Abstract
Objective
Studies have shown that Wuzi Yanzong Pill (WYP) can be used to treat neurological diseases, but its mechanisms for multiple sclerosis (MS) remain unclear. This study aims to determine the effect of WYP on MS in an animal model of experimental autoimmune encephalomyelitis (EAE), and explore its mechanism. To provide theoretical basis for the clinical treatment of MS with WYP.
Methods
C57BL/6 female mice were randomly divided into Blank control, EAE control, low dose WYP, medium dose WYP, and high dose WYP groups. One week before model generation, the mice were gavaged with saline (50 mL/kg/d) in Blank control and EAE control groups. The treatment groups was gavaged with different doses of WYP solution (4, 8, or 16 g/kg/d respectively) Clinical scores were recorded daily. Sample collection was conducted on the 14th and 28th days, respectively The expressions of IL-10, IL-17, IL-12, TNF-α and IFN-γ in spleen were detected by ELISA. The expressions of ROCKII, P-MYPT1, TLR4, NF-κB/p65, MCP-1, CCR2 in spleen, brain and spinal cord were detected by Western Blot. The types of macrophages and the contents of intracellular IL-10 and IL-12 were detected by Flow Cytometry. The contents of TNF-α and TLR4 mRNA in the spleen were detected by RT-PCR.
Results
WYP treatment improved the clinical score of EAE mice in a significant dose-dependent manner, with the WYP high-dose group showed the most significant improvement in clinical score. Compared with the EAE control group, WYP high dose group had significantly lower levels of IL-17, IFN-γ, ROCKII, P-MYPT1, TLR4, NF-κB/p65, MCP-1, and CCR2 as well as TNF-α and TLR4 mRNA, but increased the number of M2 macrophages and IL-10.
Conclusion
WYP treatment relieves clinical symptoms in EAE mice, which may be related to regulate inflammatory pathway and inhibiting expressions of inflammatory cytokines.
Keywords: Wuzi Yanzong Pill, Experimental autoimmune encephalomyelitis, Immunomodulation, anti-inflammation
1. Introduction
Multiple sclerosis (MS) is an autoimmune disease characterized by white matter demyelination in the central nervous system (CNS). With main pathological features including demyelination, axonal degeneration, and progressive nerve dysfunction [1], it affects nearly 2 million patients in the world [2]. The exact etiology and pathogenesis of MS are still not clear. EAE is recognized as the most classic animal model for MS, which resembles human MS [3,4]. During the progress of MS, autoimmune T and B cells are activated to participate in immune response. T cells through the release of proinflammatory cytokines leading to inflammatory infiltrates and demyelination in the CNS. In particular, cytokines excreted by Th1 and Th2 cells are unbalanced. In addition, the interactions between lymphocytes and endothelial cells and between cytokines and adhesion molecules also plays an important role [5]. At present, the treatment for MS mainly include glucocorticoid, fingolimod, glatiramer acetate, beta interferon, mitoxantrone and natalizumab, effective treatment for MS is still not available. It is worth mentioning that the side effects of these drugs are very severe, especially glucocorticoid [[6], [7], [8]].
Studies have shown that the pathological changes of EAE/MS, such as the destruction of Blood–brain barrier, inflammatory Cell migration, immune cell activation, demyelination reaction, etc., are all related to the activation of Rho/ROCK signaling pathway [9]. In immune inflammatory response diseases, activation of the Rho/ROCK signaling pathway activates peripheral immunity and promotes inflammatory cell infiltration into the central nervous system. Rho kinase inhibitor can reduce inflammatory reaction, regulate immune response, and improve degenerative Nervous system disease such as MS. Myosin light chain phosphatase (MLCP) and MYPT1 are the most significant ROCK protein substrates. After the Rho/ROCK signaling pathway is activated by inflammatory factors and cell mediators, MLCP is phosphorylated and inactivated. With the decrease of MLCP activity, the expression of P-MYPT1 is increased, and the structure of cell Actin microfilament skeleton and myosin activity are changed, causing cell damage [10].
TLR-4 plays an important role in the natural immune system. Neurons, oligodendrocytes, and other central nervous systems are high expression sites for TLR-4. TLR-4 can directly activate NF- κB signaling pathway exerts biological effects, releases inflammatory factors, and induces MS. TLR-4 can activate T cells, promote their proliferation, and stimulate immune responses. In the process of MS, the central Nervous tissue is damaged, the permeability of Blood–brain barrier is increased, and immune cells and inflammatory factors will infiltrate the central tissue to further damage the central system. Therefore, blocking TLR-4 activation and inhibiting NF-κB signaling pathway can alleviate the inflammatory response during the onset of MS.
Macrophages are basically derived from monocytes and exist in most tissues and organs of the human body, with strong phagocytic function. IFN- γ and TLR pathway can activate pro-inflammatory factors such as IL-12 and TNF secreted by M1 macrophages- α Play an anti infective role. IL-4 and IL-13 can activate M2 macrophages and secrete anti-inflammatory factors such as IL-10 and TGF- β. It can repair damaged tissues [11]. CCR2, a receptor for MCP1, is expressed on nerve cells, T lymphocytes, and monocytes and macrophages. Activated macrophages secrete chemokines, which recruit macrophages to aggregate into the diseased tissue and expand the inflammatory response. Kidney tonifying drugs can inhibit the activation of macrophages and reduce the secretion of chemokines.
Wuzi Yanzong Pill (WYP) is a classic kidney tonifying formula, while MS is a brain disease of central nervous system. The kidney brain related theory of Traditional Chinese medicine suggests that WYP may be a potential compound medicine for MS. In addition, WYP has been shown to boost nonspecific and specific immune functions in normal mice. It also promotes nonspecific immune function and anti-oxidative activity of immune-suppressed mice. Several studies showed that WYP increases the weight of thymus and spleen in immune-suppressed mice and enhances immune function of lymphocytes and macrophages. WYP can work for decreasing the number of white blood cells in peripheral blood and promoting the production of antibodies in spleen cells [12]. Previous studies have shown that WYP has neuroprotective effects on Parkinson's disease and cuprizone-induced demyelination [[13], [14], [15]]. In the clinical observation of WYP in the treatment of Parkinson's disease, a Nervous system disease, we found that the symptoms of Parkinson's disease in patients taking WYP were significantly improved. Thus, this study used WYP to treat mice MS under the guidance with kidney brain related theories. Our research aims to further explore the potential therapeutic effect of WYP on EAE and its molecular mechanism on the basis of previous studies.
2. Materials and methods
2.1. Experimental animals
Female C57BL/6 mice (20–22g, 8–10 weeks) were purchased from Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). This study was approved by the Ethics Committee of Shanxi University of Chinese medicine, Taiyuan, China (2018DW116). Before the experiment, all mice were reared in a pathogen free laboratory at a temperature of (25 ± 2 °C) with a 12:12 h light/dark cycle for one week. The sample size is determined by previous research and pre experiment. The mice were randomly divided into 5 groups by a random number table, including Blank control, EAE control, low dose WYP, medium dose WYP, and high dose WYP groups, 6 mice in each group.
2.2. Reagents
Mouse myelin oligodendrocyte glycoprotein peptide35-55 (MOG35-55) (#80020) was purchased from Shanghai QiangYao Biotechnology Co. Ltd., PR China. Pertussis toxin (PTX) (#115672) were purchased from Alexis company in the United States. Freund's complete adjuvant (CFA) (#70402) and saponins were purchased from Sigma company in USA. Tuberculosis bacillus (TB) (#341152) were purchased from Becton, Dickinson and company, USA. Antibodies of PE-IL-10 (#154301), FITC-CD11b (#101704), PE-CD16/32 (#101803), PE-CD206 (#141604), PE-IL-12 (#128246) used in Flow cytometry were purchased from Biolegend, Inc., USA. Beta-actin (#4970S), ROCKII (#9029S) and P-MYPT1 (#563S) antibodies were purchased from Cell Signaling Technology, USA. CCR2 (#NB100-701) antibody were purchased from Novus, USA. MCP-1(#E6487) antibody were purchased from Enzo, USA. TLR-4 (#128246) antibody were purchased from Becton, Dickinson and company, USA. NF-κB (#128246) antibody were purchased from Abcam Plc., GBR. The BCA protein assay kit (#C626DB004, BCA kit, AB protein; standard solution; RIPA lysate, PMSF) was purchased from Beyotime Institute of Biotechnology, PR China. The IL-10 (#DY417-05), IL-12 (#DY406-05), IL-17 (#DY410-05), TNF-α (#DYDY419-05) and IFN-γ (#DY421-05) ELISA kits were purchased from R&D Systems,USA. All antibodies have been KO verified or there are no hetero bands at the target location.
2.3. Induction of EAE
EAE model was induced with a water and oil mixture that was made of MOG35-55 and TB dissolved in Freund's complete adjuvant according to a 1:1 (v/v) ratio. On both flanks of 4–5 dorsal spine of a mouse, 0.1 mL of the emulsion was injected underneath the skin (MOG35-55, 250μg/mouse; TB, 350μg/mouse). On the day of immunization and 48 h later, the mice were injected with PTX via abdominal cavity (300 ng/mouse). Tail paralysis of the injected mice suggested the onset of EAE and thus successful model creation [16,17].
Clinical scores were assessed daily and blindly by at least two investigators. The scoring criteria for neurological function was based on an international 5 grade scoring system: 0. healthy; 1. limp tail; 2. ataxia of hind limbs; 3. paresis of hindlimbs and/or ataxia of forelimbs; 4. paresis of the four limbs; 5. moribund or death. When the clinical score reached 3, we do our best to reduce the sacrifice of animals and their sufferings during the experiment.
2.4. Administration of WYP
WYP consists of five herbs including Dodder(fry), medlar, Schisandra (steamed), raspberry, and salt Plantago, and is water-soluble. The pills were purchased from Taiyuan Tongrentang pharmacy (Manufacturer: Beijing Tongrentang Limited by Share Ltd Tongrentang pharmaceutical factory; Batch number: 15035044; Pill property: brown water honeyed pills; Excipient: honey; Specification: 0.1 g/piece; Adult usage: take orally, 6 g/once, 2 times/day). The pills were prepared into three solutions of high, medium and low concentrations (0.32 g/mL, 0.16 g/mL, 0.08 g/mL) with normal saline. The prepared herbal solutions were stored in a refrigerator at 4 °C and were shaken well before each use.
During the experiment, there were 6 mice in each cage, and the animals were free to eat and drink water. Treatments were started one week before the immunization. Experimental mice received WYP twice daily for 21 or 35 days through oral route, with high (16 g/kg), medium (8 g/kg) and low dose (4 g/kg) respectively (n = 12/each group). The normal mice and EAE model received respectively normal saline (50 mL/kg) as control groups in similar manner. Half of mice were sampled on day 14 post-immunization (p.i.), and the other half were sampled on day 28 p.i. All the mice were evaluated for clinical score before sacrificed.
2.5. Specimens collection
At day 14 and 28 p.i., 6 mice selected randomly from each group were anesthetized(50 mg/kg, pentobarbital sodium), and the spleens were removed under aseptic condition. The suspension of mononuclear cells (MNCs) were prepared through grinding the organs in dulbecco's modified eagle medium (DMEM) with 40 μm nylon mesh. Spinal cords and brains from 3 mice form each group were removed on ice after heart perfusion with normal saline. Organs Brain homogenates were prepared in ice-cold lysis buffer supplemented with protease inhibitors PMSF. Collected the supernatants after the lysates were centrifuged at 12,000×g for 20 min. The BCA assay was used to measure protein concentration. Spinal cords (3 mice/each group) were removed on ice after heart perfusion with normal saline, followed by 4% paraformaldehyde fixation. The sections of spinal cord (10 μm) was stained by for Hematoxylin and eosin (HE) and Luxol Fast Blue (LFB) staining.
2.6. Pathological staining
HE and LFB staining were used to observe the pathological changes. For HE staining, the sections of spinal cord were soaked in water for 2 min and then soaked in hematoxylin for 4min. After a fast wash, the sections were differentiated in 0.5% hydrochloric acid alcohol for 15 s, and then soaked in 0.5% eosin for 30 s, followed by a quick wash. After dehydration in different concentrations of ethanol for 2 min/each soaking in xylene for 5 min twice, the sections were sealed with neutral gum and observed under a light microscope.
For LFB staining, the sections of spinal cord were immersed in 95% ethanol for 15 min, then immersed in a Luxol Fast Blue liquid and incubated in thermostat box(57 °C) for 24 h. They were then soaked in 95% ethanol for 10 min, then immersed in deionized water for 5 min, in a 0.05% lithium carbonate solution for 10 s, and in deionized water for 5 min. After dehydration in ethanol with different concentrations for 2 min/each, and soaking in xylene 2 times for 5 min/each, the sections were sealed with neutral gum [18]. 3 sections/animal were imaged use a light microscope (DM4000B, Leica, Germany). The integrated optical density in the spinal cord and the number of infiltrating cells were measured in the lesion area using Image Pro Plus 6.0.
2.7. Western Blot analysis
The proteins (30 μg) was separated by 10% SDS-PAGE gel electrophoresis. Transferred the proteins to nitrocellulose membranes. The membranes were blocked with 5% defatted milk powder at room temperature (RT) for 2 h, followed by the incubation with anti-TLR4, anti–NF–κB/p65, anti-ROCKII, anti-P-MYPT1, anti-MCP-1, anti-CCR2, and anti-β-actin antibodies at 4 °C overnight. On the next day, a fluorescence secondary antibody was added and incubated at RT for 2 h. The bands were observed by using an enhanced chemiluminescence (ECL) system. The protein content were analyzed with Image Lab (3.0) software [19]. β-actin was used as the optical density of internal reference.
2.8. Flow cytometry analysis
For cellular surface staining, splenic MNCs were stained at RT for 20 min in 1% BSA-PBS buffer (50 μL) with the following antibodies: FITC-CD11b(1 μl)/PE-CD16/32 (1 μl) and FITC-CD11b(1 μl)/PE-CD206 (1 μl). For intracellular staining, splenic MNCs were stained at RT for 20 min in 0.05% saponin/1% BSA-PBS buffer (50 μl) with the following antibodies: FITC-CD11b(1 μl)/PE-IL-10(1 μl) and FITC-CD11b(1 μl)/PE-IL-12(1 μl). After washing with PBS, the labeled MNCs were detected with flow cytometry (BD FACS Calibur).
2.9. RT-PCR analysis
Total RNA was extracted from splitting splenic cells using Trizol reagent. The RNA was reverse-transcribed using ThermoScript RT-PCR System (Invitrogen Inc, USA) under a condition as following: 25 °C 5 min,50 °C 15 min,85 °C 5 min,4 °C 10 min. The reverse transcription products were used as a template for PCR amplification using the following specific primer sets (Table 1).
Table 1.
Primer sequence.
| Name | Primer | Sequence | Size |
|---|---|---|---|
| β-actin | Forward | 5‘-CACGATGGAGGGGCCGGACTCATC-3’ | 240bp |
| Reverse | 5‘-TAAAGACCTCTATGCCAACACAGT-3’ | ||
| Mus TNF-α | Forward | 5‘-CGTCAGCCGATTTGCTATCT-3’ | 206bp |
| Reverse | 5‘-CGGACTCCGCAAAGTCTAAG-3’ | ||
| Mus TLR4 | Forward | 5‘-GCCCTACCAAGTCTCAGCTA-3’ | 165bp |
| Reverse | 5‘-CTGCAGCTCTTCTAGACCCA-3’ |
18s mRNA was used as an internal control. The expressions of TLR-4 and TNF-α were calculated according to the following formula: 2-ΔΔCt = (Ct gene of interest-Ct internal control) sample A-(Ct gene of interest-Ct internal control) sample B).
2.10. Cytokines
Splenocytes (3 × 106/ml) were cultured in complete DMEM containing 10% fetal bovine serum (FBS) at 37 °C for 48 h in a CO2 incubator. After centrifugation, the supernatant was collected and used for the detection of TNF-α, IFN-γ, IL-10, IL-12 and IL-17 using ELISA kits. The results were expressed as pg/ml.
2.11. Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 software (Cabit Information Technology Co., Ltd. Shanghai, China), and normality and homogeneity of variance were tested by software before statistical analysis. One-way analysis of variance (ANOVA) with Bonferroni's post-hoc test was used for making comparison among multiple groups, LSD test was used for pairwise comparison. Data were present as mean ± SD ( ±S). P value < 0.05 was considered statistically significant.
3. Results
3.1. WYP treatment delayed EAE onset time and relieved EAE symptoms
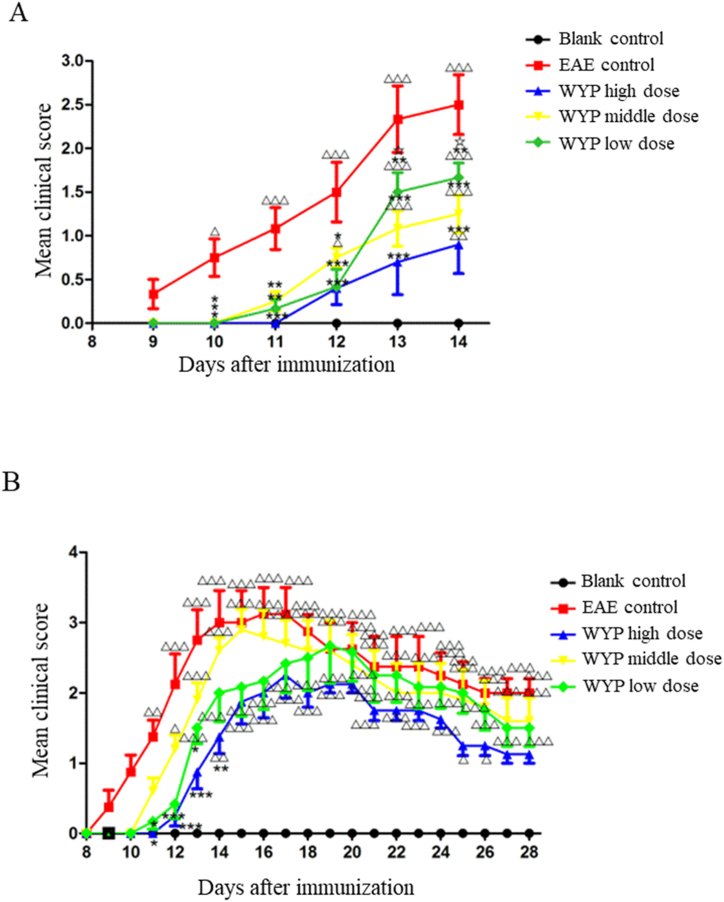
Clinical scores were used to assess the neurological function of mice. As showed in Fig. 1A, the neurological function scores of the EAE control group was higher than Blank control (P < 0.001). The symptoms of EAE control mice included. The onset time of neurological deficits in the EAE control was day 9 p.i. After treatment with WYP, the onset times of the medium and low dose WYP groups were both day 11 p.i., and that of the high dose WYP group was day 12 p.i. The neurological function scores of the three treatment groups were lower than EAE control from day 10 p.i. to day 14 p.i.(P < 0.05). The high dose WYP group had the most improvement in neurological symptoms.
Fig. 1.
Treatment with WYP delayed EAE onset time and relieved the neurological symptoms. A-B. There were progressive increases in mean neurological scores at day 9 p.i., suggesting that the EAE model induced significant neurological deficits. Treatment with three different doses of WYP for 3 weeks caused significant decreases in neurological function scores in all the three groups (A). In contrast, there were only significant decreases in neurological function scores of the high dose WYP group than the EAE control group after 5 weeks of treatment (B). △P < 0.05, △△P < 0.01, △△△P < 0.001 (compared with Blank control); *P < 0.05, **P < 0.01, ***P < 0.001 (compared with EAE control); ☆P < 0.05, ☆☆P < 0.01, ☆☆☆P < 0.001 (compared with WYP high dose).
As showed in Fig. 1B, the neurological function score of the EAE control was higher than the Blank control (P < 0.001). Treatment with WYP significantly delayed EAE onset time, with the onset times being 9 p.i. for the EAE control group, day 11 p.i. for both the middle and low WYP dose group, and day 12 p.i. for the high dose WYP group. The high dose WYP group had the most improvement in neurological symptoms.
According to Fig. 1 (A, B), the WYP high-dose group had the lowest clinical score, the latest onset of disease, and the least clinical symptoms. The high dose of WYP can be set as the optimal dose, and the follow-up detection experiments are subject to the high dose group.
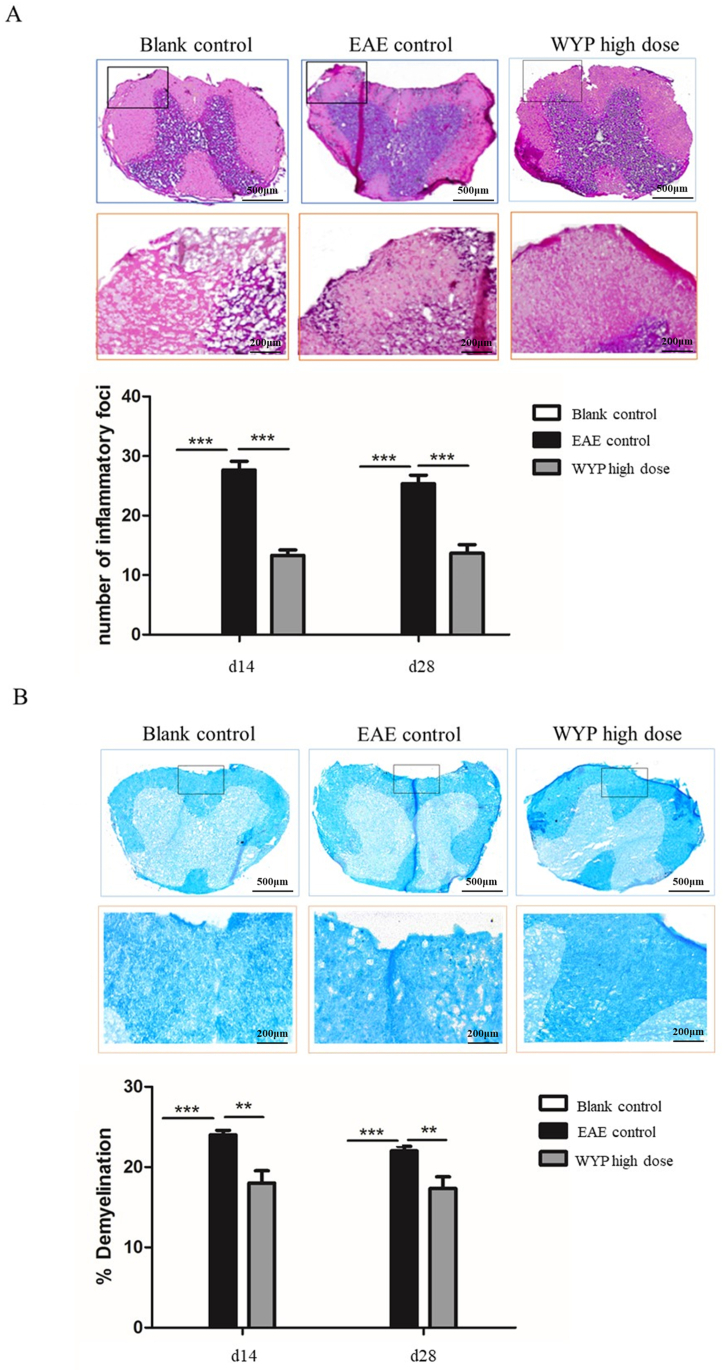
3.2. WYP treatment suppressed inflammation and improved spinal cord demyelination
EAE is accompanied by inflammatory reaction and demyelination. Inflammatory cells infiltrate the central spinal cord tissue, and the central nerve cells are injured, accompanied by demyelination. The larger the demyelination area, the more serious the disease. He staining can be used to analyze the infiltration of inflammatory cells, and solid blue staining (LFB) can be used to analyze the demyelination of white matter of spinal cord. At days 14 and 28 p.i., the mean number of inflammation foci in the spinal cord of the high dose WYP group was significantly less than those of the EAE control group (Fig. 2A. P < 0.001), and the mean area of demyelination of the high dose WYP group was also significantly smaller than those of the EAE control group (Fig. 2B. P < 0.01). The results suggest that WYP treatment significantly reduced inflammation and demyelination of the spinal cord induced by EAE.
Fig. 2.
WYP treatment improved spinal cord inflammation and demyelination in the EAE model. HE staining and Luxol Fast Blue staining were used to evaluate inflammation and demyelination of the spinal cord tissues. Among the EAE control, Blank control, and WYP high dose groups, treatment with high dose WYP for either 3 weeks (day 14 p.i.) or 5 weeks (day 28 p.i.) resulted in significant decreases in inflammatory foci (Fig. 2A) and myelin loss (Fig. 2B). The results were analyzed with Image Pro software. *P < 0.05, **P < 0.01, ***P < 0.001.
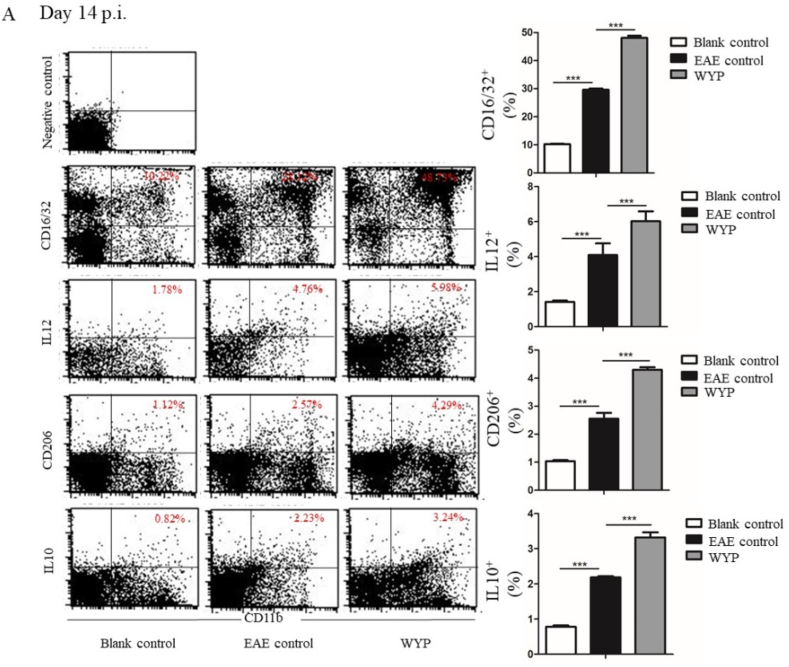
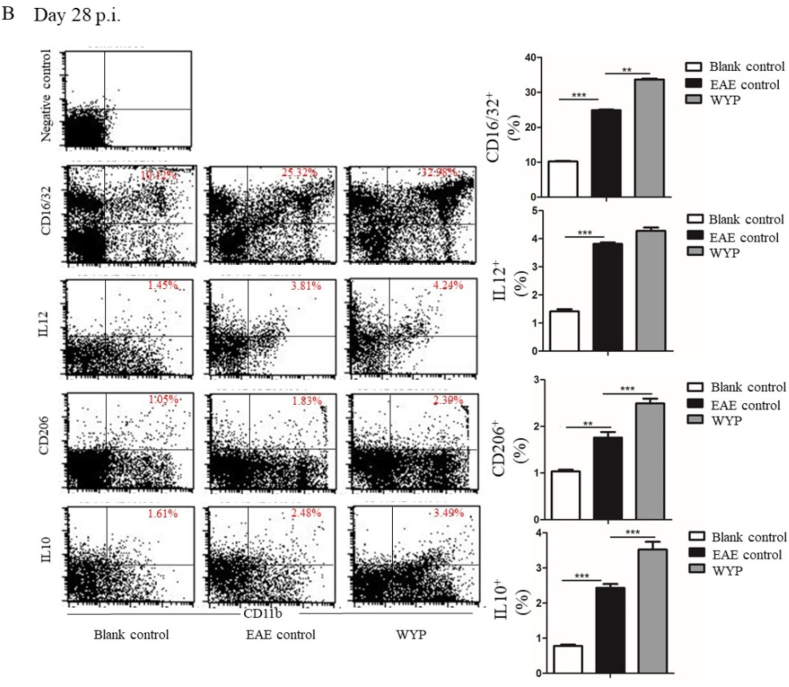
3.3. WYP promoted the expression of M2 phenotype macrophage and anti-inflammatory factors
CD11b + labeled splenic macrophages can be divided into M1 and M2 subtypes. CD16/32+ labeled M1 macrophages secreted proinflammatory factor IL-12, which aggravated the inflammatory response of EAE; CD206+ labeled M2 macrophages secrete the anti-inflammatory factor IL-10, which inhibits the inflammatory response and reduces the inflammatory degree of EAE.
At day 14 p.i., the expressions of M1 macrophage and M2 macrophage of the WYP group were significantly higher than those of the EAE control group (Fig. 3A. P < 0.001). The contents of IL-10 and IL-12 in WYP group were also significantly higher than those of the EAE control group (P < 0.001). The results obtained at day 28 p.i. were similar to that of day 14 p.i (Fig. 3B). Thus, WYP treatment increased the secretion of anti-inflammatory cytokines, but did not inhibit the secretion of pro-inflammatory cytokines.
Fig. 3.
M1 phenotype macrophage is marked with CD16/32 and excretes pro-inflammatory factor IL-12; M2 phenotype macrophage is marked with CD206 and excretes an anti-inflammation factor IL-10. The WYP group had significantly higher levels of CD206 and IL-10, suggesting that WYP improved the expression of M2 phenotype macrophage. *P < 0.05, **P < 0.01, ***P < 0.001.
3.4. WYP inhibited inflammatory pathway activity and down-regulated inflammatory chemokines and chemotactic factors and their receptors
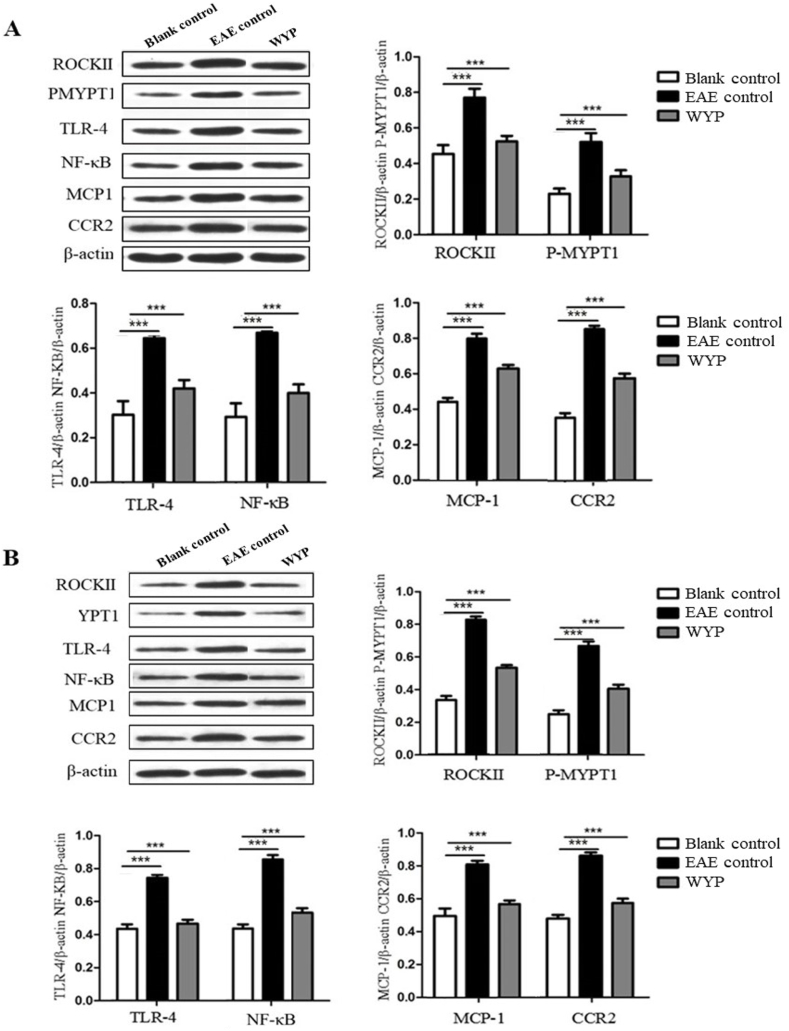
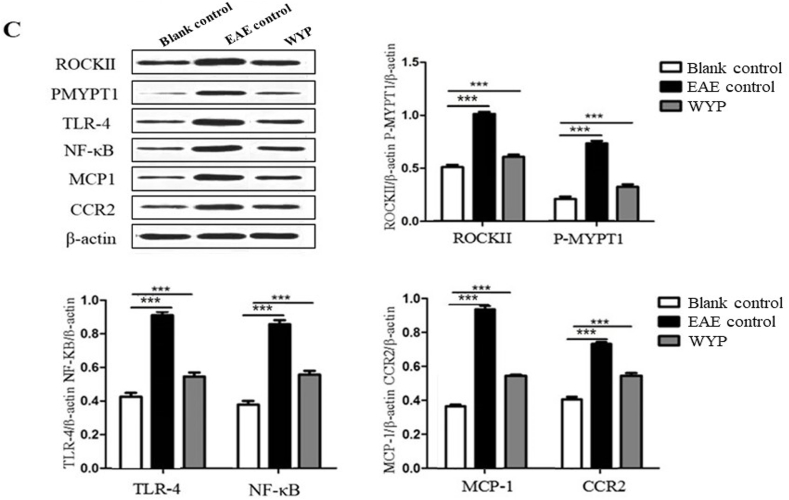
The marker proteins of Rho/ROCK signaling pathway are ROCKII and P-MYPT1; NF-κB inflammatory signal pathway marker proteins are TLR-4 and NF-κB; CCR2 and MCP-1 are chemoreceptors and chemokines that promote the development of inflammation.
As shown in Fig. 4, the expressions of TLR-4 receptor and NF-κB in brain, spinal cord, and the macrophages of spleen of the EAE mice were much higher than those of the Blank control group, but their expressions in the WYP group were lower than the EAE control (P < 0.001). The contents of ROCKII and P-MYPT1 in brain, spinal cord, and the macrophages of spleen of the WYP group were significantly lower than the EAE control (P < 0.001). The contents of MCP-1and CCR2 in brain, spinal cord, and the macrophages of spleen of the WYP group were lower than the EAE control (P < 0.001). The results indicate that treatment with WYP has the effects of normalizing inflammatory pathway activity and chemokine release.
Fig. 4.
Treatment with WYP for 3 weeks inhibited inflammatory pathway activity and down-regulated inflammatory chemokines and chemotactic factors and their receptors. At day 14 p.i., the contents of ROCKII, P-MYPT1, TLR-4, NF-κB, CCR2, and MCP-1 in brain (A), spinal cord (B), and the macrophages of spleen (C) were detected by Western blot. The results were analyzed from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 (Compared with EAE control).
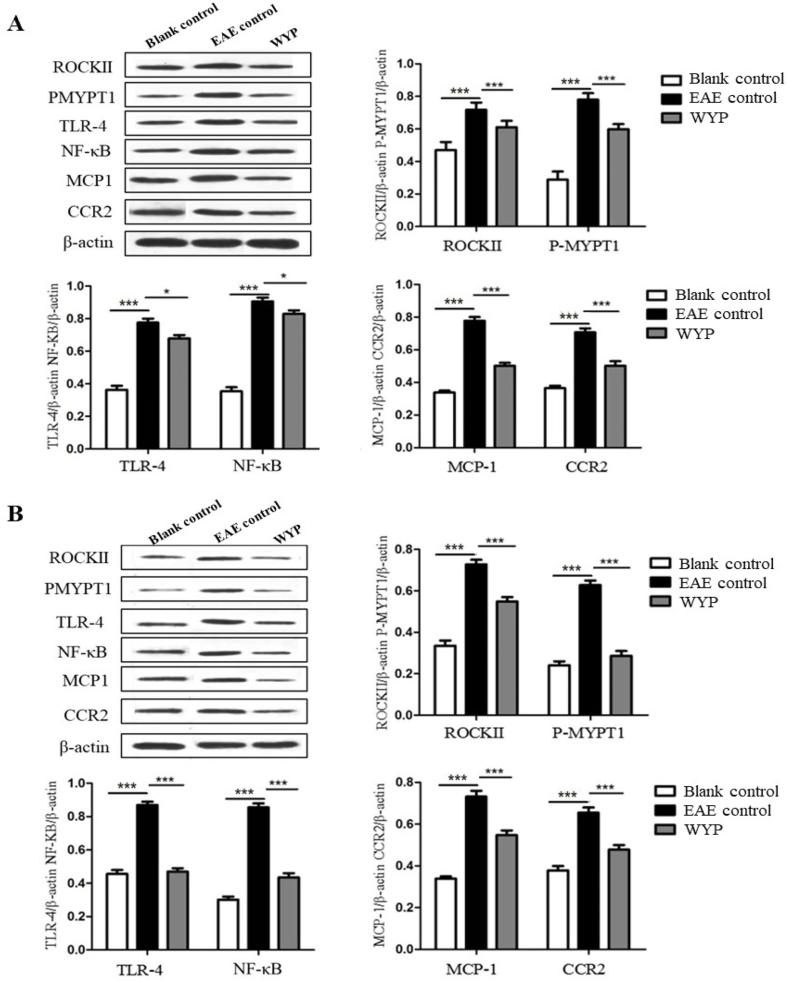
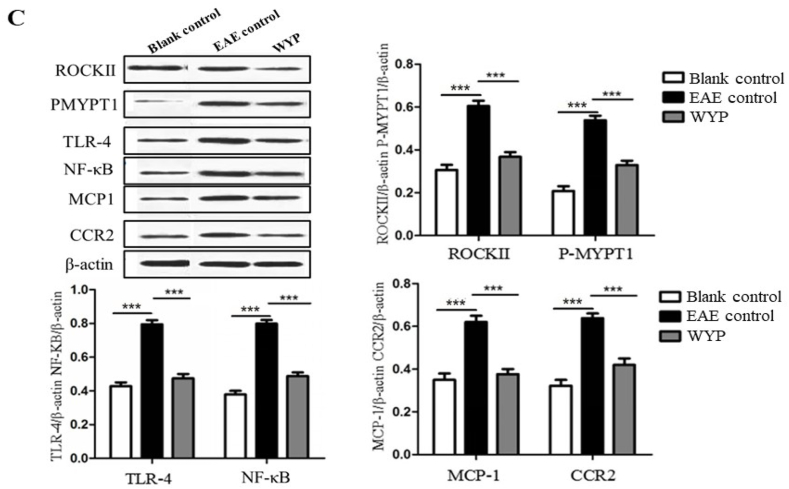
At 5 weeks p.i., the expressions of TLR-4 receptor and NF-κB in brain (Fig. 5A), spinal cord (Fig. 5B), and macrophages of spleen (Fig. 5C) of the WYP group were significantly lower than the EAE control group (P < 0.001). The contents of ROCKII and P-MYPT1 in brain, spinal cord, and macrophages of spleen in the WYP group were lower than the EAE control (P < 0.001). The contents of MCP-1and CCR2 in the WYP group of brain, spinal cord, and macrophages of spleen were lower than the EAE control group (P < 0.001).
Fig. 5.
Treatment with WYP for 5 weeks inhibited inflammatory pathway activity and down-regulateed inflammatory chemokines and chemotactic factors and their receptors. At day 28 p.i., the contents of ROCKII, P-MYPT1, TLR-4, NF-κB, CCR2, and MCP-1 in brain (A), spinal cord (B) and macrophages of spleen (C) were detected respectively by Western blot. The results were obtained from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 (Compared with EAE control).
3.5. WYP regulated the expression of inflammatory factor of splenocytes supernatant
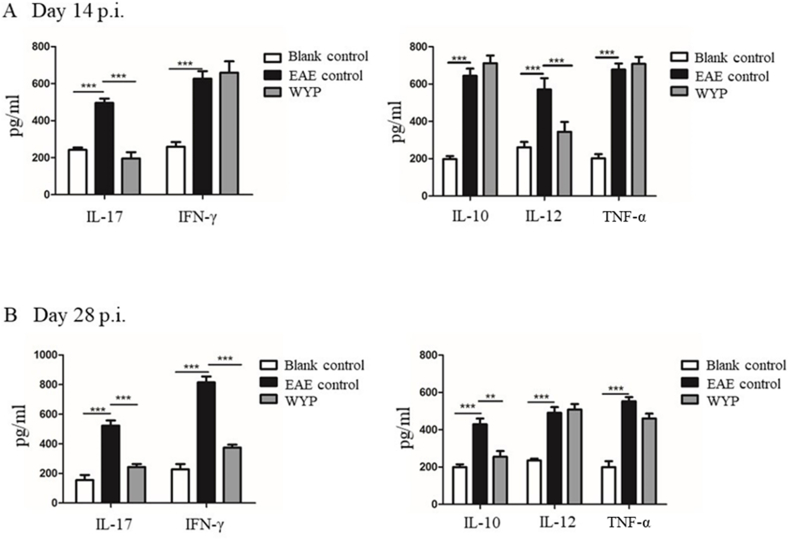
The content of inflammatory factors in splenocyte supernatant was detected by ELISA. T cells secrete IL-17 and IFN-γ; Macrophages secrete IL-10, IL-12 and TNF-α. The greater the secretion of extracellular proinflammatory factors, the more intense the local tissue inflammatory response. On the contrary, the greater the secretion of extracellular anti-inflammatory factors, the degree of local tissue inflammatory response can be alleviated. At day 14 p.i. (Fig. 6A), the content of IL-17 was lower in the WYP group than the EAE control group. There was no significant difference in the expression of IFN-γ between the two groups. The content of IL-12 excreted by macrophages was lower in the WYP group than the EAE control group, but there was no significant differences in the expressions of IL-10 and TNF-α between the two groups. At day 28 p.i. (Fig. 6B), the contents of IL-17, IFN-γ and IL-10 were lower in the WYP group than the EAE control group, but there was no significant differences in the expressions of IL-12 and TNF-α between the WYP group and the EAE control group.
Fig. 6.
Expressions of inflammatory factors in splenocytes supematant. IL-10, IL-12, IL-17, TNF-α and IFN-γ were detected by ELISA kits. The results were expressed as pg/mL. *P < 0.05; **P < 0.01; ***P < 0.001.
3.6. WYP inhibited the expressions of genes of inflammatory factors and receptors
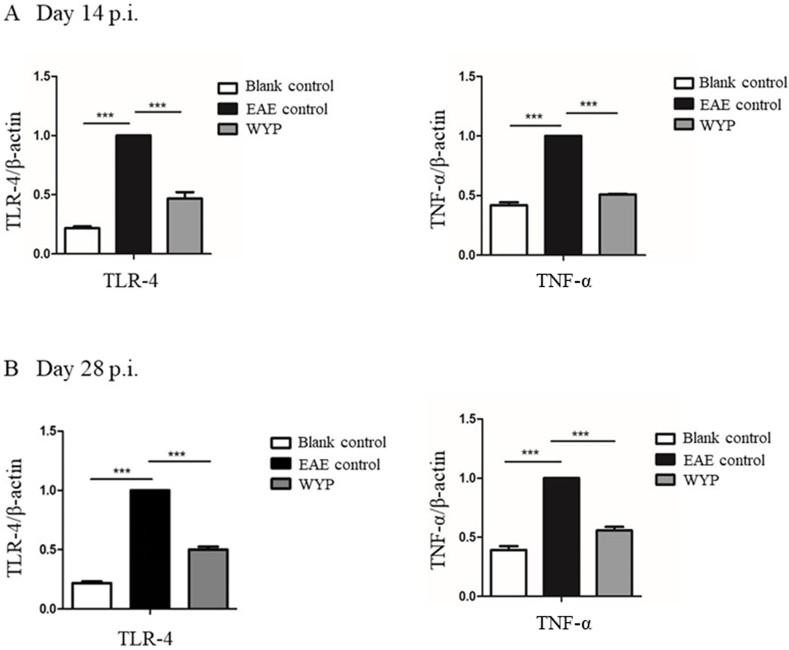
TLR-4 is an inflammatory receptor and TNF-α is a proinflammatory factor. The higher the severity of EAE, the higher the expression levels of TLR-4 and TNF-α. WYP inhibited the expressions of inflammatory factors and their receptors at gene level. As showed in Fig. 7, the contents of TLR-4 and TNF-α of the WYP group were significantly lower than those of the EAE control group at day 14 p.i.; the contents of TLR-4 and TNF-α in the WYP group were significantly lower than those of the EAE control group.
Fig. 7.
Expressions of TLR-4 and TNF-α genes in spleen cells were detected by RT-PCR technique. The results were obtained from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
4. Discussion
Multiple sclerosis is a central nervous system demyelinating disease [20,21]. There is no effective treatment available until now. The affected regions of MS center in white matter of the spinal cord and brain, with inflammatory cells infiltration and demyelination but relative sparing of axons. In addition, MS is associated with damage of blood-brain barrier, activation of T cells, and the poor repair of oligodendrocytes [22]. Chinese herbal compound WYP has protective effect on several Nervous system disease. The mechanistic inhibition of WYP to investigate its role in Immune regulation and inflammation in the EAE model of C57 mice has not been reported previously. Hence, the present study was designed to investigate the possible protective role of WYP on the neurological function and immune inflammation developed in EAE Mice.
WYP consists of Chinese wolfberry, Cuscuta (fried), Raspberry, Schisandra (steamed), Plantago (salt fried). This prescription was originally found in Tang dynasty. Chinese wolfberry and Cuscuta have a function of immunoregulation [[23], [24], [25]]. Schisandra can strengthen the kidney and restrain the central nerve, as well as enhance phagocytosis of macrophage and improve immunity [26,27]; Raspberry can warm kidney Yang and supplement kidney essence, which can activate lymphocytes [28,29]. Some toxicology studies showed that WYP is safe and non-toxic [30]. The theory of traditional Chinese medicine acknowledges that the function of the kidney greatly affects immune-regulation function of the human body. According to traditional Chinese medicine, the pathogeny of inflammation of spinal cord in MS is a weakness of kidney essence and brain and a damage of meridians after the body being affected by external factors. Because WYP has the effect of nourishing kidney essence, it can relieve the symptoms of MS [31].
Studies have shown that the pathological changes of MS are related to the activation of Rho/ROCK signal pathway. Rho kinase (ROCK) is the substrate of small G protein Rho. There are two subtypes of ROCK: ROCKⅠ and ROCKⅡ. ROCK is abundant in neural tissue. The Rho/ROCK signaling pathway is involved in the regulation of cell contraction, differentiation, migration and gene expression [32]. After nerve cell injury, inhibitory factors are generated and the Rho/ROCK signaling pathway is activated through NOGO protein. When ROCK is activated or its expression level is increased, disease can be induced, and the growth of nerve axons is inhibited, leading to degenerative changes [33]. In immune-inflammatory diseases, activation of the Rho/ROCK signaling pathway initiates peripheral immunity and promotes inflammatory cell infiltration into the central nervous system [34].
This study found that the levels of ROCK II receptor and its effector substrate P-MYPT1 in the brain and spinal cord tissue of the WYP group were significantly reduced after treatment. To some extent, these prevent further damage to nerve cells and improve the microenvironment of axon growth. At the same time, it inhibits the occurrence of local immune responses, thereby slowing down the clinical symptoms of MS. Therefore, WYP has a certain degree of Rho kinase inhibitor effect.
In MS patients, the TLR-4 content in peripheral blood increases. The TLR-4 content in the cerebrospinal fluid of MS patients significantly increased [35]. Thus, WYP can regulate the NF-κB inflammatory pathway by reducing the nuclear transfer of NF-κB, which exerting anti-inflammatory and antioxidant neuroprotective effects. Studies have shown that Rho kinase inhibitors can down regulate TLR-4 and NF-κB and reduce the infiltration of inflammatory cells, as well as promote axonal regeneration in EAE mice [36,37]. The results of this study showed that WYP treatment significantly reduced the expressions of TLR-4 and NF-κB. Therefore, WYP may inhibit inflammatory reaction by reducing the activation of Toller like receptor signaling pathway to achieve the neuroprotective effects in the central nervous system.
The activation level of CCR2 is positively correlated with the expression of MCP1. Research has shown that the activation of CCR2 is positively correlated with the successful induction of EAE models and the onset of MS. The combination of highly expressed MCP1 and CCR2 will increase the permeability of the Blood–brain barrier, enhance the infiltration of macrophages and lymphocytes into central tissues, strengthen the immune inflammatory lesions of the nervous system, and aggravate the demyelination. The results of this experiment suggest that the protein content of MCP1 and CCR2 in brain and spinal cord tissues and Splenocyte of mice in WYP group is significantly reduced, which further suggests that Kidney Tonifying Prescription can inhibit the activation of chemoattractants, reduce the secretion of chemokines, thereby reducing the inflammatory lesions of central tissues and inhibiting the autoimmune response of central environment.
IFN-γ is an inflammatory cytokine secreted by Th1 cells, IL-17 is a pro-inflammatory cytokine excreted by Th17 cells. These factors initiate inflammation and tissue damage in immune response [38]. Our results showed that WYP treatment significantly reduced the expressions of both inflammatory and proinflammatory cytokines. WYP may inhibit inflammatory reaction and regulate immune response by reducing the activation of T cells, increasing anti-inflammatory cytokine IL-10, and changing the phenotype of macrophages. We also observed an increased expression of IL-12, which may be caused by hypersplenism and related to multi-target effects of Chinese medicine.
Our study shows that WYP can improve neurological function and alleviate clinical symptoms in EAE mice. WYP regulates rho/rock signaling pathway and NF-κB signaling pathway, reduce nerve tissue damage and inhibit the expression of inflammatory proteins. WYP can promote the expression of anti-inflammatory macrophages, increase the expression of anti-inflammatory factors and reduce the expression of pro-inflammatory factors. The mechanism of WYP in preventing and treating EAE may be to regulate immunity and suppress inflammatory reaction.
5. Conclusion
The present study showed that WYP is effective in delaying the onset time of EAE and alleviating its clinical symptoms. The mechanism of action may include reducing the secretion of inflammatory factors, inhibiting the activation of inflammatory pathway, and regulating the immune system. Overall, the current research supports WYP protective effects against neurological function and immune inflammatory reactions caused by EAE in this animal model owing to its neuroprotective potential. This study provides an experimental basis for clinical application of WYP for the treatment of MS and for further exploration of its immune regulation function. However, there is still a lack of research on the specific points of action of WYP in regulating the Rho/ROCK and NF-κB signaling pathways. And it is not clear in the relationship between the two signaling pathways involved in the neuroprotective effect of WYP on MS. This is the deficiency in the study, and to better recognize the role of WYP in MS, we will continue to improve it in the follow-up study.
Author contribution statement
Yan-Rong Li, Ruo-Nan Zhang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Rui-Rui Sun, Yan-Yan Li, Bo Zhang, Xiao-Ming Jin, Hai-Fei Zhang: Contributed reagents, materials, analysis tools or data. Bao-Guo Xiao, Cun-Gen Ma, Hui-Jie Fan and Zhi Chai: Conceived and designed the experiments; Analyzed and interpreted the data.
Data availability statement
Data will be made available on request.
Funding
This study was supported by the Key National Science and Technology Cooperation Projects in Shanxi Province (202204041101002), the National Young Qihuang Scholars Training Program (No.❲2022❳256), the Outstanding Youth Talents Program of Shanxi Province (No.❲2019❳35), the Natural Science Foundation of Shanxi Province (No.20210302124293, 202203021212346, 202203021222269), the Research Project supported by Shanxi Scholarship Council of China (No.2021–142), the Key Science and Technology R&D Project of Jinzhong (No.Y213004), the Science and Technology Innovation Ability Cultivation Project, Shanxi University of Chinese Medicine, China (No.2020PY-JC-03, No.2021PY-QN-03, No.2022PY-TH-16, No.2022TD1013).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Cun-Gen Ma, Email: macungen@sxtcm.edu.cn.
Hui-Jie Fan, Email: fanhuijie@sxtcm.edu.cn.
Zhi Chai, Email: chaizhi@sxtcm.edu.cn.
References
- 1.He A., Merkel B., Brown J., et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307–316. doi: 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 2.Mathur D., Mishra B.K., Rout S., et al. Potential biomarkers associated with multiple sclerosis pathology. Int. J. Mol. Sci. 2021;22(19) doi: 10.3390/ijms221910323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traboulsee A., Létourneau-Guillon L., Freedman M.S., et al. Canadian expert panel recommendations for MRI use in MS diagnosis and monitoring. Can. J. Neurol. Sci. 2015;42(3):159–167. doi: 10.1017/cjn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klaren R.E., Motl R.W., Woods J.A., et al. Effects of exercise in experimental autoimmune encephalomyelitis (an animal model of multiple sclerosis) J. Neuroimmunol. 2014;274(1):14–19. doi: 10.1016/j.jneuroim.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann-Horn K., Kinzel S., Weber M.S. Deciphering the role of B cells in multiple sclerosis-towards specific targeting of pathogenic function. Int. J. Mol. Sci. 2017;18(10):2048. doi: 10.3390/ijms18102048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brola W., Steinborn B. Pediatric multiple sclerosis-current status of epidemiology, diagnosis and treatment. Neurol. Neurochir. Pol. 2020;54(6):508–517. doi: 10.5603/PJNNS.a2020.0069. [DOI] [PubMed] [Google Scholar]
- 7.Shirani Afsaneh, Okuda Darin T., Stüve Olaf. Therapeutic advances and future prospects in progressive forms of multiple sclerosis. Neurotherapeutics. 2016;13:58–69. doi: 10.1007/s13311-015-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor T., Mehan S. Neuroprotective methodologies in the treatment of multiple sclerosis current status of clinical and pre-clinical findings. Curr. Drug Discov. Technol. 2021;18(1):31–46. doi: 10.2174/1570163817666200207100903. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Schittenhelm J., Meyermann R., et al. Lesional accumulation of Rho A(+)cells in brains of experimental autoimmune encephalomyelitis and multiple sclerosis. Neuropathol. Appl. Neurobiol. 2008;34(2):231–240. doi: 10.1111/j.1365-2990.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 10.Godin C.M., Ferguson S.S. The angiotensinⅡtype 1 receptor induces membrane blebbing by coupling to Rho A, Rho kinase, and myosin light chain kinase. Mol. Pharmacol. 2010;77:903–911. doi: 10.1124/mol.110.063859. 06. [DOI] [PubMed] [Google Scholar]
- 11.Sica A., Mantovani A. Macrophage plasticity andpolarization: in vivo veritas. J. Clin. Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang N., Zhang S.C., Chen X.H., et al. Analysis the immune-promoting effect of Wuzi Yanzong pill for the aspermia mice by gene expression. Journal of Shanghai University of Traditional Chinese Medicine. 2014;27(4):63–67. [Google Scholar]
- 13.Hang W., Fan H.J., Li Y.R., et al. Wuzi Yanzong pill attenuates MPTP-induced Parkinson's Disease via PI3K/Akt signaling pathway. Metab. Brain Dis. 2022;37(5):1435–1450. doi: 10.1007/s11011-022-00993-8. [DOI] [PubMed] [Google Scholar]
- 14.Li Y.R., Fan H.J., Sun R.R., et al. Wuzi Yanzong pill plays A neuroprotective role in Parkinson's disease mice via regulating unfolded protein response mediated by endoplasmic reticulum stress. Chin. J. Integr. Med. 2023;29(1):19–27. doi: 10.1007/s11655-022-3727-0. [DOI] [PubMed] [Google Scholar]
- 15.Li Y.R., Sun M.Y., Hang W., et al. Wuzi Yanzong Pill relieves CPZ-induced demyelination by improving the microenvironment in the brain. Heliyon. 2022;8(12) doi: 10.1016/j.heliyon.2022.e12277. Liu, C. Y., Guo, S. D., Yu, J. Z., Li, et al. (2015). Fasudil mediates cell therapy of EAE by immunomodulating encephalomyelitic T cells and macrophages. European journal of immunology. 45(1): 142-152. DOI: 10.1002/eji.201344429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo M.F., Meng J., Li Y.H., et al. The inhibition of Rho kinase blocks cell migration and accumulation possibly by challenging inflammatory cytokines and chemokines on astrocytes. J. Neurol. Sci. 2014;343(1–2):69–75. doi: 10.1016/j.jns.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y.F., Zhang X., Ding Z.B., et al. The therapeutic potential of Rho kinase inhibitor fasudil derivative FaD-1 in experimental autoimmune encephalomyelitis. J. Mol. Neurosci.: M. Inc. 2015;55(3):725–732. doi: 10.1007/s12031-014-0411-7. [DOI] [PubMed] [Google Scholar]
- 18.Yu J.W., Li Y.H., Song G.B., et al. Synergistic and superimposed effect of bone marrow-derived mesenchymal stem cells combined with fasudil in experimental autoimmune encephalomyelitis. J. Mol. Neurosci.: M. Inc. 2016;60(4):486–497. doi: 10.1007/s12031-016-0819-3. [DOI] [PubMed] [Google Scholar]
- 19.Li Y.H., Yu J.Z., Xin Y.L., et al. Protective effect of a novel Rho kinase inhibitor WAR-5 in experimental autoimmune encephalomyelitis by modulating inflammatory response and neurotrophic factors. Exp. Mol. Pathol. 2015;99(2):220–228. doi: 10.1016/j.yexmp.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 20.McKeon A., Zekeridou A. Autoimmune encephalitis management: MS centers and beyond. Mult. Scler. 2020;26(13):1618–1626. doi: 10.1177/1352458520905485. [DOI] [PubMed] [Google Scholar]
- 21.Passali M., Josefsen K., Frederiksen, et al. Current evidence on the efficacy of gluten-free diets in multiple sclerosis, psoriasis, type 1 diabetes and autoimmune thyroid diseases. Nutrients. 2020;12(8):2316. doi: 10.3390/nu12082316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann M., Evans H., Schaupp A.L., et al. Identifying CNS-colonizing T cells as potential therapeutic targets to prevent progression of multiple sclerosis. Méd. 2021;2(3):296–312. doi: 10.1016/j.medj.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Sun M., Jin H., et al. Effects of lycium barbarum polysaccharides on immunity and the gut microbiota in cyclophosphamide-induced immunosuppressed mice. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.701566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceccarini M.R., Codini M., Cataldi S., et al. Acid sphingomyelinase as target of Lycium Chinense: promising new action for cell health. Lipids Health Dis. 2016;15(1):183–193. doi: 10.1186/s12944-016-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Y., Yan Y., Chen D., et al. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019;10(6):3671–3683. doi: 10.1039/c9fo00638a. [DOI] [PubMed] [Google Scholar]
- 26.Huang S., Zhang D., Li Y., et al. Schisandra sphenanthera: a comprehensive review of its botany, phytochemistry, pharmacology, and clinical applications. Am. J. Chin. Med. 2021;49(7):1577–1622. doi: 10.1142/S0192415X21500749. [DOI] [PubMed] [Google Scholar]
- 27.Song F., Zeng K., Liao L., et al. Schizandrin A. Inhibits microglia-mediated neuroninflammation through inhibiting TRAF6-NF-κb and jak2-stat3 signaling pathways. PLoS One. 2016;13(4):266–279. doi: 10.1371/journal.pone.0149991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J.H., Kim Y.S., Kim T.I., et al. Unripe black raspberry (rubus coreanus miquel) extract and its constitute, ellagic acid induces T cell activation and antitumor immunity by blocking PD-1/PD-L1 interaction. Foods. 2020;9(11):1590. doi: 10.3390/foods9111590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y.W., Lin C.W., Pan P., et al. Black raspberries suppress colorectal cancer by enhancing Smad4 expression in colonic epithelium and natural killer cells. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hai J., Dong M.W., Yu P.W., et al. Wuzi Yanzong pill,a Chinese polyherbal formula, alleviates testicular damage in mice induced by ionizing radiation. BMC Compl. Alternative Med. 2016;16:509–511. doi: 10.1186/s12906-016-1481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng K.W., Wan Y.J., Liao L.X., et al. Identification and function analysis of neuroprotective targets group of modified Wuzi Yanzong pill. Zhongguo Zhongyao Zazhi. 2017;42(19):3656–3660. doi: 10.19540/j.cnki.cjcmm.2017.0143. [DOI] [PubMed] [Google Scholar]
- 32.Liu C., Li Y. Propofol relieves inflammation in MIRI rats by inhibiting Rho/Rock signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2021;25(2):976–984. doi: 10.26355/eurrev_202101_24667. [DOI] [PubMed] [Google Scholar]
- 33.Kubo T., Hata K., Yamaguchi A., et al. Rho-ROCK inhibitors as emerging strategies to promote nerve regeneration. Curr. Pharmaceut. Des. 2007;13(24):2493–2499. doi: 10.2174/138161207781368657. [DOI] [PubMed] [Google Scholar]
- 34.Porazinski S., Parkin A., Pajic M. Rho-ROCK signaling in normal physiology and as a Key player in shaping the tumor microenvironment. Adv. Exp. Med. Biol. 2020;1223:99–127. doi: 10.1007/978-3-030-35582-1_6. [DOI] [PubMed] [Google Scholar]
- 35.Miranda H.S., Baxter A.G. Role of toll-like receptors in multiple sclerosis. Afr. J. Clin. Exp. Immunol. 2013;2(1):75–93. [PMC free article] [PubMed] [Google Scholar]
- 36.Xin Y.L., Yu J.Z., Yang X.W., et al. FSD-C10: a more promising novel ROCK inhibitor than Fasudil for treatment of CNS autoimmunity. Biosci. Rep. 2015;35:533–545. doi: 10.1042/BSR20150032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren X., Meng T., Ren X., et al. Fasudil alleviates acetaminophen-induced liver injury via targeting Rhoa/ROCK signal pathway. J. Toxicol. Sci. 2021;46(6):255–262. doi: 10.2131/jts.46.255. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad S.F., Nadeem A., Ansari M.A., et al. CC chemokine receptor 5 antagonist alleviates inflammation by regulating IFN-γ/IL-10 and STAT4/Smad3 signaling in a mouse model of autoimmune encephalomyelitis. Cell. Immunol. 2022;379 doi: 10.1016/j.cellimm.2022.104580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.