CHM extractions also promote angiogenesis and regulate autophagy to protect IS against a variety of threats. In a word, it is important to explore the mechanisms and effective molecular targets of CHM, which will provide new insights into the treatment of IS and facilitate the process of discovering new active drugs from CHM. Thus, we cover the effects of CHM extractions in ischemic brain damage in this part (Table 3) [[108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118]].
Table 3.
The role of natural bioactive compounds in ischemic brain injury.
| Compounds | CHM | Major results | Targets | Model | Dosage | Refs. |
|---|---|---|---|---|---|---|
| Sesamin | Sesame | Inflammatory, oxidative stress, infarct volume | COX-2, Cleaved-Caspase-3, SOD, iNOS, TNF-α, IL-6, p-ERK1/2, p-P38 | MCAO (I 2 h/R 22 h) (Male mice) | 30 mg/kg (i.p.) | [108] |
| Tanshinone IIA | Salviae Miltiorrhizae Bunge | Infarct volume, cell apoptosis | HMGB1, NF-κB | MCAO (I 2 h/R 22 h) (Male mice) | 5 or 10 mg/kg (i.p.) | [109] |
| Salvianolic acid A | Salviae Miltiorrhizae Bunge | Apoptosis, inflammation, nitrosative stress, infarct volume, BBB permeability, neurogenesis | Caspase-3, p35, nitrotyrosine, GSK3, p35/p25, Cdk5, -catenin, DCX, Bcl-2, PI3K, Akt | MCAO (I 2 h/R 22 h) (Male mice) | 100 μg/kg (i.v.) | [110] |
| Salvianolic acid B | Salviae Miltiorrhizae Bunge | Neuroinflammation | CD40, ICAM-1, IL-1β, IL-6, IL-8, MCP-1, NF-κB/p65, IκBα | MCAO (I 1 h/R 24 h) (Male Wistar rats) | 3, 6 and 12 mg/kg (i.p.) | [111] |
| 6-paradol | Zinginber | Infarct Volume, neurological deficit, neuroinflammation | IL-6, TNF-α, iNOS | MCAO (I 1.5 h/R 22 h) (Male ICR mice) | 10 mg/kg (i.g.) | [112] |
| Baicalin | Scutellaria baicalensis Georgi | Infarct volume | NF-κB | MCAO (I 1 h/R 24 h) (Male Waster rats) | 50, 100 and 200 mg/kg (i.v.) | [113] |
| Astragaloside IV | Radix Astragali | Neurological dysfunction, infarct volume, neuroprotective | SIRT1, MAPT | MCAO (I 2 h/R 7 days) (Male SD) rats) | 20 mg/kg (i.p.) | [114] |
| Totarol | Podocarpus totara | Infarct volume Neuroprotective | Akt, GSK-3β, Nrf2, HO-1, GSH, SOD | MCAO (I 2 h/R 24 h) (Male SD) rats) OGD/R (OGD 6 h/R 24 h) | 0.1, 1 and 10 μg/kg (i.v.) 5 μM |
[115] |
| Ginkgetin aglycone | Ginkgo | Brain edemag, oxidative stress, neuroinflammation | NF-κB, IL-1β, TNF-α, SOD, MDA, Bcl-2, Akt, TLR-4, STAT-3, SIRT-1 | MCAO (I 0.5 h/R 24 h) (Male Waster rats) | 100 or 200 mg/kg (i.p.) | [116] |
| Naringin | Grapefruit | Infarct volume, cell apoptosis | Superoxide, peroxynitrite, nitric oxide, NADPH oxidase, eNOS, Parkin, LCI/II | MCAO (I 1.5 h/R 24 h) (Male SD Rat) | 80, 120, or 160 mg/kg (i.v.) | [117] |
| 6′-O-galloylpaeoniflorin | Peony | Apoptosis, oxidative stress, inflammatory response, infarct volume Apoptosis, oxidative stress, inflammation, cell viability |
SOD, MDA, GSH, Nrf2, p-P38, p-JNK, Iba1, IL-1, TNF-α, Caspase-3 Nrf2, p-Akt, MDA, SOD, IL-1β, TNF-α, p-Akt | MCAO (I 2 h/R 24 h) (Male Waster rats) OGD/R (OGD 4 h/R 24 h) | 2.5, 5 or 10 mg/kg (i.p.) 10, 50 or 100 μM |
[118] |
CHM: Chinese herbal medicine; COX-2: cyclooxygenase-2; SOD: superoxide dismutase; iNOS: inducible nitric xide synthase; TNF-α: tumor necrosis factor-α; IL-6: interleukin 6; p-ERK1/2: phospho extracellular regulated protein kinases; p-P38: phospho mitogen-activated protein kinases; HMGB1: high mobility group box 1; NF-κB: nuclear factor kappa-B; Caspase-3: cysteinyl aspartate specific proteinase-3; GSK-3: glycogen synthase kinase-3; MCAO: middle cerebral artery occlusion; Cdk5: cyclin dependent kinase 5; DCX: recombinant doublecortin; BCL-2: B-cell lymphoma-2; PI3k: phosphatidylinositol 3-kinase; Akt: protein kinase B; CD40: tumor necrosis factor superfamily, member 5; ICAM-1: intercellular cell adhesion molecule-1; IL-1β: interleukin-1β; IL-8: interleukin 8; MCP-1: monocyte chemoattractant protein-1; IκBα: inhibitory subunit of NF Kappa B alpha; SIRT1: silent mating type information regulation 2 homolog-1; MAPT: microtubule-associated protein tau; Nrf2: nuclear factor erythroid 2-related factor 2; HO-1: heme oxygenase 1; GSH: Glutathione; MDA: macdonald dettweiler associates; TLR-4: toll-like receptor 4; STAT-3: signal transducer and activator of transcription 3; NADPH: nicotinamide adenine dinucleotide phosphate; eNOS: endothelial nitric oxide synthase; p-JNK: phospho-jun n-terminal kinase; Iba1: ionized calcium-binding adapter molecule 1; OGD/R: oxygen-glucose deprivation and reperfusion.
6.2. Metabolomics approach to explore the natural compounds and CHM formulae for IS treatment
Finding the metabolic biomarkers and signaling pathways, as well as understanding the therapeutic tenets of natural compounds and CHM formulations for IS, is made possible by the high throughput and high sensitivity of metabolomics. Numerous metabolites, including glutamate, isoleucine, leucine, valine, glycine, lysine, LysoPC (16:0), LysoPC (18:2), serine, uric acid, citrate, and palmitic acid, have the potential to act as biomarkers for ischemic brain injury. These metabolites have been linked to apoptosis, oxidative stress, excitotoxicity and inflammation [119]. Comprehensive profiles of the molecular targets of natural compounds and CHM formulations for the treatment of IS are provided by combining advanced metabolomics and network analysis.
Famous Chinese herbal remedy Huang-Lian-Jie-Du Decoction (HLJDD) has been used to treat IS [120]. HLJDD consists of Radix Scutellariae, Cortex Phellodendri, Rhizoma Coptidis and Fructus Gardenia [121]. Its neuroprotective benefits against brain ischemia-reperfusion damage are aided by HLJDD's antioxidant and anti-inflammatory properties [122,123]. Zhu et al. [124] used a metabolomics approach to identify 19 biomarkers associated with metabolic stress, glutamate/GABA-glutamine cycle, and enhancement of cholinergic neuron function. The disturbance of amino acid metabolism in MCAO ischemic rats was also reversed by HLJDD, as demonstrated by global and amino acid-targeted metabolomics combined with correlation network analysis [125]. In addition, berberine, baicalin, and jasminoidin, which are the main active ingredients in HLJDD, were indicated to have neuroprotective benefits. A recent study looked at the effects of berberine, baicalin and jasminoidin on neuroprotection. 1H NMR metabolomics indicated that these three components may regulate oxidative stress, aberrant metabolism, neuron autophagy and inflammatory response in cerebral ischemia-reperfusion damage [126,127].
A well-known CHM formula called Angong Niuhuang Wan (AGNHW) is frequently applied as emergency care in China for stroke and traumatic brain injury [128]. Clinical research suggests that AGNHW may reduce brain damage in IS patients [129,130]. The protective effects of AGNHW against cerebral ischemia-reperfusion injury included maintaining BBB integrity, reducing infarct size, and improving neurological function by inhibiting ROS/RNS-mediated MMP activation and maintaining tight junction proteins [131]. Utilizing ultra-performance liquid chromatography coupled with quadrupole time of flight mass spectrometry (UPLC/Q-TOF MS) and pattern recognition technique, the detoxicant components of AGNHW was discovered. According to the results, 36 of the 41 endogenous metabolites associated with liver toxicity in cinnabar and realgar might be regulated by the herbal components of AGNHW [132]. Additionally, Zhang et al. [133] used network pharmacology and molecular docking methods to study AGNHW, which identified quercetin, β-estradiol, berberine, baicalein and β-sitosterol as the main active ingredients and identified the key targets, including interleukin 6 (IL-6), AKT1, mitogen-activated protein kinase 3 (MAPK3), PIK3CA and tumor necrosis factor (TNF). Until now, the use of metabolomics to study the active ingredient of AGNHW and its application in ischemic stroke has not been reported.
When treating cerebral infarction, the CHM formulae Naodesheng (NDS) is frequently utilized and has positive therapeutic results [89]. In rats with localized cerebral ischemia, NDS was reported to significantly reduce the area of infarction and improve the impairment of neurological function [134]. NMR-based metabolomics approach to determine the protective effect of multiple components from NDS has been shown to regulate oxidative stress, energy metabolism, lipid metabolism, amino acid metabolism, and inflammation in cerebral ischemia-reperfusion injuries [89]. In another study, it was also shown that a bioactive extract of NDS had protective effects in rats with ischemic stroke, with potential mechanisms involving multiple metabolic pathways, like energy metabolism, amino acid metabolism, oxidative stress, and inflammatory damage [135]. Other CHM formulas, such as Naoshuantong Capsule [136], Fu-Fang-Dan-Zhi Tablet [137] and Mi-Jian-Chang-Pu Decoction [138] were used to understand the molecular mechanisms of CHM in the treatment of IS through metabolomics methods. However, there is still a need to use metabolomics approaches to explore the molecular mechanisms of the active ingredients in CHM and CHM formulations for the treatment of IS.
7. Data analysis and processing methods for metabolomics
Metabolomics is a omics approach that aims to qualitatively and quantitatively cellular metabolites [36]. Along with other omics such as genomes, transcriptomics, and proteomics, metabolomics provides essential insights into the make-up of organisms. Research on metabolomics has so far been conducted using a range of methods, mostly concentrating on the two popular platforms, NMR and MS. Although non-destructive and high reproducibility are demonstrated using NMR [21,139], MS-based metabolomics is a common technique because of its broad analytical coverage, high sensitivity, high throughput, and capability of molecular structure identification. Because metabolomics raw data is complicated, ongoing improvements to the analytical process are required for the best possible information retrieval. The complexity stems from the structure of the spectrographic data as well as the linear and nonlinear interactions between metabolites. There are some challenges with the data structure due to high noise levels, batch effects, and missing findings. The metabolomics community has thus always been keen to adopt fresh computational and statistical techniques to improve data processing.
Machine learning (ML) is a computer method to finding meaningful patterns in data [140]. For instance, assume that we get genome-wide gene expression measurements from the blood of individuals who have received a certain medication. Forecast whether a new patient would be receptive or unresponsive to the therapy based on his or her gene expression profile would be a typical issue that may be handled using a ML method [141]. To make credible predictions about unknown data, statistical models are trained on data in ML. Artificial neural networks (ANNs) were among the first ML methods to be used; in 1990, Curry and Rumelhart [142] released “MSnet” to discern between different classes of metabolite composition and ANNs were continuously used and improved [143,144]. A few years later, random forest and support vector machines were introduced into the field of omics [145,146]. The common supervised and unsupervised ML models are summarized in Table 4 [[147], [148], [149], [150], [151], [152]].
Table 4.
Description of statistical models in machine learning (ML).
| ML model | Approach | Features | Advantage | Disadvantage | Refs. |
|---|---|---|---|---|---|
| Supervised | PLS, PLS-DA, OPLS-DA | When the factors are collinear, PLS is a supervised method for building predictive models. To maximize the covariance between classes, PLS-DA, an extension of PLS, is used. OPLS-DA is designed to increase the interpretability of latent features. | High classification accuracy, low overfitting risk, and easy implementation | Collinear data | [147] |
| RF | It consists of several decision trees. The samples are separated by each decision tree based on the features of the measurements. | High classification accuracy, medium overfitting risk, and easy implementation. | The sample has a low classification. | [148] | |
| SVM | It determines the boundaries of the separate classes. A kernel function is used to add more dimensions to the data for nonlinear separation. | The sample has a high classification and easy implementation. | High overfitting risk, low data interpretation | [149] | |
| ANN | By using hidden nodes with the linear equation “z” and the nonlinear function “g”, features are transformed. There may be several layers after that, each having nodes containing transformations by the function “z”nd “g”. A “softmax” function generates the output. | The sample has a medium classification. | High overfitting risk, medium data interpretation and complex implementation | [150] | |
| Unsupervised | PCA | PCA is a method of reducing the dimensionality of datasets that minimizes information loss while improving interoperability. | Easy implementation | After the PCA transformation, it is difficult to estimate how many latent variables were ultimately retrieved. | [151] |
| Hierarchical clustering | It organizes the variable information in the form of data sets, so that objects in the same cluster are strongly related and objects in different clusters are weakly related. | Easy implementation, good interpretability | Outliers are more sensitive and the k value is not easy to grasp. | [152] |
PLS: partial least squares; PLS-DA: partial least squares discriminant analysis; OPLS-DA: orthogonal partial least squares discriminant analysis; RF: random forest; SVM: support vector machine; ANN: artificial neural network; PCA: principal component analysis.
ML has advanced quickly and now offers multiple algorithms. The vast of ML solutions feature intuitive user interfaces that let chemometricians evaluate different ML strategies and improve applications for spectrum analysis. Utilizing the proper statistical techniques, metabolic data is evaluated to identify biomarkers and determine connections between various factors [153]. In general, univariate statistics are used to analyze only one variable, whereas multivariate statistics are used to analyze many variables. Skewed or jagged datasets are utilized for nonparametric testing, whereas normally distributed datasets are used for parametric tests [154]. Unsupervised multivariate statistical analysis techniques include K-means, hierarchical clustering, self-organizing maps, and principal component analysis (PCA). These approaches make the data less dimensional and provide clusters based on sample-to-sample data similarity [[155], [156], [157]]. Using supervised multivariate statistical analysis techniques, the relationship between a given etiology and metabolites was investigated. The methods for regression and classification are included in the supervised multivariate statistical analysis. Regression between response and predictor variables was performed using partial least squares (PLS) and partial least squares discriminant analysis (PLS-DA) [158]. Orthogonal projections to latent structures (OPLS), an improved PLS method, employs an orthogonal signal correction filter to eliminate uncorrelated variables and reduce model complexity [159]. Different algorithms are used to simultaneously create classifiers, such as discriminative analysis, support vector machine (SVM), K-nearest neighbor classification, and soft independent modeling of class analogies [160,161].
8. Metabolomics and precision medicine
The goal of precision or customized medicine is to use advanced omics testing to customize a patient's medical care to their specific profile. Using high-throughput omics approaches such as genomics, proteomics, and metabolomics in conjunction with computational techniques such as bioinformatics, we may be able to learn more about specific targets in biological systems [162]. Among them, metabolomics involves the identification and quantification of small molecules, and the resulting network interactions, representing the functional genome of an individual. The entire collection of metabolites in a biological sample is called the “metabolome” and is highly dynamic. Significant changes in multiple metabolic pathways essential for maintaining cellular homeostasis may be evaluated at the metabolic level. Metabolomics combined with clinical chemistry actually provides over 190 clinically approved metabolite biomarkers [163]. The success of metabolomics in biomarker translation relative to other omics approaches appears to be that the underlying instrument (tandem mass spectrometer) is robust, greatly quantitative, readily adaptable to different detection methods and has been used in numerous clinical tests laboratory [163,164]. In addition, a lot of clinical chemistry laboratories have the necessary expertise, regulatory clearance and personnel qualifications to perform accurate chemical biomarker validation. Future clinical approval of several more drugs is expected, in line with further recognition of the inherent advantages of metabolomics. This is due to continued advances in metabolomic automation and quantification, as well as improvements in software and protocols for biomarker selection and validation.
Drug response and monitoring is another area where metabolomics has an impact on precision medicine. The individual's capacity to metabolize or utilize the medicine has a significant impact on drug response, which is very diverse. Despite the discipline of pharmacogenomics, which has genotyping as its major focus, is widely known, the developing of pharmacogenomics demonstrates how metabolomics may be utilized to augment this genetic information [165]. Measurements of medication dosage and response have been made possible by pharmacometabolomics, which has produced several exciting and beneficial findings. For instance, to optimize patient dose, fast MS-based monitoring of immunosuppressant medications and their metabolites is being used [166]. In addition, MS-based assays have been shown to be slightly faster, more sensitive and more robust than conventional immunoassays [167]. In drug dose determination, the metabolomics approach allows one to phenotype an individual's likely drug response prior to administration [168]. This metabolomics approach avoids the time-consuming and occasionally risky approach that many physicians employ when figuring out specific dosages for medications with constrained therapeutic windows. Recently, pharmacometabolomics has begun to benefit from several large-scale genome-wide association studies and metabolome-wide association studies [169,170]. Such studies not only help explain individual differences in metabolite levels, but also highlight how endogenous metabolites combined with single nucleotide polymorphism typing can be used to predict or better understand an individual's response to a drug or therapeutic intervention [170].
9. Single cell metabolomics: the future approach in IS research
“Metabolome” refers to the collection of all small molecule metabolites from a single cell to a whole organism [171]. These metabolites represent the physiological state of the sample and are necessary ingredients for many intracellular processes and activities. Cells are the basic components of organisms [[172], [173], [174]]. The downstream products of the genome, transcriptome, and proteome are the cellular metabolomes, and the results of these studies provide crucial biological information [175]. Due to their inherent complexity as biological systems, cells are frequently used as model systems in a wide range of metabolomics studies [176,177]. As a result, quantitative metabolomics has long been a potent analytical tool in many fields. Due to sensitivity issues, current metabolomics approaches often require a high number of cells per sample, especially for certain methods such as NMR [178]. Although the fact that multicellular samples enable efficient detection of chemical compositions, cellular heterogeneity is neglected. Differences between individual cells may be the result of genetic, epigenetic, developmental and environmental factors. There is growing evidence that cells are highly heterogeneous, and that they are incredibly diverse, even when they come from the same tissue [179,180]. Single cell analysis enables the investigation of cell-to-cell differences within a cell population and may provide crucial clues to identify disease causes that are hidden in bulk analyses [181,182]. Single cell genomics, transcriptomics, proteomics and metabolomics have experienced a boom in recent years due to advances in single cell isolation techniques and analytical tools. Single cell omics provides insights into the heterogeneity of cell phenotypes and genotypes [[183], [184], [185]]. Particularly, single cell metabolite profiling helps us better understand how different types of cells respond to environmental cues and may also provide us with crucial data for the diagnosis of diseases and the development of new drugs [173,174,186].
9.1. Methods for single cell metabolomics
Due to the small cell size (usually many tens of micrometers in diameter for a mammalian cell) [171,187], intrinsic complexity of intracellular species [188], and rapid turnover of cellular metabolites, sampling analysis is one of the most challenging steps in single cell studies [189,190]. Thus, an efficient sampling strategy often requires a high magnification microscope to watch cells, a finely controlled mechanical system to move the sample, and an effective extraction or desorption procedure to acquire data on cellular content.
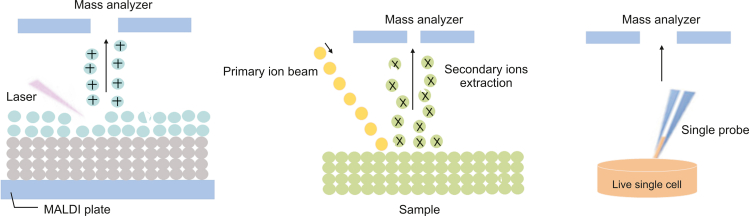
To present, a variety of approaches have been described for sampling and ionizing molecules from individual cells in both vacuum and natural environments. Ion beam [191], laser [192] and probe [193] sampling approaches are among them (Fig. 3). The method used for single cell separation is arguably the most crucial stage, as improper operation may disturb the surrounding environment and lead to inaccurate metabolic profiles [171,185]. Now, the two most common methods for sampling and ionization in vacuum settings are ion beam and laser techniques. For these methods, the sampling and ionization processes occur concurrently. The ion-beam based methods, such as nanoscale secondary ionization MS (SIMS) and time-of-flight SIMS, may achieve high resolution (100 nm–1 μm) [194]. These techniques provide high spatial resolution and outstanding sensitivity, making them effective tools for single cell imaging and intracellular studies [195,196].
Fig. 3.
Examples of single cell metabolomics techniques. (A) matrix-assisted laser desorption-ionization mass spectrometry (MALDI MS), (B) secondary ionization mass spectrometry (SIMS), and (C) single probe mass spectrometry.
Since SIMS-based approaches are generally more energetic, they often produce a high number of fragments, which makes data processing of most biomolecules challenging. The main laser-based technique for single cell research is matrix-assisted laser desorption-ionization (MALDI) MS, which relies on interactions between the laser beam and organic matrix molecules to start analyte desorption and ionization. This method offers great measurement sensitivity and throughput while having exceptional salt tolerance [197,198]. Additionally, when used in conjunction with a flash-freezing sample preparation technique, MALDI MS may deliver high-fidelity findings that accurately reflect the native distribution of cellular analytes [199]. Despite their advanced equipment and wide variety of applications, SIMS and MALDI based techniques require rigorous sample preparation due to vacuum working conditions, which may alter the chemical composition of the cells compared to those in the original biological environment. Contrary to vacuum-based methods, ambient sampling techniques may successfully preserve many the cellular microenvironment while sampling in situ. For metabolites with a high turnover rate, this distinctive feature becomes increasingly advantageous [187]. Numerous creative approaches have been created due to the flexibility of the work environment. These procedures typically involve distinct sampling and ionization phases, with nanoelectrospray ionization serving as the primary basis for ionization.
Monosaccharides, disaccharides, and trisaccharides are among the cellular metabolites from A. cepa cells discovered and identified using laser-ablation electrospray mass spectrometry (LAESI-MS) by Shrestha et al. [200]. There are several designs for probe-based approaches. For instance, Pan et al. [193] and Liu et al. [201] exploited a microscale liquid connection between two fused silica capillaries to detect lipids and amino acids from single cells using nano-desorption electrospray ionization. In addition, other tools such as single probes and T probes are used to detect intracellular metabolites as well as drug compounds from the natural microenvironment of single cells. Microfluidic-based technologies provide a more thorough inspection of microscale devices by using microfabrication techniques. To identify diverse cellular lipids and better distinguish variations in cellular metabolic profiles across cell types, a flow-assisted cellular classification system has been created [202].
9.2. Single-cell metabolomics for metabolic analysis of IS
Single-cell measurements advance our understanding of the function and microstructure of highly complex biological systems [203]. Brain cells exhibit significant heterogeneity, including neuronal, glial cells, and epithelial cells of cerebral blood vessels, even cells that are close to each other may exhibit significant differences in synaptic connections and metabolic product profiles. The study of cell heterogeneity is of great significance for elucidating neurochemical pathways, to better understand relevant mechanisms, and further improving the diagnosis and treatment of brain diseases [204].
Single-cell metabolomics with mass spectrometry enables a large variety of metabolites to be simultaneously detected from individual cells, without any preselection or labelling, to map phenotypes on the single cell level. Although the field is relatively young, it is steadily progressing with an increasing number of active research groups, techniques for cell sampling and ionization, tools for data analysis, and applications to answer important biomedical and environmental questions. The research strategies for single-cell metabolomics include cell handling, sampling, detection, quantification, and data analysis [205]. For example, the strategies for processing and sampling individual neuronal metabolites in the single-cell metabolomics of IS are as follows. Firstly, primary neuronal cells were obtained from neonatal rats using a culture method based on the previous description [206]. Secondly, neuronal cells induced by oxygen/glucose deprivation and reoxygenation (OGD/R) were used to simulate the pathological process of cerebral ischemia/reperfusion. Thirdly, optical imaging, sample preparation, MALDI matrix application, and MS analysis are performed sequentially. Fourthly, conduct data analysis and statistics.
10. Conclusion and prospective
IS is a multi-faceted, complex disease that causes many deaths and cases of severe impairment worldwide. Current disease-specific diagnostics and treatments are urgently needed to facilitate the development of more precise and personalized care.
In systems biology research, metabolomics is a potent tool that offers fresh perspectives on the pathological state of disease. Measuring the metabolites in an organism is part of the developing subject of metabolomics, which has enormous potential in the field of IS, whether for disease early detection or for tailored medication. Currently, metabolomics has provided a novel approach for the diagnosis, prediction and prognosis of cancer, cardiovascular diseases, neurological disorders diseases. Studying abnormal metabolic changes in IS and finding the potential metabolic biomarkers of IS provides a deeper understanding of the IS pathogenesis. A clinical biomarker has not yet been found, despite the vast number of IS-related metabolites have so far been found. Diet and comorbidities, which have a large impact on metabolomic profiles and biomarker discovery, have also been confounding factors in studies. Due to variations in the experimental setup and the methods used for metabolic analysis, the results are occasionally conflicting. Thus, the results are far from real-world practice.
CHM is widely used in China and Southeast Asia, and there are a variety of herbal medicines used to treat diseases in Europe, North America and Australia. Many compounds have been isolated from CHM, and most of these compounds have not yet been used in pharmacological studies aimed at the development of new drugs. The discovery of lead compounds from CHM faces several challenges. In addition, due to the lack of scientific research methods, the true value of CHM has not been fully recognized globally. Fortunately, metabolomics has become a hot topic in the world of life sciences research and is also extensively employed in research on CHM as the post-genome age approach. Metabolomics has been developed in recent years for innovative drug discovery and provides powerful tools to understand the nature and function of herbal compound formulations. Its characteristics are in concert with the holistic of CHM use, indicating that it is seen to have the potential to improve the study of CHM. The introduction of the concept of metabolomics, which enables the study of living systems from a holistic perspective, has opened a unique and novel opportunity to investigate CHM. The use of metabolomics strategies in CHM has started to offer fresh perspectives into the essence and molecular underpinnings of the system of CHM.
The heterogeneity of IS is highly influenced by the complexity of its etiology and pathophysiology. IS remains a challenging and deadly disease, in part due to its heterogeneous nature, despite the ongoing development of novel therapies and treatments. Thus, we need to conduct studies that look at the heterogeneity of IS tissues to better understand the pathophysiology of IS and create more effective treatments. Because one of the hallmarks of IS is its metabolic changes, metabolomics has become a promising research direction. Particularly, the development of spatial metabolomics has given the opportunity to identify molecular localizations in addition to their relative abundances. In this way, small-molecule alterations may be directly correlated with anatomical features. This may lead to the invention of personalized medicine and faster diagnostic methods, as well as further understanding of heterogeneous diseases.
As the most fundamental structural and functional units found in biology, cells may be affected by a variety of factors that can alter their proliferation, differentiation, and metabolism, leading to a wide range of heterogeneity between each individual cell, even within the same organism. Despite being genetically identical, the composition and concentration of chemical substances found in two homologous cells can differ. Population cell analysis provides only averaged results, and information regarding cellular individuality is often lacking. Bulk analysis of many cells has more material available for analysis. Single-cell analysis is required to identify phenotypic heterogeneity among individual cells and find subpopulations of seemingly similar cells to decipher the origin of drug resistance, differentiation, and disease progression. However, there are many further requirements for single cell analysis to be truly useful in systems biology and medical diagnosis. Challenges in single-cell analysis include the low picoliter volume of material available for analysis, the rapid shift, properties, and concentrations, which range from nanomolar to millimolar [207]. Despite the challenges, single cell analysis can be successfully achieved through the development of a range of technologies. Studying cell morphology and composition at the single cell level provides accurate information about the specific microenvironment in a single cell and applications to answer important biomedical questions.
Metabolomics research is clearly moving towards single cell analysis. Due to its use as an effective tool for determining the precise mechanisms behind cellular and molecular activity, single cell analysis has attracted increasing interest from researchers working in a variety of biological domains. MS methods are highly sensitive and selective, enabling the analysis and measurement of substances in individual cells. The variety of MS methods available for single cell analysis has grown over the years of research. Since each method has advantages and disadvantages of its own, the variety of different approaches is advantageous for researchers working on single cell analysis. In addition, the study of cellular environments and individual cells is accompanied by higher technical requirements, thus requiring continuous improvement of MS systems, especially in terms of detection sensitivity and temporal and spatial resolution. As technologies develop, MS will become increasingly crucial in single cell analysis with greater spatial resolution and sensitivity, which will facilitate biological research. Single cell metabolomics is expected to be a powerful tool for early detection of IS in the future and provide valuable insights into the pathogenesis of IS and single cell metabolism.
CRediT author statement
Wentao Li: Writing - Original draft preparation; Chongyu Shao, Chang Li, and Huifen Zhou: Project administration; Li Yu and Jiehong Yang: Formal analysis; Haitong Wan: Supervision, Funding acquisition; Yu He: Conceptualization, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.: 81873226, and 81874366), and the Zhejiang Provincial Science and Technology Innovation Leading Talent Project of “Ten Thousand Talents Plan” (2019).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Haitong Wan, Email: whtong@126.com.
Yu He, Email: heyu0923@hotmail.com.
References
- 1.German J.B., Hammock B.D., Watkins S.M. Metabolomics: Building on a century of biochemistry to guide human health. Metabolomics. 2005;1:3–9. doi: 10.1007/s11306-005-1102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wishart D.S. Applications of metabolomics in drug discovery and development. Drugs R D. 2008;9:307–322. doi: 10.2165/00126839-200809050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kaddurah-Daouk R., Kristal B.S., Weinshilboum R.M. Metabolomics: A global biochemical approach to drug response and disease. Annu. Rev. Pharmacol. Toxicol. 2008;48:653–683. doi: 10.1146/annurev.pharmtox.48.113006.094715. [DOI] [PubMed] [Google Scholar]
- 4.Paul S., Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021;335 doi: 10.1016/j.expneurol.2020.113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katan M., Luft A. Global burden of stroke. Semin. Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 6.De Lima L.G., Soares B.G.O., Saconato H., et al. Beta-blockers for preventing stroke recurrence. Cochrane Database Syst. Rev. 2013:CD007890. doi: 10.1002/14651858.CD007890.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Manolescu, Oprea, Mititelu, et al. Dietary anthocyanins and stroke: A review of pharmacokinetic and pharmacodynamic studies. Nutrients. 2019;11 doi: 10.3390/nu11071479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S., Lu J., Shao A., et al. Glial cells: Role of the immune response in ischemic stroke. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo T., Felzani G., Marini C. Stroke in the very old: A systematic review of studies on incidence, outcome, and resource use. J. Aging Res. 2011;2011 doi: 10.4061/2011/108785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Wang Z., Shi J., et al. Inhibition of proprotein convertase subtilisin/kexin type 9 attenuates neuronal apoptosis following focal cerebral ischemia via apolipoprotein E receptor 2 downregulation in hyperlipidemic mice. Int. J. Mol. Med. 2018:2098–2106. doi: 10.3892/ijmm.2018.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deguchi I., Mizuno S., Kohyama S., et al. Drip-and-ship thrombolytic therapy for acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2018;27:61–67. doi: 10.1016/j.jstrokecerebrovasdis.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Li P., Stetler R.A., Leak R.K., et al. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2018;134:208–217. doi: 10.1016/j.neuropharm.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gori A.M., Giusti B., Piccardi B., et al. Inflammatory and metalloproteinases profiles predict three-month poor outcomes in ischemic stroke treated with thrombolysis. J. Cereb. Blood Flow Metab. 2017;37:3253–3261. doi: 10.1177/0271678X17695572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou D., Luo M., Han Z., et al. Activation of alpha-7 nicotinic acetylcholine receptor reduces brain edema in mice with ischemic stroke and bone fracture. Mol. Neurobiol. 2017;54:8278–8286. doi: 10.1007/s12035-016-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H., Qi S., Shen J. One-compound-multi-target: Combination prospect of natural compounds with thrombolytic therapy in acute ischemic stroke. Curr. Neuropharmacol. 2016;15:134–156. doi: 10.2174/1570159X14666160620102055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Sun H., Zhang A., et al. Potential role of metabolomics apporoaches in the area of traditional Chinese medicine: As Pillars of the bridge between Chinese and Western medicine. J. Pharm. Biomed. Anal. 2011;55:859–868. doi: 10.1016/j.jpba.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Zhang A., Sun H., Wang Z., et al. Metabolomics: Towards understanding traditional Chinese medicine. Planta Med. 2010;76:2026–2035. doi: 10.1055/s-0030-1250542. [DOI] [PubMed] [Google Scholar]
- 18.Luan H., Wang X., Cai Z. Mass spectrometry-based metabolomics: Targeting the crosstalk between gut microbiota and brain in neurodegenerative disorders. Mass Spectrom. Rev. 2019;38:22–33. doi: 10.1002/mas.21553. [DOI] [PubMed] [Google Scholar]
- 19.Wishart D.S. Advances in metabolite identification. Bioanalysis. 2011;3:1769–1782. doi: 10.4155/bio.11.155. [DOI] [PubMed] [Google Scholar]
- 20.Nagana Gowda G.A., Raftery D. Springer; Cham: 2021. NMR-based metabolomics. Cancer Metabolomics; pp. 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wishart D.S. NMR metabolomics: A look ahead. J. Magn. Reson. 2019;306:155–161. doi: 10.1016/j.jmr.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang A., Sun H., Wang P., et al. Modern analytical techniques in metabolomics analysis. Analyst. 2012;137:293–300. doi: 10.1039/c1an15605e. [DOI] [PubMed] [Google Scholar]
- 23.Dunn W.B., Bailey N.J.C., Johnson H.E. Measuring the metabolome: Current analytical technologies. Analyst. 2005;130:606–625. doi: 10.1039/b418288j. [DOI] [PubMed] [Google Scholar]
- 24.Weaver E.M., Hummon A.B. Imaging mass spectrometry: From tissue sections to cell cultures. Adv. Drug Deliv. Rev. 2013;65:1039–1055. doi: 10.1016/j.addr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Balog J., Sasi-Szabó L., Kinross J., et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005623. [DOI] [PubMed] [Google Scholar]
- 26.Cullen C.H., Ray G.J., Szabo C.M. A comparison of quantitative nuclear magnetic resonance methods: Internal, external, and electronic referencing. Magn. Reson. Chem. 2013;51:705–713. doi: 10.1002/mrc.4004. [DOI] [PubMed] [Google Scholar]
- 27.Liang Q., Wang Q., Wang Y., et al. Quantitative 1H-NMR spectroscopy for profiling primary metabolites in mulberry leaves. Molecules. 2018;23 doi: 10.3390/molecules23030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S., Roy R. The application of absolute quantitative 1H NMR spectroscopy in drug discovery and development. Expert Opin. Drug Discov. 2016;11:695–706. doi: 10.1080/17460441.2016.1189899. [DOI] [PubMed] [Google Scholar]
- 29.Simmler C., Napolitano J.G., McAlpine J.B., et al. Universal quantitative NMR analysis of complex natural samples. Curr. Opin. Biotechnol. 2014;25:51–59. doi: 10.1016/j.copbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao Y., Le W. Recent advances and perspectives of metabolomics-based investigations in Parkinson’s disease. Mol. Neurodegener. 2019;14 doi: 10.1186/s13024-018-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimes J.H., O’Connell T.M. The application of micro-coil NMR probe technology to metabolomics of urine and serum. J. Biomol. NMR. 2011;49:297–305. doi: 10.1007/s10858-011-9488-2. [DOI] [PubMed] [Google Scholar]
- 32.Xi Y., de Ropp J.S., Viant M.R., et al. Improved identification of metabolites in complex mixtures using HSQC NMR spectroscopy. Anal. Chim. Acta. 2008;614:127–133. doi: 10.1016/j.aca.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theodoridis G., Gika H.G., Wilson I.D. Mass spectrometry-based holistic analytical approaches for metabolite profiling in systems biology studies. Mass Spectrom. Rev. 2011;30:884–906. doi: 10.1002/mas.20306. [DOI] [PubMed] [Google Scholar]
- 34.Yu Z., Huang H., Reim A., et al. Optimizing 2D gas chromatography mass spectrometry for robust tissue, serum and urine metabolite profiling. Talanta. 2017;165:685–691. doi: 10.1016/j.talanta.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neese R.A., Gertz E.W., Wisneski J.A., et al. A stable isotope technique for investigating lactate metabolism in humans, Biomed. Mass Spectrom. 1983;10:458–462. doi: 10.1002/bms.1200100804. [DOI] [PubMed] [Google Scholar]
- 36.Fiehn O., Kopka J., Dörmann P., et al. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000;18:1157–1161. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- 37.Theodoridis G.A., Gika H.G., Want E.J., et al. Liquid chromatography-mass spectrometry based global metabolite profiling: A review. Anal. Chim. Acta. 2012;711:7–16. doi: 10.1016/j.aca.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 38.Venti M., Parnetti L., Gallai V. Genetics of ischemic stroke. Clin. Exp. Hypertens. 2002;24:531–534. doi: 10.1081/ceh-120015329. [DOI] [PubMed] [Google Scholar]
- 39.Djebali S., Davis C.A., Merkel A., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palazzo A.F., Lee E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015;6 doi: 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao J., Xu J., Wang W., et al. Long non-coding RNA LINC-01572: 28 inhibits granulosa cell growth via a decrease in p27 (Kip1) degradation in patients with polycystic ovary syndrome. SSRN Electron. J. 2018:526–538. doi: 10.1016/j.ebiom.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akella A., Bhattarai S., Dharap A. Long noncoding RNAs in the pathophysiology of ischemic stroke. NeuroMolecular Med. 2019;21:474–483. doi: 10.1007/s12017-019-08542-w. [DOI] [PubMed] [Google Scholar]
- 43.Chen X., Chen H., Xu M., et al. Targeting reactive nitrogen species: A promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol. Sin. 2013;34:67–77. doi: 10.1038/aps.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen C.L., Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 45.Sun M., Jin H., Sun X., et al. Free radical damage in ischemia-reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/3804979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cherubini A., Ruggiero C., Polidori M.C., et al. Potential markers of oxidative stress in stroke. Free. Radic. Biol. Med. 2005;39:841–852. doi: 10.1016/j.freeradbiomed.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Coyle J.T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 48.Sugawara T., Chan P.H. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid. Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 49.Faraci F.M. Reactive oxygen species: Influence on cerebral vascular tone. J. Appl. Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- 50.Neumar R.W. Molecular mechanisms of ischemic neuronal injury. Ann. Emerg. Med. 2000;36:483–506. doi: 10.1067/mem.2000.110995. [DOI] [PubMed] [Google Scholar]
- 51.Gariballa S.E., Hutchin T.P., Sinclair A.J. Antioxidant capacity after acute ischaemic stroke. QJM. 2002;95:685–690. doi: 10.1093/qjmed/95.10.685. [DOI] [PubMed] [Google Scholar]
- 52.Margaill I., Plotkine M., Lerouet D. Antioxidant strategies in the treatment of stroke. Free. Radic. Biol. Med. 2005;39:429–443. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Duris K., Splichal Z., Jurajda M. The role of inflammatory response in stroke associated programmed cell death. Curr. Neuropharmacol. 2018;16:1365–1374. doi: 10.2174/1570159X16666180222155833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuttolomondo A., Di Raimondo D., di Sciacca R., et al. Inflammatory cytokines in acute ischemic stroke. Curr. Pharm. Des. 2008;14:3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 55.N.T. Lee, L.K. Ong, P. Gyawali, et al., Role of purinergic signalling in endothelial dysfunction and thrombo-inflammation in ischaemic stroke and cerebral small vessel disease, Biomolecules 11 (2021), 994. [DOI] [PMC free article] [PubMed]
- 56.Kim J.Y., Kawabori M., Yenari M.A. Innate inflammatory responses in stroke: Mechanisms and potential therapeutic targets. Curr. Med. Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okada T., Suzuki H., Travis Z.D., et al. The stroke-induced blood-brain barrier disruption: Current progress of inspection technique, mechanism, and therapeutic target. Curr. Neuropharmacol. 2020;18:1187–1212. doi: 10.2174/1570159X18666200528143301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-de-Puig I., Miró-Mur F., Ferrer-Ferrer M., et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129:239–257. doi: 10.1007/s00401-014-1381-0. [DOI] [PubMed] [Google Scholar]
- 59.Cuartero M.I., Ballesteros I., Moraga A., et al. N2 neutrophils, novel players in brain inflammation after stroke: Modulation by the PPARγ agonist rosiglitazone. Stroke. 2013;44:3498–3508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- 60.Morris G., Berk M., Carvalho A.F., et al. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav. Brain Res. 2018;341:154–175. doi: 10.1016/j.bbr.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y., Liu J., Liu H., et al. Investigation of cerebral iron deposition in aged patients with ischemic cerebrovascular disease using susceptibility-weighted imaging. Ther. Clin. Risk Manag. 2016;12:1239–1247. doi: 10.2147/TCRM.S107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang Y.L., Hung S.H., Ling W., et al. Association between ischemic stroke and iron-deficiency Anemia: A population-based study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrova J., Manolov V., Vasilev V., et al. Ischemic stroke, inflammation, iron overload–connection to a hepcidin. Int. J. Stroke. 2016;11:NP16–NP17. doi: 10.1177/1747493015607509. [DOI] [PubMed] [Google Scholar]
- 64.Tuo Q.Z., Lei P., Jackman K.A., et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 65.Li N., Wang X., Sun C., et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19:1–8. doi: 10.1186/s12866-019-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Q., Elson C.O. Adaptive immune education by gut microbiota antigens. Immunology. 2018;154:28–37. doi: 10.1111/imm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bartley A., Yang T., Arocha R., et al. Increased abundance of lactobacillales in the colon of beta-adrenergic receptor knock out mouse is associated with increased gut bacterial production of short chain fatty acids and reduced IL17 expression in circulating CD4+ immune cells. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carabotti M., Scirocco A., Maselli M.A., et al. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H., Wang Y. Gut microbiota-brain axis. Chin. Med. J. 2016;129:2373–2380. doi: 10.4103/0366-6999.190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh V., Roth S., Llovera G., et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 2016;36:7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin J., Liao S., He Y., et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y., Liang J., Ouyang F., et al. Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in Cynomolgus monkeys. Front. Neurol. 2019;10 doi: 10.3389/fneur.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Machiels K., Joossens M., Sabino J., et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitziidefines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 74.Gophna U., Konikoff T., Nielsen H.B. Oscillospira and related bacteria - From metagenomic species to metabolic features. Environ. Microbiol. 2017;19:835–841. doi: 10.1111/1462-2920.13658. [DOI] [PubMed] [Google Scholar]
- 75.Bourassa M.W., Alim I., Bultman S.J., et al. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan F., Zhang L., Li M., et al. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome. 2018;6 doi: 10.1186/s40168-018-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chesnokova V., Pechnick R.N., Wawrowsky K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016;58:1–8. doi: 10.1016/j.bbi.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiryk A., Pluta R., Figiel I., et al. Transient brain ischemia due to cardiac arrest causes irreversible long-lasting cognitive injury. Behav. Brain Res. 2011;219:1–7. doi: 10.1016/j.bbr.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Medeiros G.C., Roy D., Kontos N., et al. Post-stroke depression: A 2020 updated review. Gen. Hosp. Psychiatry. 2020;66:70–80. doi: 10.1016/j.genhosppsych.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Radenovic L., Nenadic M., Ułamek-Kozioł M., et al. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging. 2020;12:12251–12267. doi: 10.18632/aging.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Houten S.M. Metabolomics: Unraveling the chemical individuality of common human diseases. Ann. Med. 2009;41:402–407. doi: 10.1080/07853890902729794. [DOI] [PubMed] [Google Scholar]
- 82.Wishart D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016;15:473–484. doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- 83.Wishart D.S., Feunang Y.D., Marcu A., et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018;46:D608–D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shah S.H., Kraus W.E., Newgard C.B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: Form and function. Circulation. 2012;126:1110–1120. doi: 10.1161/CIRCULATIONAHA.111.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang R., Meng J., Wang X., et al. Metabolomics of ischemic stroke: Insights into risk prediction and mechanisms. Metab. Brain Dis. 2022;37:2163–2180. doi: 10.1007/s11011-022-01011-7. [DOI] [PubMed] [Google Scholar]
- 86.Guo W., Wang Y., Fan M., et al. Integrating metabolomics and network pharmacology to explore the protective effect of gross saponins of Tribulus terrestris L. fruit against ischemic stroke in rat. J. Ethnopharmacol. 2020;263 doi: 10.1016/j.jep.2020.113202. [DOI] [PubMed] [Google Scholar]
- 87.Wu L., Chen C., Li Y., et al. UPLC-Q-TOF/MS-based serum metabolomics reveals the anti-ischemic stroke mechanism of nuciferine in MCAO rats. ACS Omega. 2020;5:33433–33444. doi: 10.1021/acsomega.0c05388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Zhao H., Liu Y., et al. GC-MS-based metabolomics to reveal the protective effect of gross saponins of Tribulus terrestris fruit against ischemic stroke in rat. Molecules. 2019;24 doi: 10.3390/molecules24040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luo L., Kang J., He Q., et al. A NMR-based metabonomics approach to determine protective effect of a combination of multiple components derived from Naodesheng on ischemic stroke rats. Molecules. 2019;24 doi: 10.3390/molecules24091831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jung J.Y., Lee H.S., Kang D.G., et al. 1H-NMR-based metabolomics study of cerebral infarction. Stroke. 2011;42:1282–1288. doi: 10.1161/STROKEAHA.110.598789. [DOI] [PubMed] [Google Scholar]
- 91.Jiang Z., Sun J., Liang Q., et al. A metabonomic approach applied to predict patients with cerebral infarction. Talanta. 2011;84:298–304. doi: 10.1016/j.talanta.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 92.Kimberly W.T., Wang Y., Pham L., et al. Metabolite profiling identifies a branched chain amino acid signature in acute cardioembolic stroke. Stroke. 2013;44:1389–1395. doi: 10.1161/STROKEAHA.111.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu M., Zhou K., Li H., et al. Potential of serum metabolites for diagnosing post-stroke cognitive impairment. Mol. BioSyst. 2015;11:3287–3296. doi: 10.1039/c5mb00470e. [DOI] [PubMed] [Google Scholar]
- 94.Ding X., Liu R., Li W., et al. A metabonomic investigation on the biochemical perturbation in post-stroke patients with depressive disorder (PSD) Metab. Brain Dis. 2016;31:279–287. doi: 10.1007/s11011-015-9748-z. [DOI] [PubMed] [Google Scholar]
- 95.Zhang W., Zhang X. A novel urinary metabolite signature for non-invasive post-stroke depression diagnosis. Cell Biochem. Biophys. 2015;72:661–667. doi: 10.1007/s12013-014-0472-9. [DOI] [PubMed] [Google Scholar]
- 96.Hu Z., Zhu Z., Cao Y., et al. Rapid and sensitive differentiating ischemic and hemorrhagic strokes by dried blood spot based direct injection mass spectrometry metabolomics analysis. J. Clin. Lab. Anal. 2016;30:823–830. doi: 10.1002/jcla.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruiz-Canela M., Toledo E., Clish C.B., et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin. Chem. 2016;62:582–592. doi: 10.1373/clinchem.2015.251710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao J., Zhang J., Sun D., et al. Discriminating poststroke depression from stroke by nuclear magnetic resonance spectroscopy-based metabonomic analysis. Neuropsychiatr. Dis. Treat. 2016;12:1919–1925. doi: 10.2147/NDT.S110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guasch-Ferré M., Zheng Y., Ruiz-Canela M., et al. Plasma acylcarnitines and risk of cardiovascular disease: Effect of Mediterranean diet interventions1. Am. J. Clin. Nutr. 2016;103:1408–1416. doi: 10.3945/ajcn.116.130492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng Y., Hu F.B., Ruiz-Canela M., et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvención con DIeta MEDiterránea (PREDIMED) trial. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang D.D., Toledo E., Hruby A., et al. Correction to: Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (prevención con dieta mediterránea) Circulation. 2019;140:2028–2040. doi: 10.1161/CIRCULATIONAHA.116.024261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang L., Lv P., Ai W., et al. Lipidomic analysis of plasma in patients with lacunar infarction using normal-phase/reversed-phase two-dimensional liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2017;409:3211–3222. doi: 10.1007/s00216-017-0261-6. [DOI] [PubMed] [Google Scholar]
- 103.Lee Y., Khan A., Hong S., et al. A metabolomic study on high-risk stroke patients determines low levels of serum lysine metabolites: A retrospective cohort study. Mol. BioSyst. 2017;13:1109–1120. doi: 10.1039/c6mb00732e. [DOI] [PubMed] [Google Scholar]
- 104.Liu P., Li R., Antonov A.A., et al. Discovery of metabolite biomarkers for acute ischemic stroke progression. J. Proteome Res. 2017;16:773–779. doi: 10.1021/acs.jproteome.6b00779. [DOI] [PubMed] [Google Scholar]
- 105.Kell D.B. Systems biology, metabolic modelling and metabolomics in drug discovery and development. Drug Discov. Today. 2006;11:1085–1092. doi: 10.1016/j.drudis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 106.Cao H., Zhang A., Zhang H., et al. The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytother. Res. 2015;29:159–166. doi: 10.1002/ptr.5240. [DOI] [PubMed] [Google Scholar]
- 107.Verpoorte R., Crommelin D., Danhof M., et al. Commentary: “a systems view on the future of medicine: Inspiration from Chinese medicine?”. J. Ethnopharmacol. 2009;121:479–481. doi: 10.1016/j.jep.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 108.Ahmad S., Elsherbiny N.M., Haque R., et al. Sesamin attenuates neurotoxicity in mouse model of ischemic brain stroke. NeuroToxicology. 2014;45:100–110. doi: 10.1016/j.neuro.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 109.Wang J., Bondy S.C., Zhou L., et al. Protective effect of Tanshinone IIA against infarct size and increased HMGB1, NFκB, GFAP and apoptosis consequent to transient middle cerebral artery occlusion. Neurochem. Res. 2014;39:295–304. doi: 10.1007/s11064-013-1221-y. [DOI] [PubMed] [Google Scholar]
- 110.Chien M., Chuang C.H., Chern C.M., et al. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free. Radic. Biol. Med. 2016;99:508–519. doi: 10.1016/j.freeradbiomed.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 111.Xu S., Zhong A., Ma H., et al. Neuroprotective effect of salvianolic acid B against cerebral ischemic injury in rats via the CD40/NF-κB pathway associated with suppression of platelets activation and neuroinflammation. Brain Res. 2017;1661:37–48. doi: 10.1016/j.brainres.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 112.Gaire B.P., Kwon O.W., Park S.H., et al. Neuroprotective effect of 6-paradol in focal cerebral ischemia involves the attenuation of neuroinflammatory responses in activated microglia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xue X., Qu X., Yang Y., et al. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochem. Biophys. Res. Commun. 2010;403:398–404. doi: 10.1016/j.bbrc.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y., Zhang P., Liu Y. Neuroprotective effect of WYY026B on cerebral ischemia/reperfusion injury in rodents through activation of Nrf2/HO-1 pathway. SSRN Electron. J. 2022:639–898. [Google Scholar]
- 115.Gao Y., Xu X., Chang S., et al. Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: Potential roles of Akt activation and HO-1 induction. Toxicol. Appl. Pharmacol. 2015;289:142–154. doi: 10.1016/j.taap.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Xu B., He X., Sui Y., et al. Ginkgetin aglycone attenuates neuroinflammation and neuronal injury in the rats with ischemic stroke by modulating STAT3/JAK2/SIRT1. Folia Neuropathol. 2019;57:16–23. doi: 10.5114/fn.2019.83827. [DOI] [PubMed] [Google Scholar]
- 117.Feng J., Chen X., Lu S., et al. Naringin attenuates cerebral ischemia-reperfusion injury through inhibiting peroxynitrite-mediated mitophagy activation. Mol. Neurobiol. 2018;55:9029–9042. doi: 10.1007/s12035-018-1027-7. [DOI] [PubMed] [Google Scholar]
- 118.Wen Z., Hou W., Wu W., et al. 6’-O-galloylpaeoniflorin attenuates cerebral ischemia reperfusion-induced neuroinflammation and oxidative stress via PI3K/akt/Nrf2 activation. Oxid. Med. Cell. Longev. 2018;2018:1–14. doi: 10.1155/2018/8678267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ke C., Pan C., Zhang Y., et al. Metabolomics facilitates the discovery of metabolic biomarkers and pathways for ischemic stroke: A systematic review. Metabolomics. 2019;15 doi: 10.1007/s11306-019-1615-1. [DOI] [PubMed] [Google Scholar]
- 120.Fu X., Wang J., Liao S., et al. 1H NMR-based metabolomics reveals refined-Huang-Lian-Jie-du-decoction (BBG) as a potential ischemic stroke treatment drug with efficacy and a favorable therapeutic window. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qi Y., Zhang Q., Zhu H. Huang-Lian Jie-Du Decoction: A review on phytochemical, pharmacological and pharmacokinetic investigations. Chin. Med. 2019;14:1–22. doi: 10.1186/s13020-019-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hwang Y.S., Shin C.Y., Huh Y., et al. Hwangryun-Hae-Dok-Tang (Huanglian-Jie-Du-Tang) extract and its constituents reduce ischemia-reperfusion brain injury and neutrophil infiltration in rats. Life Sci. 2002;71:2105–2117. doi: 10.1016/s0024-3205(02)01920-3. [DOI] [PubMed] [Google Scholar]
- 123.Wang P., Wang J., Zhang C., et al. Huang-Lian-Jie-Du-Decotion induced protective autophagy against the injury of cerebral ischemia/reperfusion via MAPK-mTOR signaling pathway. J. Ethnopharmacol. 2013;149:270–280. doi: 10.1016/j.jep.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 124.Zhu B., Cao H., Sun L., et al. Metabolomics-based mechanisms exploration of Huang-Lian Jie-Du Decoction on cerebral ischemia via UPLC-Q-TOF/MS analysis on rat serum. J. Ethnopharmacol. 2018;216:147–156. doi: 10.1016/j.jep.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Q., Wang J., Liao S., et al. Optimization of Huang-Lian-Jie-du-decoction for ischemic stroke treatment and mechanistic study by metabolomic profiling and network analysis. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Q., Wang J., Zhang C., et al. The components of Huang-Lian-Jie-Du-Decoction act synergistically to exert protective effects in a rat ischemic stroke model. Oncotarget. 2016;7:80872–80887. doi: 10.18632/oncotarget.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q., Fu X., Wang J., et al. Treatment effects of ischemic stroke by berberine, baicalin, and jasminoidin from Huang-Lian-Jie-du-decoction (HLJDD) explored by an integrated metabolomics approach. Oxid. Med. Cell. Longev. 2017;2017:1–20. doi: 10.1155/2017/9848594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo Y., Yan S., Xu L., et al. Use of Angong Niuhuang in treating central nervous system diseases and related research. Evid. Based Complementary. Altern. Med. 2014;2014 doi: 10.1155/2014/346918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luo Y. Angongniuhuang pill treatment of acute stroke clinical observation of 64 cases. Chin. J. Ethnomed. Ethnopharmacy. 2009;10:45–46. [Google Scholar]
- 130.Wei Z. Therapeutic effects of Angongniuhuang pill on 34 patients with cerebral stroke. Hebei J. Tradit. Chin. Med. 2005;27:13–14. [Google Scholar]
- 131.Tsoi B., Chen X., Gao C., et al. Neuroprotective effects and hepatorenal toxicity of Angong Niuhuang Wan against ischemia-reperfusion brain injury in rats. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xia F., Li A., Chai Y., et al. UPLC/Q-TOFMS-based metabolomics approach to reveal the protective role of other herbs in an-Gong-Niu-Huang Wan against the hepatorenal toxicity of cinnabar and realgar. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y., Liu X., Long J., et al. Exploring active compounds and mechanisms of Angong Niuhuang Wan on ischemic stroke based on network pharmacology and molecular docking. Evid. Based Complementary Altern. Med. 2022;2022:1–13. doi: 10.1155/2022/2443615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang L., Cheng X., Chen R., et al. Protective effect of effective composite of Chinese medicine prescription Naodesheng (脑得生) against focal cerebral ischemia in rats. Chin. J. Integr. Med. 2009;15:377–383. doi: 10.1007/s11655-009-0377-4. [DOI] [PubMed] [Google Scholar]
- 135.Luo L., Zhen L., Xu Y., et al. 1H NMR-based metabonomics revealed protective effect of Naodesheng bioactive extract on ischemic stroke rats. J. Ethnopharmacol. 2016;186:257–269. doi: 10.1016/j.jep.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 136.Luo L., Wu S., Zhu X., et al. The profiling and identification of the absorbed constituents and metabolites of Naoshuantong capsule in mice biofluids and brain by ultra- fast liquid chromatography coupled with quadrupole-time-of-flight tandem mass spectrometry. J. Chromatogr. B. 2019;1129 doi: 10.1016/j.jchromb.2019.121791. [DOI] [PubMed] [Google Scholar]
- 137.Gu Y., Huang P., Cheng T., et al. A multiomics and network pharmacological study reveals the neuroprotective efficacy of Fu-Fang-Dan-Zhi Tablets against glutamate-induced oxidative cell death. Comput. Biol. Med. 2022;148 doi: 10.1016/j.compbiomed.2022.105873. [DOI] [PubMed] [Google Scholar]
- 138.Liu J., Yang L., Niu Y., et al. Potential therapeutic effects of mi-Jian-Chang-Pu decoction on neurochemical and metabolic changes of cerebral ischemia-reperfusion injury in rats. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/7319563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giraudeau P. NMR-based metabolomics and fluxomics: Developments and future prospects. Analyst. 2020;145:2457–2472. doi: 10.1039/d0an00142b. [DOI] [PubMed] [Google Scholar]
- 140.Williamson D.J., Burn G.L., Simoncelli S., et al. Machine learning for cluster analysis of localization microscopy data. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-15293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sealfon R.S.G., Mariani L.H., Kretzler M., et al. Machine learning, the kidney, and genotype-phenotype analysis. Kidney Int. 2020;97:1141–1149. doi: 10.1016/j.kint.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Curry B., Rumelhart D.E. MSnet: A neural network which classifies mass spectra. Tetrahedron Comput. Methodol. 1990;3:213–237. [Google Scholar]
- 143.Cirovic D.A. Feed-forward artificial neural networks: Applications to spectroscopy. Trac Trends Anal. Chem. 1997;16:148–155. [Google Scholar]
- 144.Mendez K.M., Broadhurst D.I., Reinke S.N. The application of artificial neural networks in metabolomics: A historical perspective. Metabolomics. 2019;15 doi: 10.1007/s11306-019-1608-0. [DOI] [PubMed] [Google Scholar]
- 145.Truong Y., Lin X., Beecher C. Proceedings of the tenth ACM SIGKDD international conference on Knowledge discovery and data mining. August 22 - 25, 2004. ACM; Seattle, WA, USA. New York: 2004. Learning a complex metabolomic dataset using random forests and support vector machines; pp. 835–840. [Google Scholar]
- 146.Brown M.P.S., Grundy W.N., Lin D., et al. Knowledge-based analysis of microarray gene expression data by using support vector machines. Proc. Natl. Acad. Sci. U. S. A. 2000;97:262–267. doi: 10.1073/pnas.97.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saccenti E., Hoefsloot H.C.J., Smilde A.K., et al. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics. 2014;10:361–374. [Google Scholar]
- 148.Touw W.G., Bayjanov J.R., Overmars L., et al. Data mining in the Life Sciences with Random Forest: A walk in the park or lost in the jungle? Brief. Bioinform. 2013;14:315–326. doi: 10.1093/bib/bbs034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brereton R.G., Lloyd G.R. Support Vector Machines for classification and regression. Analyst. 2010;135:230–267. doi: 10.1039/b918972f. [DOI] [PubMed] [Google Scholar]
- 150.Min S., Lee B., Yoon S. Deep learning in bioinformatics. Brief. Bioinform. 2017;18:851–869. doi: 10.1093/bib/bbw068. [DOI] [PubMed] [Google Scholar]
- 151.Jolliffe I.T., Cadima J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. A. 2016;374 doi: 10.1098/rsta.2015.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McLachlan G.J. Cluster analysis and related techniques in medical research. Stat. Meth. Med. Res. 1992;1:27–48. doi: 10.1177/096228029200100103. [DOI] [PubMed] [Google Scholar]
- 153.Au A. Metabolomics and lipidomics of ischemic stroke. Adv. Clin. Chem. 2018;85:31–69. doi: 10.1016/bs.acc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 154.Shin T.H., Kim H.A., Jung J.Y., et al. Analysis of the free fatty acid metabolome in the plasma of patients with systemic lupus erythematosus and fever. Metabolomics. 2018;14 doi: 10.1007/s11306-017-1308-6. [DOI] [PubMed] [Google Scholar]
- 155.Ben Salem K., Ben Abdelaziz A. Principal component analysis (PCA) Tunis. Med. 2021;99:383–389. [PMC free article] [PubMed] [Google Scholar]
- 156.Ivosev G., Burton L., Bonner R. Dimensionality reduction and visualization in principal component analysis. Anal. Chem. 2008;80:4933–4944. doi: 10.1021/ac800110w. [DOI] [PubMed] [Google Scholar]
- 157.Bartel J., Krumsiek J., Theis F.J. Statistical methods for the analysis of high-throughput metabolomics data. Comput. Struct. Biotechnol. J. 2013;4 doi: 10.5936/csbj.201301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fonville J.M., Richards S.E., Barton R.H., et al. The evolution of partial least squares models and related chemometric approaches in metabonomics and metabolic phenotyping. J. Chemom. 2010;24:636–649. [Google Scholar]
- 159.Trygg J., Wold S. Orthogonal projections to latent structures (O-PLS) J. Chemom. 2002;16:119–128. [Google Scholar]
- 160.Bylesjö M., Rantalainen M., Cloarec O., et al. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006;20:341–351. [Google Scholar]
- 161.Wang T., Shao K., Chu Q., et al. Automics: An integrated platform for NMR-based metabonomics spectral processing and data analysis. BMC Bioinform. 2009;10 doi: 10.1186/1471-2105-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jacob M., Lopata A.L., Dasouki M., et al. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019;38:221–238. doi: 10.1002/mas.21548. [DOI] [PubMed] [Google Scholar]
- 163.Xia J., Broadhurst D.I., Wilson M., et al. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics. 2013;9:280–299. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.la Marca G. Mass spectrometry in clinical chemistry: The case of newborn screening. J. Pharm. Biomed. Anal. 2014;101:174–182. doi: 10.1016/j.jpba.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 165.Kaddurah-Daouk R., Weinshilboum R., Network P.R. Metabolomic signatures for drug response phenotypes: Pharmacometabolomics enables precision medicine. Clin. Pharmacol. Ther. 2015;98:71–75. doi: 10.1002/cpt.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sallustio B.C. LC-MS/MS for immunosuppressant therapeutic drug monitoring. Bioanalysis. 2010;2:1141–1153. doi: 10.4155/bio.10.58. [DOI] [PubMed] [Google Scholar]
- 167.Moes D.J.A.R., Press R.R., de Fijter J.W., et al. Liquid chromatography-tandem mass spectrometry outperforms fluorescence polarization immunoassay in monitoring everolimus therapy in renal transplantation, Ther. Drug Monit. 2010;32:413–419. doi: 10.1097/FTD.0b013e3181e5c656. [DOI] [PubMed] [Google Scholar]
- 168.Zgheib N.K., Frye R.F., Tracy T.S., et al. Validation of incorporating flurbiprofen into the Pittsburgh cocktail. Clin. Pharmacol. Ther. 2006;80:257–263. doi: 10.1016/j.clpt.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 169.Suhre K., Shin S.Y., Petersen A.K., et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shin S.Y., Fauman E.B., Petersen A.K., et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014;46:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zenobi R. Single-cell metabolomics: Analytical and biological perspectives. Science. 2013;342 doi: 10.1126/science.1243259. [DOI] [PubMed] [Google Scholar]
- 172.Misra B.B., Assmann S.M., Chen S. Plant single-cell and single-cell-type metabolomics. Trends Plant Sci. 2014;19:637–646. doi: 10.1016/j.tplants.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 173.Rubakhin S.S., Lanni E.J., Sweedler J.V. Progress toward single cell metabolomics. Curr. Opin. Biotechnol. 2013;24:95–104. doi: 10.1016/j.copbio.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oikawa A., Saito K. Metabolite analyses of single cells. Plant J. 2012;70:30–38. doi: 10.1111/j.1365-313X.2012.04967.x. [DOI] [PubMed] [Google Scholar]
- 175.Pinu F.R., Beale D.J., Paten A.M., et al. Systems biology and multi-omics integration: Viewpoints from the metabolomics research community. Metabolites. 2019;9 doi: 10.3390/metabo9040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Luo X., Li L. Metabolomics of small numbers of cells: Metabolomic profiling of 100, 1000, and 10000 human breast cancer cells. Anal. Chem. 2017;89:11664–11671. doi: 10.1021/acs.analchem.7b03100. [DOI] [PubMed] [Google Scholar]
- 177.Huang T., Armbruster M., Lee R., et al. Metabolomic analysis of mammalian cells and human tissue through one-pot two stage derivatizations using sheathless capillary electrophoresis-electrospray ionization-mass spectrometry. J. Chromatogr. A. 2018;1567:219–225. doi: 10.1016/j.chroma.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pan Z., Raftery D. Comparing and combining NMR spectroscopy and mass spectrometry in metabolomics. Anal. Bioanal. Chem. 2007;387:525–527. doi: 10.1007/s00216-006-0687-8. [DOI] [PubMed] [Google Scholar]
- 179.Svatoš A. Single-cell metabolomics comes of age: New developments in mass spectrometry profiling and imaging. Anal. Chem. 2011;83:5037–5044. doi: 10.1021/ac2003592. [DOI] [PubMed] [Google Scholar]
- 180.Wang D., Bodovitz S. Single cell analysis: The new frontier in ‘omics. Trends Biotechnol. 2010;28:281–290. doi: 10.1016/j.tibtech.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li Y., Jang J.H., Wang C., et al. Microfluidics cell loading-dock system: Ordered cellular array for dynamic lymphocyte-communication study. Adv. Biosys. 2017;1 doi: 10.1002/adbi.201700085. [DOI] [PubMed] [Google Scholar]
- 182.Li Y., Motschman J.D., Kelly S.T., et al. Injection molded microfluidics for establishing high-density single cell arrays in an open hydrogel format. Anal. Chem. 2020;92:2794–2801. doi: 10.1021/acs.analchem.9b05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Feng X., Liu B., Li J., et al. Advances in coupling microfluidic chips to mass spectrometry. Mass Spectrom. Rev. 2015;34:535–557. doi: 10.1002/mas.21417. [DOI] [PubMed] [Google Scholar]
- 184.Gavasso S., Gullaksen S.E., Skavland J., et al. Single-cell proteomics: Potential implications for cancer diagnostics. Expert Rev. Mol. Diagn. 2016;16:579–589. doi: 10.1586/14737159.2016.1156531. [DOI] [PubMed] [Google Scholar]
- 185.Gross A., Schoendube J., Zimmermann S., et al. Technologies for single-cell isolation. Int. J. Mol. Sci. 2015;16:16897–16919. doi: 10.3390/ijms160816897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fessenden M. Metabolomics: Small molecules, single cells. Nature. 2016;540:153–155. doi: 10.1038/540153a. [DOI] [PubMed] [Google Scholar]
- 187.Zhang L., Vertes A. Single-cell mass spectrometry approaches to explore cellular heterogeneity. Angew. Chem. Int. Ed. 2018;57:4466–4477. doi: 10.1002/anie.201709719. [DOI] [PubMed] [Google Scholar]
- 188.Duncan K.D., Fyrestam J., Lanekoff I. Advances in mass spectrometry based single-cell metabolomics. Analyst. 2019;144:782–793. doi: 10.1039/c8an01581c. [DOI] [PubMed] [Google Scholar]
- 189.Muschet C., Möller G., Prehn C., et al. Removing the bottlenecks of cell culture metabolomics: Fast normalization procedure, correlation of metabolites to cell number, and impact of the cell harvesting method. Metabolomics. 2016;12 doi: 10.1007/s11306-016-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang L., Vertes A. Energy charge, redox state, and metabolite turnover in single human hepatocytes revealed by capillary microsampling mass spectrometry. Anal. Chem. 2015;87:10397–10405. doi: 10.1021/acs.analchem.5b02502. [DOI] [PubMed] [Google Scholar]
- 191.Passarelli M.K., Newman C.F., Marshall P.S., et al. Single-cell analysis: Visualizing pharmaceutical and metabolite uptake in cells with label-free 3D mass spectrometry imaging. Anal. Chem. 2015;87:6696–6702. doi: 10.1021/acs.analchem.5b00842. [DOI] [PubMed] [Google Scholar]
- 192.Amantonico A., Urban P.L., Fagerer S.R., et al. Single-cell MALDI-MS as an analytical tool for studying intrapopulation metabolic heterogeneity of unicellular organisms. Anal. Chem. 2010;82:7394–7400. doi: 10.1021/ac1015326. [DOI] [PubMed] [Google Scholar]
- 193.Pan N., Rao W., Kothapalli N.R., et al. The single-probe: A miniaturized multifunctional device for single cell mass spectrometry analysis. Anal. Chem. 2014;86:9376–9380. doi: 10.1021/ac5029038. [DOI] [PubMed] [Google Scholar]
- 194.Kleinfeld A.M., Kampf J.P., Lechene C. Transport of 13C-oleate in adipocytes measured using multi imaging mass spectrometry. J. Am. Soc. Mass Spectrom. 2004;15:1572–1580. doi: 10.1016/j.jasms.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 195.Schoffelen N.J., Mohr W., Ferdelman T.G., et al. Single-cell imaging of phosphorus uptake shows that key harmful algae rely on different phosphorus sources for growth. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-35310-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li H., Hua X., Long Y. Graphene quantum dots enhanced ToF-SIMS for single-cell imaging. Anal. Bioanal. Chem. 2019;411:4025–4030. doi: 10.1007/s00216-019-01686-5. [DOI] [PubMed] [Google Scholar]
- 197.Dueñas M.E., Essner J.J., Lee Y.J. 3D MALDI mass spectrometry imaging of a single cell: Spatial mapping of lipids in the embryonic development of zebrafish. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang B., Patterson N.H., Tsui T., et al. Single-cell mass spectrometry reveals changes in lipid and metabolite expression in RAW 264.7 cells upon lipopolysaccharide stimulation. J. Am. Soc. Mass Spectrom. 2018;29:1012–1020. doi: 10.1007/s13361-018-1899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zimmerman T.A., Monroe E.B., Tucker K.R., et al. Elsevier; Amsterdam: 2008. Chapter 13 imaging of cells and tissues with mass spectrometry. Methods in Cell Biology; pp. 361–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shrestha B., Vertes A. in situ metabolic profiling of single cells by laser ablation electrospray ionization mass spectrometry. Anal. Chem. 2009;81:8265–8271. doi: 10.1021/ac901525g. [DOI] [PubMed] [Google Scholar]
- 201.Liu R., Pan N., Zhu Y., et al. T-probe: An integrated microscale device for online in situ single cell analysis and metabolic profiling using mass spectrometry. Anal. Chem. 2018;90:11078–11085. doi: 10.1021/acs.analchem.8b02927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huang Q., Mao S., Khan M., et al. Dean flow assisted cell ordering system for lipid profiling in single-cells using mass spectrometry. Chem. Commun. 2018;54:2595–2598. doi: 10.1039/c7cc09608a. [DOI] [PubMed] [Google Scholar]
- 203.Neumann E.K., Ellis J.F., Triplett A.E., et al. Lipid analysis of 30 000 individual rodent cerebellar cells using high-resolution mass spectrometry. Anal. Chem. 2019;91:7871–7878. doi: 10.1021/acs.analchem.9b01689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Qi M., Philip M.C., Yang N., et al. Single cell neurometabolomics. ACS Chem. Neurosci. 2018;9:40–50. doi: 10.1021/acschemneuro.7b00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.I. Lanekoff, V.V. Sharma, C. Marques, Single-cell metabolomics: Where are we and where are we going? Curr. Opin. Biotechnol. 75 (2022), 102693. [DOI] [PubMed]
- 206.Y. Fangma, H. Zhou, C. Shao, et al., Hydroxysafflor yellow A and anhydrosafflor yellow B protect against cerebral ischemia/reperfusion injury by attenuating oxidative stress and apoptosis via the silent information regulator 1 signaling pathway, Front. Pharmacol. 12 (2021), 739864. [DOI] [PMC free article] [PubMed]
- 207.Taylor M.J., Lukowski J.K., Anderton C.R. Spatially resolved mass spectrometry at the single cell: Recent innovations in proteomics and metabolomics. J. Am. Soc. Mass Spectrom. 2021;32:872–894. doi: 10.1021/jasms.0c00439. [DOI] [PMC free article] [PubMed] [Google Scholar]