Abstract
Objective
To explore the associations between adiposity indices, assessed at or after a diagnosis of prostate cancer, and mortality.
Design
Systematic review and meta-analysis.
Data sources
PubMed and Embase, from inception to 16 November 2022.
Eligibility criteria for selecting studies
Cohort studies or randomised controlled trials of men with a diagnosis of prostate cancer that investigated the associations between adiposity (body mass index, waist and hip circumference, waist-to-hip ratio, and subcutaneous and visceral adipose tissue) after diagnosis and mortality outcomes. A modified version of the risk of bias for nutrition observational studies tool was used to assess risk of bias.
Results
79 studies were identified that investigated adiposity indices after a diagnosis of prostate cancer in relation to mortality. No randomised controlled trials were found. A non-linear dose-response meta-analysis indicated a J shaped association between body mass index and all cause mortality (33 910 men, 11 095 deaths, 17 studies). The highest rate of all cause mortality was found at the lowest and upper range of the distribution: 11-23% higher rate for a body mass index of 17-21 and 4-43% higher rate for a body mass index of 30-40. The association between body mass index and mortality specific to prostate cancer was flat until body mass index reached 26-27, and then increased linearly by 8-66% for a body mass index of 30-40 (33 137 men, 2947 deaths, 13 studies), but the 95% confidence intervals were wide. These associations did not differ in most predefined subgroups by study design, number of deaths, anthropometric assessment, follow-up time, geographical location, prostate cancer risk group, and adjustment variables. No associations were found in meta-analyses between 10 cm increases in waist circumference and all cause mortality or mortality specific to prostate cancer, but only three studies were available. The few studies with data on change in weight, waist-to-hip ratio, and subcutaneous and visceral adipose tissue reported conflicting results.
Conclusions
This review suggests that patients with prostate cancer might benefit from maintaining a healthy weight and avoiding obesity. Future studies should investigate adiposity across different stages of cancer survivorship and use various parameters for distribution of adipose tissue.
Systematic review registration
Open Science Framework https://osf.io/qp3c4.
Keywords: Epidemiology, Prostatic diseases
What is already known on the topic
Adiposity (based on body mass index) has been associated with a higher risk of advanced and lower risk of localised prostate cancer
The association between adiposity and the prognosis of prostate cancer is less well understood and observational studies have reported conflicting results
None of the existing meta-analyses investigated the potential non-linearity of associations between adiposity and prognosis of prostate cancer
What this study adds
This meta-analysis provides evidence of a J shaped association between body mass index after a diagnosis of prostate cancer and all cause mortality, and a similar non-linear shape for mortality specific to prostate cancer
Limited and inconsistent evidence exists for other adiposity indices, including waist circumference, waist-to-hip ratio, and change in weight in relation to mortality outcomes
None of the studies performed time varying exposure analyses
How this study might affect research, practice, or policy
Future studies should investigate adiposity across different stages of cancer survivorship and use various parameters for distribution of adipose tissue
Introduction
In 2020, 1.4 million men received diagnoses of prostate cancer (14% of all incident cancers (10 million) among men) and 376 000 deaths from prostate cancer (7% of all cancer deaths (5.5 million) among men) occurred worldwide.1 Prostate cancer has generally high survival rates but is the leading cause of death related to cancer in 46 countries.2 Most individuals with a diagnosis of prostate cancer are expected to survive for at least five years after diagnosis, and extended survivorship is often accompanied by comorbidities, including adiposity.3 Men with prostate cancer continue to have excess mortality up to 15 years after diagnosis.4 Adiposity and prostate cancer affect substantial proportions of the male population, making the association between the two important for public health.5 When the relation between adiposity and prostate cancer prognosis is clarified, the existing limited survivorship evidence based lifestyle recommendations and tailored weight management interventions could be improved and implemented to assist men to cope with the disease and further improve prognosis.3 6
Currently, patients with prostate cancer are advised to avoid gaining weight, to maintain a healthy weight, or both.7 8 Prostate cancer is highly prevalent and has a substantial clinical, economic, and societal burden, but few risk factors (mainly non-modifiable, such as advanced age, ethnic group, family history, and genetics) have been identified so far, and several questions remain about its aetiology.9 Excess body fat, commonly defined by body mass index, is a well characterised modifiable risk factor of advanced prostate cancer,7 10 11 but has been inversely associated with localised prostate cancer.10 12 The association between body mass index and the prognosis of prostate cancer, however, is less well understood.13
Three meta-analyses14–16 of patients with prostate cancer investigated body mass index after diagnosis and mortality specific to prostate cancer, and found some evidence of an association with the 95% confidence intervals crossing the null (hazard ratio per 5 unit increase in body mass index=1.20, 95% confidence interval 0.99 to 1.46, I2=74, six studies14; 1.10, 0.99 to 1.22, I2=35, seven studies=715; 1.07, 1.00 to 1.14, I2=49, 10 studies16). Two meta-analyses15 16 investigated body mass index after diagnosis and all cause mortality; one15 found no association (hazard ratio per 5 unit increase in body mass index=1.05, 95% confidence interval 0.98 to 1.13, I2=65, 10 studies) and the other16 reported a small increase in the risk of all cause mortality (1.03, 1.01 to 1.05, I2=24, 10 studies). None of these meta-analyses investigated non-linearity.
Much less is known and no published meta-analysis has investigated the influence of abdominal adiposity (waist circumference, hip circumference, and waist-to-hip ratio) on survival after prostate cancer.13 Also, data on body composition parameters and the prognosis of prostate cancer are emerging but are currently limited, making it difficult to draw firm conclusions.17–19 For the scientific community to develop evidence based recommendations tailored to people living with and beyond cancer, obtaining evidence focusing on modifiable factors after the development of cancer that could affect survival is important. This systematic review and meta-analysis was carried out to explore whether adiposity, assessed after a diagnosis of prostate cancer, is associated with mortality outcomes and to assess the shape of the associations.
Materials and methods
This work was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines20 21 (online supplemental appendix 1, supplementary table 1) and in line with the Global Cancer Update Programme22 standard methods. The review protocol is registered in the Open Science Framework (https://osf.io/qp3c4).
bmjmed-2022-000339supp001.pdf (6.3MB, pdf)
Search strategy and selection criteria
We searched PubMed and Embase, from inception to 16 November 2022, for prospective or retrospective cohort studies or randomised controlled trials in men with a diagnosis of prostate cancer. Online supplemental appendix 1, supplementary text gives the full search strategy. The titles and abstracts of the retrieved publications were screened by one investigator to determine eligibility for full text review. About 10% (~1500/18 000) of the retrieved publications and full texts were checked in duplicate by a second investigator. Minor discrepancies were identified and resolved by discussion. References from relevant reviews and meta-analyses were checked to ensure completeness.
The primary adiposity variable of interest was body mass index. Secondary adiposity variables were waist circumference, hip circumference, waist-to-hip ratio, change in weight, and subcutaneous or visceral adipose tissue. Primary outcomes were all cause mortality and mortality specific to prostate cancer; secondary outcomes were death from cardiovascular disease and mortality not specific to prostate cancer. We excluded studies that reported on adiposity only before diagnosis; ecological, cross sectional, and case-control studies; case reports; surveys; conference abstracts; commentaries; studies with <100 participants; studies of individuals without cancer (that recruited healthy participants who were followed up for development of prostate cancer and death); and non-mortality outcomes, such as biochemical recurrence, metastasis, and quality of life. We also excluded from the analysis or descriptive synthesis, or both, studies that had prognostication aims and specifically focused on building prognostic models to achieve these aims.
Data extraction
From each included study, information was captured by three reviewers working independently on the retrieved records: first author’s last name, publication year, country, number of deaths and participants, disease characteristics, information related to the adiposity variable-outcome association, treatment, whether studies included individuals who were underweight, any exclusions for early follow-up (lagged analysis), hazard ratios, 95% confidence intervals for each category of the adiposity variable, and variables that were adjusted for. All extracted information included in this review was double checked for accuracy and completeness by one investigator before data analysis.
Data analysis
We conducted non-linear dose-response meta-analyses when at least five studies provided sufficient data for at least three quantitative categories of the adiposity variable. If a study only provided a dichotomous or linear effect estimate, we did not include it in the non-linear analysis. We used restricted cubic splines, with knots at 10th, 50th, and 90th centiles of the overall dose distribution (based on the reported or estimated quartile midpoints across studies).23 24 All of the available body mass index categories (including underweight) were used to model the association between body mass index and mortality across the full body mass index range. Hazard ratio estimates and their 95% confidence intervals derived from the most comprehensively adjusted Cox proportional hazards model were used in the analyses. We did not perform meta-analyses of unadjusted hazard ratios. We prioritised estimates from the Cox regression model over those derived from competing risk models, but if only the latter was available, we included it in our analyses.
When multiple publications with the necessary data were available from the same study, we used the publication with the largest number of deaths. The median or mean of each adiposity variable category was taken directly from studies that provided this information. If studies reported the range of the adiposity variable category, the midpoint was calculated. For open ended highest or lowest categories, the width was assumed to be the same as the adjacent one. The Hamling25 method was used to re-scale estimates when the lowest category was not the referent. We found the nadir of the dose-response curve for each outcome and re-scaled the analysis with the nadir as the referent.26 The Wald test (P<0.05) and visual inspection of the generated plot26 were used to test the hypothesis of deviation from linearity.
As a secondary analysis, we conducted linear dose-response meta-analyses by calculating summary hazard ratios and 95% confidence intervals with the random effects model.27 Estimates were obtained directly from studies that examined the adiposity variable on a continuous scale, re-scaling to the appropriate increment unit if needed. If publications only reported categorised adiposity variables, dose-response estimates and their 95% confidence intervals were derived from the Greenland and Longnecker method24 28 that requires the distribution of events and non-events and the hazard ratios with the variance estimates for at least three categories of each adiposity variable. When information was missing, standard imputation procedures were used where possible.29
Assessment of heterogeneity and small study effects
Cochran’s Q and the I2 statistics were used to examine heterogeneity between studies. In non-linear analyses, common heterogeneity, across the whole body mass index range of the studies, was taken into account by using the random effects approach.30 Cochran’s Q was also used to test potential heterogeneity among the subgroup analyses. A P value <0.10 for the Q test was considered significant.31 The 95% confidence intervals around I2 were calculated to help interpretation.32 Because I2 depends on the precision of a study (proportional to the sample size),33 its interpretation could be misleading. Therefore, for each meta-analysis, we calculated 95% prediction intervals to better account for heterogeneity between studies as these help to evaluate how consistent an observed estimate would be in a future study investigating the same association.34 35 Also, we provided τ2 estimates that indicated the underlying variability between studies. This metric does not systematically increase with either the size or number of studies in a meta-analysis.33
Egger’s regression asymmetry test36 and visual inspection of the generated funnel plots were used to assess whether small study effects such as publication bias were present in meta-analyses with >10 studies.37 A P value <0.10 indicated evidence of small study effects but the test has limited power when the number of studies in a meta-analysis is small.37 We also used the Debray’s D-FIV test that uses the total number of observed events per study as the independent variable, instead of the inverse of the standard error (1/SE) as the Egger’s test, which has been shown to perform better in meta-analyses of survival data.38
Subgroup and sensitivity meta-analyses
We performed predefined subgroup linear dose-response meta-analyses: by method of assessment of adiposity, geographical location, study design, median number of deaths, length of follow-up, prostate cancer risk groups (based on stage or grade information, or both, as given in the studies), adjustment variables, and by studies that included or excluded (or could be excluded by us) men who were underweight. We also conducted a non-linear dose-response meta-analysis by prostate cancer risk group (homogeneous high risk (advanced/metastatic) and all other risk groups combined). We performed leave-one-out analyses to explore the potential influence of single studies on results.39 Summary estimates were also obtained with the fixed effects model for comparisons with the random effects models.40 For the non-linear dose-response meta-analysis, when the lowest, upper, or both categories were open ended, commonly reported mean or median body mass index values were used in a sensitivity analysis for the specific category range, after performing a review of the relevant literature (online supplemental appendix 1, supplementary table 2).
We performed categorical meta-analyses to compare the risk of all cause mortality and mortality specific to prostate cancer in men who were overweight compared with those with a normal weight (body mass index 25-30 v <25 or body mass index 25-30 v 18.5-25) and in men with obesity versus those with a normal weight (body mass index ≥30 v <25 or body mass index ≥30 v 18.5-25) by synthesising the hazard ratio estimates and 95% confidence intervals of these categories. If studies subdivided any of the categories (eg, obese, severely obese) we combined them first with the Hamling method.25 Studies that provided a dichotomous estimate or different categories than the World Health Organization were excluded from categorical meta-analyses.
Risk-of-bias assessment
We used a modified version of the risk of bias for nutrition observational studies tool (RoB-NObs) considering the most influential sources of bias in cancer survival studies41 42 to evaluate the studies included in the meta-analyses. The tool is a domain based evaluation that considers the following aspects of bias: confounding, selection of participants, exposure misclassification (misclassification of variables under investigation), departures from intended exposures (eg, any changes in the adiposity variables investigated that might have occurred among participants and if these changes were unbalanced across groups and could have possibly impacted the outcome), missing data, outcome measurement error, and selection of reported results. We operationalised and tested the RoB-NObs tool to ensure that the questions in each bias domain sufficiently addressed the adiposity variable-outcome associations that we investigated. This process involved adding further explanations or remarks to the tool’s prompting questions, and guidance to cover studies on adiposity as well as diet and nutrition. A triplicate rating was done on 15% (5/33) of the studies. Minor discrepancies were identified, which were resolved by discussion.
Software
We conducted the meta-analyses with R version 4.0.5, packages meta, metafor, dosresmeta, rms, tidyverse, and metamisc.
Patient and public involvement
We have not involved patients or the public in the design, or conduct, or reporting of our research, because we used only data from previously published studies. The research will be disseminated via academic and social media avenues including a press release.
Results
Search results
We identified 79 publications that explored the association between adiposity indices after a diagnosis of prostate cancer and mortality outcomes (figure 1). Non-linear dose-response meta-analyses were possible only for body mass index and the primary outcomes of interest. Linear dose-response meta-analyses were possible for body mass index and waist circumference and the primary outcomes of interest. For other adiposity variable-outcome associations listed in the search strategy and selection criteria section, we could not perform a meta-analysis because the data were limited or heterogenous, or both. Online supplemental appendix 2 provides an overview of the findings. No relevant randomised controlled trials were identified. Online supplemental appendix 1, supplementary table 3 shows the main characteristics of the studies included in the meta-analyses of body mass index after a diagnosis of prostate cancer and mortality.
Figure 1.
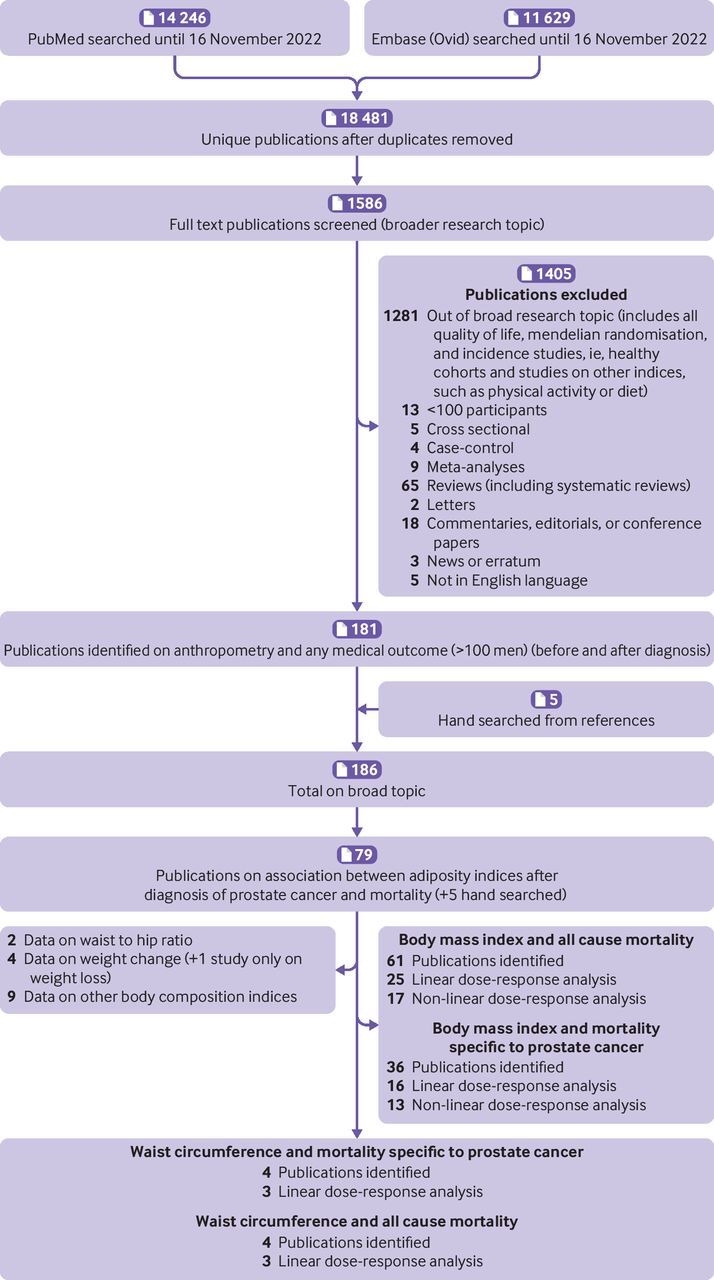
Flowchart of study selection process
Body mass index and all cause mortality
We found 61 publications that reported data on body mass index after a diagnosis of prostate cancer and all cause mortality (figure 1). Online supplemental appendix 1, supplementary tables 4A-B list the studies that were excluded from the meta-analyses with reasons for exclusion.
Non-linear dose-response meta-analysis
Seventeen studies13 43–58 were included in the non-linear dose-response meta-analysis comprising 33 910 men and 11 095 deaths (33%) from all causes. Of the 33 910 men, 11 831 (35%) had a normal weight, 14 305 (42%) were overweight, and 7774 (23%) had obesity. Evidence of non-linearity (Wald test P<0.001, I2=64%) and a J shaped association were found. We saw the highest rate of all cause mortality at the lowest and upper range of the distribution: 11-23% higher rate for a body mass index of 17-21 and 4-43% higher rate for a body mass index of 30-40. The lowest mortality ratio was seen in the high normal/low overweight group (body mass index 23-27) (figure 2 and online supplemental appendix 1, supplementary table 5). The shape of the curve was similar when we used midpoint values directly from the literature for open ended categories (Wald test P<0.00, I2=65%) (online supplemental appendix 1, supplementary figure 1A and supplementary table 5). Analysis of eight studies of high risk (advanced or metastatic) prostate cancer at diagnosis showed evidence of a reverse J shaped association, but the confidence intervals were wide.43–45 48 49 54 57 58 The shape of the curve in analysis of the other risk groups combined (10 studies) was similar to the main analysis (online supplemental appendix 1, supplementary figure 2).
Figure 2.
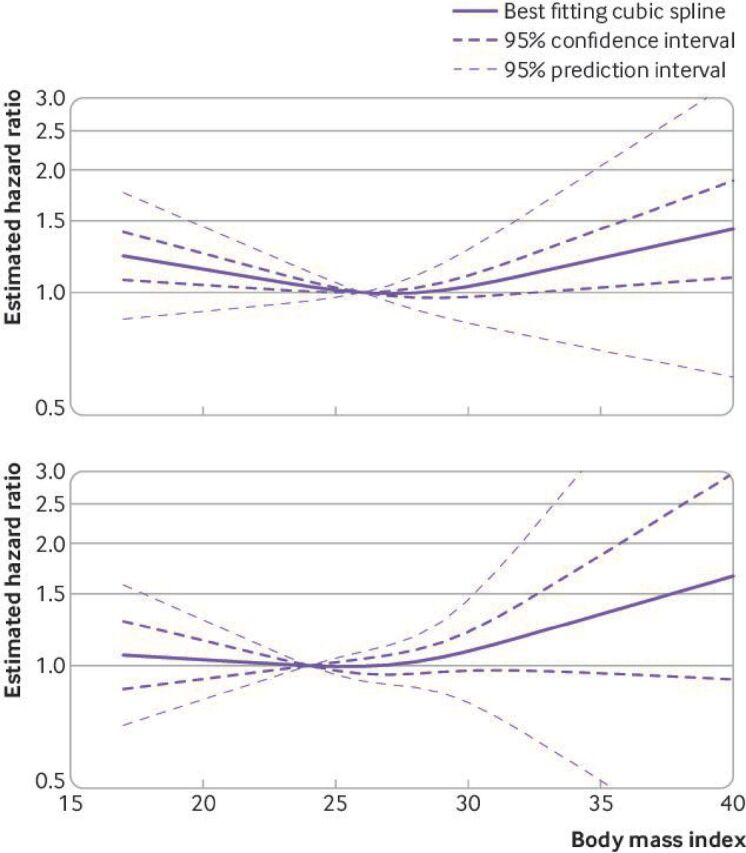
Non-linear dose-response meta-analysis for the association between body mass index and all cause mortality (top) and between body mass index and mortality specific to prostate cancer (bottom)
Linear dose-response meta-analysis
We included 25 studies13 17 43–65 comprising 58 574 men (range 17864-15 56565) and 13 811 deaths (6064-385543). We found no association between 5 unit increases in body mass index and risk of all cause mortality (hazard ratio 0.98, 95% confidence interval 0.93 to 1.04, I2=77%, P value for heterogeneity <0.01, Egger’s P=0.13, Debray’s P=0.49) (table 1, figure 3, and online supplemental appendix 1, supplementary figure 3A).
Table 1.
Linear dose-response meta-analyses of body mass index and mortality outcomes: summary of main results
| No of studies | No of deaths/total No of men |
Summary hazard ratio (95% CI) for random effects |
Summary hazard ratio (95% CI) for fixed effects |
Heterogeneity | Small study effects: Egger’s P value, Debray’s P value |
τ2 | |||
| I2 (%) (95% CI) |
95% PI | Q value, P value | |||||||
| All cause mortality | |||||||||
| Linear dose- response meta-analysis (per 5 unit increase in body mass index)* |
25 | 13811/58 574 | 0.98 (0.93 to 1.04) | 1.02 (0.99 to 1.04) | 77 (66 to 84) | 0.77 to 1.26 | 103,<0.01 | 0.13, 0.49 | 0.01 |
| Mortality specific to prostate cancer | |||||||||
| Linear dose- response meta-analysis (per 5 unit increase in body mass index) |
16 | 3412/55 457 | 1.08 (1.00 to 1.17) | 1.06 (1.01 to 1.10) | 54 (19 to 74) | 0.84 to 1.38 | 33,<0.01 | 0.23, 0.32 | 0.01 |
CI=confidence interval; PI=prediction interval.
*Number of deaths in an included publication was not given or could not be estimated, or both.
Figure 3.
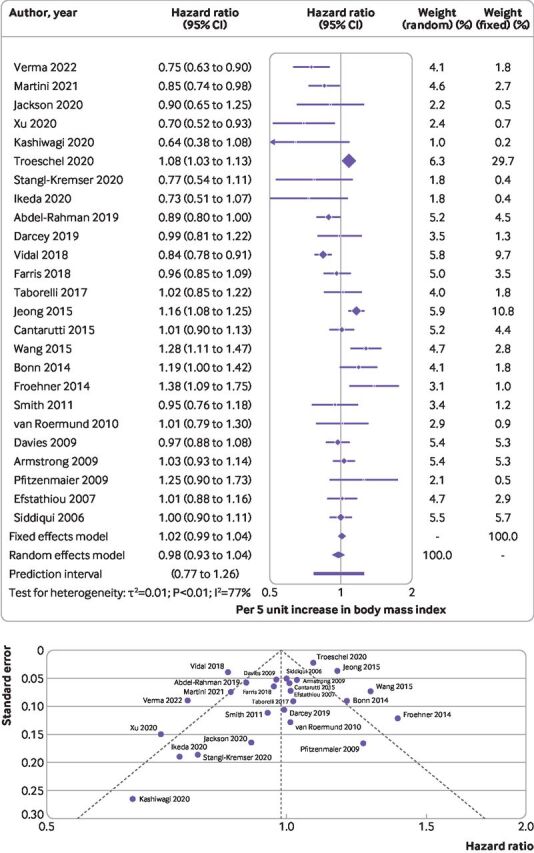
(Top) Summary hazard ratio (95% confidence interval) of all cause mortality for every 5 unit increase in body mass index after diagnosis of prostate cancer. Forest plot shows results from the random and fixed effects models. Diamond symbol represents the summary hazard ratio. Each symbol represents the hazard ratio estimate of each study and the horizontal line across each symbol represents the 95% confidence interval of the hazard ratio estimate. Horizontal line across the hazard ratio axis represents the 95% prediction interval. (Bottom) Funnel plot shows logit transformed hazard ratios plotted on the horizontal axis against standard error of the logit transformed hazard ratios plotted on the vertical axis. Each symbol represents an individual study, and the vertical line represents the summary hazard ratio from a random effects meta-analysis. The diagonal lines represent pseudo 95% confidence limits (Egger’s P=0.13)
Categorical meta-analyses
Subgroup analysis of men who were overweight versus those of normal weight13 43–46 48 49 51–58 66–69 gave an 11% lower rate of all cause mortality (hazard ratio 0.89, 95% confidence interval 0.82 to 0.95, I2=47%, P value for heterogeneity=0.01, 19 studies; online supplemental appendix 1, supplementary table 6 and supplementary figure 4). We found evidence of small study effects (Egger’s P=0.04) (online supplemental appendix 1, supplementary table 6 and supplementary figure 5). Analysis of men with obesity versus those with normal weight and all cause mortality gave a null association13 43–46 48 49 51–58 66 68 69 (hazard ratio 0.94, 95% confidence interval 0.83 to 1.08, I2=73%, P value for heterogeneity <0.01, 18 studies, Egger’s P=0.26; online supplemental appendix 1, supplementary table 6 and supplementary figures 6-7).
Body mass index and mortality specific to prostate cancer
We identified 36 publications that reported data on body mass index after diagnosis and mortality specific to prostate cancer (figure 1). Online supplemental appendix 1, supplementary tables 4C-D list the studies excluded from the meta-analyses with reasons for exclusion. Two studies that used competing risk models were replaced with the results from earlier publications of the same cohort that used the Cox proportional hazards model. We included one study70 from the Health Professionals Follow-up Study that reported on a composite outcome of distant metastasis or death from prostate cancer.
Non-linear dose-response meta-analysis
Thirteen studies13 43 45 47 48 51–53 55 58 70–72 were included comprising 33 137 men and 2947 (9%) deaths from prostate cancer. Of the 33 137 men, 11 454 (35%) had normal weight, 14 843 (45%) were overweight, and 6840 (21%) had obesity. The association between body mass index and mortality specific to prostate cancer was flat until body mass index reached 26-27 and then increased linearly by 8-66% for a body mass index of 30-40 (Wald test P=0.23, I2=50%), but the 95% confidence intervals were wide (figure 2). The shape of the curve was similar in the sensitivity analysis with midpoint values for open ended categories obtained from the literature (Wald test P=0.27, I2=48%) (online supplemental appendix 1, supplementary figure 1B and supplementary table 7). We could not perform non-linear analysis of high risk prostate cancer (advanced or metastatic) because of limited data but the curve for the other three risk groups (10 studies) had a similar shape to the main analysis (online supplemental appendix 1, supplemental figure 8).
Linear dose-response meta-analysis
We included 16 studies13 43 45 47 48 51–53 55 58 61 62 65 70–72 comprising 55 457 men (range 23913-15 56565) and 3412 deaths (2471-65847). An indication of a positive association for every 5 unit increase in body mass index and higher rate of mortality specific to prostate cancer was found, but the 95% confidence interval included the null value (hazard ratio 1.08, 95% confidence interval 1.00 to 1.17, I2=54%, P value for heterogeneity <0.01, Egger’s P=0.23, Debray’s P=0.32). Excluding two separate studies51 58 from the leave-one-out analysis slightly altered the summary estimate, resulting in a positive association not including the null value (table 1, figure 4, and online supplemental appendix 1, supplementary figure 3B).
Figure 4.
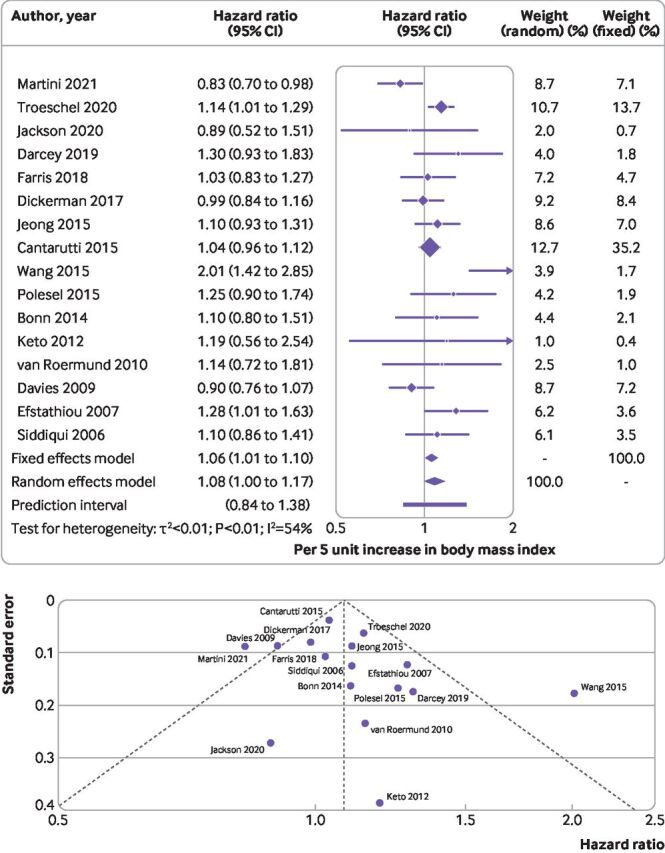
(Top) Summary hazard ratio (95% confidence interval) of mortality specific to prostate cancer for every 5 unit increase in body mass index after diagnosis of prostate cancer. Forest plot shows results from the random and fixed effects models. Diamond symbol represents the summary hazard ratio. Each symbol represents the hazard ratio estimate of each study and the horizontal line across each square represents the 95% confidence interval of the hazard ratio estimate. Horizontal line across the hazard ratio axis represents the 95% prediction interval. (Bottom) Funnel plot shows logit transformed hazard ratios plotted on the horizontal axis against the standard error of the logit transformed hazard ratios plotted on the vertical axis. Each symbol represents an individual study, and the vertical line represents the summary hazard ratio from a random effects meta-analysis. The diagonal lines represent pseudo 95% confidence limits (Egger’s P=0.23)
Categorical meta-analyses
We did not find an association in the analysis of men with overweight versus those with normal weight (hazard ratio 1.02, 95% confidence interval 0.89 to 1.17, I2=52%, P value for heterogeneity <0.01, 15 studies,13 43 45 48 51–53 55 58 67–72 Egger’s P=0.27) or in the analysis of men with obesity versus those with normal weight (1.08, 0.89 to 1.31, I2=54%, P value for heterogeneity <0.01, 14 studies,13 43 45 48 51–53 55 58 68–72 Egger’s P=0.03; online supplemental appendix 1, supplementary table 6 and supplementary figures 9-12).
Sensitivity and subgroup analyses
We found little difference among the estimates of linear dose-response subgroup meta-analyses with few exceptions. Heterogeneity was evident in the subgroup analysis of prostate cancer risk groups for all cause mortality (P<0.01), where an inverse association was seen in patients with advanced prostate cancer (including metastatic) and positive associations in all other subgroups. In the analysis by geographical location for all cause mortality (P=0.03) and mortality specific to prostate cancer (P=0.02), meta-analysis of multinational studies and studies from Japan showed an inverse association for all cause mortality. Similarly, for mortality specific to prostate cancer, we found an inverse association in the multinational study compared with positive associations in other country groups. Heterogeneity was also seen in the subgroup analysis of studies that adjusted for or did not adjust for Gleason score (P<0.01) (online supplemental appendix 1, supplementary figures 13-50 and supplementary tables 8-9). We found no substantial evidence of publication bias with Egger’s or Debray’s test (online supplemental appendix 1, supplementary table 10).
Waist circumference, and all cause mortality and mortality specific to prostate cancer
We identified four studies and three13 45 73 were included in the linear dose-response meta-analysis for all cause mortality and mortality specific to prostate cancer. Meta-analyses gave null associations between 10 cm increases in waist circumference and all cause mortality (hazard ratio 1.06, 95% confidence interval 0.95 to 1.19, I2=0%, P value for heterogeneity=0.42, 910 deaths, online supplemental appendix 1, supplementary figure 51A) and mortality specific to prostate cancer (0.97, 0.80 to 1.16, I2=0%, P value for heterogeneity=0.96, 317 deaths, online supplemental appendix 1, supplementary figure 51B). Non-linear analysis was not conducted because of limited data, but individual study estimates suggested non-linearity (online supplemental appendix 1, supplementary figure 52).
Risk-of-bias assessment overview
Most studies were rated as having a moderate risk of bias because of confounding, with about 30% rated as having a severe or critical risk of bias. In our review, age, stage of disease, grade of disease, prostate specific antigen, and cancer treatment were the most relevant confounders. Most studies had a serious risk of bias for selection of participants, but this was expected because in this population participation in the study was conditional on survival. None of the included studies used adjustment techniques to correct for potential selection bias. Most of the studies had a low or moderate risk of exposure (adiposity variable) and outcome misclassification but about a third did not provide sufficient information to judge the risk of bias because of missing data. All studies were judged as having a critical risk of bias owing to departures from intended exposures (adiposity variables) because none performed a time varying exposure analysis (online supplemental appendix 1, supplementary figure 53).
Discussion
Principal findings
In this systematic review and meta-analysis, we found evidence of a J shaped association between body mass index and all cause mortality, and a similar but flatter non-linear shape in the underweight and normal weight groups for body mass index and mortality specific to prostate cancer. These associations did not differ in most predefined subgroups. Linear dose-response meta-analysis for waist circumference in the few available studies gave null results. The few studies on change in weight, waist-to-hip ratio, subcutaneous adipose tissue, and visceral adipose tissue reported inconsistent results.
Study implications
Non-linear meta-analyses of body mass index suggested that men with obesity who survived prostate cancer have higher rates of all cause mortality and mortality specific to prostate cancer. Several mechanisms might explain this association, linked to either the direct consequences of obesity or implications related to cancer treatment in men with obesity. Individuals with obesity are prone to insulin resistance, a driver of hyperinsulinaemia.44 High levels of insulin are linked to reduced plasma levels of sex hormones that have critical roles in the development of poorly differentiated prostate cancer.74 Diabetes has been associated with increased mortality in patients with prostate cancer,73 75 and worse outcomes (mortality or progression) in patients with prostate cancer with obesity versus those without obesity.76 Chronic low grade inflammation induced by obesity enhances the development of androgen resistance, aggressive tumour phenotype, progression, and death.5 61 71 77 Also, because of the difficulty of performing a thorough digital rectal examination in men with obesity, the clinical disease severity of the localised tumour might be underestimated or misclassified,10 51 78 resulting in undertreatment62 and a poorer prognosis.53 78 Men with obesity and prostate cancer undergoing radical treatments could be at higher risk of complications, undesirable outcomes, or more serious side effects compared to patients without obesity.79
The non-linear analysis also showed a higher all cause mortality rate for a body mass index of 17-21. Cachectic conditions alter catabolic and anabolic processes, and studies have indicated imbalances between protein synthesis and degradation as a result of overactivation of proteolysis pathways.80 The blood transports tissue wasting mediators, including factors involved in systemic inflammation associated with cancer cachexia. Loss of skeletal muscle and raised blood coagulation under cachectic conditions contribute to a worse prognosis.80 Treatments for prostate cancer adversely affect nutritional status and cause hormonal, cellular, and immune imbalances.47 Patients with prostate cancer who are malnourished as a result of their tumour or cancer treatments, or both, have a higher risk of progression of prostate cancer and subsequently death.47 81 This finding is supported by studies43 47 53 excluding the initial follow-up years.
Patients in the high normal/low overweight (body mass index 23-27) group had the lowest risk of mortality. A higher than normal body mass index could indicate lack of cachexia or protection against the development of cachexia,44 and men in the upper body mass index range might benefit from extra energy reserves, protecting them against catabolism, especially in advanced prostate cancer.3 59 Individuals with a similar body mass index can have considerably different distributions of adipose tissue and distinct metabolic profiles.82 83 Previous studies suggested that higher body fat composition is linked to better survival, likely because of improved tolerance to chemotherapy.17 69 This finding is particularly important in the treatment of metastatic castration resistant prostate cancer where docetaxel, a lipophilic agent, has high affinity for adipose tissue17 and possibly increased uptake in these patients. Overall survival of patients with advanced prostate cancer who were overweight and treated with docetaxel was reported to be better than in patients who had a normal weight.57 This suggested interaction between body mass index or body composition and response to cancer drug treatment requires further study. These associations need to be elucidated and any differential effect on survival that might be caused by pharmacodynamic interactions of the drug and body fat should be considered.57 59 66 71
Although the observed association among the high normal/low overweight group is biologically plausible, methodological limitations84 85 of the included studies could partly explain the findings. Most studies adjusted for age, stage of disease, grade of disease, and cancer treatments, which are critical confounders, but few adjusted for smoking and other relevant variables before diagnosis of prostate cancer. Collider bias could also explain the observed associations, whereby being overweight leads to a higher risk of prostate cancer but other unmeasured or uncontrolled risk factors (such as smoking or pre-existing comorbidities) occurring disproportionally in patients having a normal weight might be more strongly related to mortality than being overweight, making overweight seem protective.3 Also, the most unwell patients with prostate cancer and overweight might have been less likely to enter the study compared with the most unwell patients with normal weight, or those who had the poorest prognosis might have died before enrolment in the studies.
Strengths and limitations
The aim of this meta-analysis was to characterise the shape of the body mass index-mortality associations through non-linear analyses. The strengths of this work include the detailed subgroup analyses, which allowed better assessment of the magnitude and direction of associations across important characteristics of the individuals or the tumour, and inclusion of adiposity indices that could influence the prognosis of prostate cancer, apart from body mass index.17 Null results in some meta-analyses (eg, for waist circumference) or subgroup analyses with few studies and wide confidence intervals might not imply the absence of an association but that more studies are needed to draw definitive conclusions. We reviewed mortality related to cardiovascular disease and not specific to prostate cancer as outcomes, despite the small number of studies identified. No firm conclusions could be drawn for these outcomes but the need for clarifications from future studies was highlighted. Future Global Cancer Update Programme reviews will expand the variables investigated to also include diet and physical activity, and cover other important outcomes, such as progression to metastasis or recurrence. The combined evidence will then be assessed by the independent Global Cancer Update Programme Expert Panel for conclusions and development of lifestyle recommendations tailored to patients with prostate cancer.
We acknowledge that risk of bias assessment tools have limitations and currently no consensus exists on what is the best approach to assess risk of bias in observational studies.86 Based on our research question, however, we judged that the RoB-NObs tool, which was developed by the US Department of Agriculture (USDA) Nutrition Evidence Systematic Review after modifications to the Cochrane’s collaboration ROBINS-I, was the most appropriate to use because it provided the basis of a thorough domain based assessment covering the most important biases related to adiposity, physical activity, diet, and cancer survival studies.
Because of the observational nature of the included studies, residual confounding (eg, by screening behaviour, severity of disease, smoking information, treatment dose or duration, diseases before diagnosis, lifelong v adulthood onset adiposity) cannot be excluded. Most of the studies lacked data on adiposity and other lifestyle changes beyond the time of diagnosis that could have affected the associations.13 Anthropometric indices were mostly assessed once at baseline instead of at multiple crucial time points (eg, before, during, and after treatment or each treatment cycle). This approach prevented performance of time varying analyses which are important because single time point anthropometry assessments might not be representative of the cumulative effect on cancer survival.84 Because of limited data, we also could not investigate the cumulative effect of adiposity (combined before and after diagnosis) on all cause mortality and mortality specific to prostate cancer. Most of the available published studies have assessed adiposity before or after diagnosis separately, not in combination.
Another limitation of our review was that despite the relatively large number of articles identified, only a fraction (28-45%, [17/61-16/36]) were included in the meta-analyses of all cause mortality or mortality specific to prostate cancer. Many of the studies excluded from analyses did not provide the necessary data (14-20%, 5/35 on all cause mortality and 4/20 on mortality specific to prostate cancer) or only provided univariable estimates or Kaplan-Meier figures (35-50%, 7/20 on mortality specific to prostate cancer and 18/35 on all cause mortality). Future survival studies should conduct and report results in a more complete and standardised way, which allows for inclusion in future updated meta-analyses. Our search covered two electronic databases and excluded studies not published in English, and therefore we might not have identified all publications, although the most influential studies are likely to have been captured.
Self-reported body mass index in some studies could be a limitation, but self-reported and measured weight and height have been shown to be strongly correlated.44 47 Our subgroup analyses of measured versus self-reported body mass index gave associations that did not substantially differ. Most studies in our review only used body mass index as the marker of adiposity and few incorporated other measures of body fat, providing limited statistical power. The reason for the scarcity of evidence on other adiposity indices could be that body mass index is a widely used proxy measure of general adiposity and is easy to measure. Body mass index is commonly recorded in patients’ health records,87 but it cannot differentiate between lean and fat mass (body composition) and could misclassify patients.17 87–89 Integrating measures of body composition other than body mass index into clinical practice would be beneficial.90 This practice would allow future studies to incorporate alternative adiposity indices91 and more sophisticated body composition parameters that might characterise adiposity better than body mass index, and would clarify the associations between adiposity and outcomes after a diagnosis of cancer.90 Only two studies13 45 reported data on general and central adiposity after diagnosis, and therefore we could not investigate the associations between body mass index and mortality outcomes in different stratums of central adiposity.
Few subgroup analyses in our review showed evidence of small study effects with the Egger’s test.37 38 92 When we applied the Debray’s test, which has been shown to perform better when survival data are pooled, the results were similar. All tests for funnel plot asymmetry have limitations and simulation studies showed that even with many studies in a meta-analysis (≥50) their power is <50%.38 Patients with prostate cancer in the included studies received multimodal treatments but detailed data were not provided, and we could not perform subgroup analyses by different treatments. The inability to carry out this particular subgroup analysis, however, was to a degree compensated by carrying out the subgroup analysis of tumour risk groups.93 More studies are needed to clarify the effect of intentional and unintentional changes in weight on cancer treatment and consequently the prognosis of prostate cancer. This information is particularly important for men with a diagnosis of localised prostate cancer that are less likely to progress to clinically significant disease, because lifestyle changes such as weight management might influence progression of the tumour.53 94 Because of limited available data, we could not explore further whether weight management can complement active surveillance or other specific treatments in men with localised prostate cancer and lead to better prognosis.
The linear subgroup dose-response meta-analyses that we performed gave generally consistent results. Some heterogeneity existed in the analysis by prostate cancer risk groups (inverse association between body mass index and all cause mortality among the high risk/advanced group and positive association in the other groups). A general limitation of this subgroup analysis is that most studies included a mixture of aggressive and non-aggressive prostate cancer tumours, often defined with a combination of stage, grade, and sometimes information on levels of prostate specific antigen.
Our observation of improved overall survival in patients with advanced prostate cancer with a higher body mass index agrees with previous studies.57 59 66 95 Potential explanations include better response to treatment, protection from greater caloric reserve, or favourable metabolic profiles. Higher body mass index has been associated with improved prognosis in advanced melanoma and kidney cancer, and mechanisms proposed involve downregulation of certain oncogenes or factors affecting responses related to immunity after inhibitor drug treatments are given.58 96 Selection bias is another potential explanation and could be more prevalent in studies of patients with advanced prostate cancer that most likely have included individuals with the best possible health status in their samples.
We found some heterogeneity in analysis by geographical location although most studies originated from the US or Europe. The two Japanese studies in men with metastatic prostate cancer showed inverse associations (body mass index and all cause mortality). None of the studies from Europe or the US included a homogenous sample of patients with metastatic prostate cancer. In Japan, screening for prostate specific antigen is reported to be low.97 This observation could explain the higher number of metastatic cancers at diagnosis in Japan in comparison to western countries.98 However the differences observed might be related to the different participation of patients with metastatic disease in each region or study.
Most studies in this review were conducted in white patients and therefore studies in ethnically diverse under-represented populations with different body compositions are necessary to resolve ethnic differences in cancer survival84 and obtain more widely generalisable results. Finally, associations between adiposity and other causes of death should be evaluated further.
Conclusions
This systematic review and meta-analysis provides evidence of a J shaped association between body mass index and all cause mortality, and a similar non-linear association for mortality specific to prostate cancer. Evidence on the associations between waist circumference, waist-to-hip ratio, change in weight, visceral adipose tissue, and subcutaneous adipose tissue and mortality is limited and inconsistent. Data from well designed longitudinal observational studies that better account for reverse causality, selection bias, and confounding are necessary to confirm the associations found in our analyses. Future studies should assess adiposity across different stages of cancer survivorship84 99 by using various parameters for distribution of adipose tissue and incorporate analyses by different treatment regimens.
Footnotes
Twitter: @margarita_cario
DSMC and KKT contributed equally.
Contributors: KKT and DSMC were responsible for the conception, initial design, and supervision of the study. DCM and MC contributed to the study design. MC and RV did the study selection. MC, RV, and KB extracted the data. MC, GM, and NB-T assessed the risk of bias in the included studies. MC performed the analysis. MC and KKT wrote the manuscript with contributions from all authors. All authors critically revised and approved the manuscript. KKT attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. KKT is the guarantor. DSMC and KKT share last authorship. Transparency: KKT affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding: This work was funded by the World Cancer Research Fund network of charities (American Institute for Cancer Research, World Cancer Research Fund, and Wereld Kanker Onderzoek Fonds). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the World Cancer Research Fund network of charities (American Institute for Cancer Research, World Cancer Research Fund, and Wereld Kanker Onderzoek Fonds) for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics approval
No ethical approval needed.
References
- 1. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2020. Available: https://gco.iarc.fr/today [Accessed 12 May 2022].
- 2. Langlais CS, Graff RE, Van Blarigan EL, et al. Post-diagnostic dietary and lifestyle factors and prostate cancer recurrence, progression, and mortality. Curr Oncol Rep 2021;23:37. 10.1007/s11912-021-01017-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson AS, Martin RM, Renehan AG, et al. Cancer survivorship, excess body fatness and weight-loss intervention-where are we in 2020? Br J Cancer 2021;124:1057–65. 10.1038/s41416-020-01155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Husson O, van Steenbergen LN, Koldewijn EL, et al. Patients with prostate cancer continue to have excess mortality up to 15 years after diagnosis. BJU Int 2014;114:691–7. 10.1111/bju.12519 [DOI] [PubMed] [Google Scholar]
- 5. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol 2013;63:800–9. 10.1016/j.eururo.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schatten H. Brief overview of prostate cancer statistics, grading, diagnosis and treatment strategies. Adv Exp Med Biol 2018;1095:1–14. 10.1007/978-3-319-95693-0_1 [DOI] [PubMed] [Google Scholar]
- 7. World Cancer Research Fund International/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Report. 2018. Available: https://www.wcrf.org/dietandcancer
- 8. Skolarus TA, Wolf AMD, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin 2014;64:225–49. 10.3322/caac.21234 [DOI] [PubMed] [Google Scholar]
- 9. Markozannes G, Tzoulaki I, Karli D, et al. Diet, body size, physical activity and risk of prostate cancer: an umbrella review of the evidence. Eur J Cancer 2016;69:61–9. 10.1016/j.ejca.2016.09.026 [DOI] [PubMed] [Google Scholar]
- 10. Perez-Cornago A, Appleby PN, Pischon T, et al. Tall height and obesity are associated with an increased risk of aggressive prostate cancer: results from the EPIC cohort study. BMC Med 2017;15:115. 10.1186/s12916-017-0876-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao Y, Giovannucci E. Obesity and prostate cancer. Recent Results Cancer Res 2016;208:137–53. 10.1007/978-3-319-42542-9_8 [DOI] [PubMed] [Google Scholar]
- 12. Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer -- a dose-response meta-analysis of prospective studies. Ann Oncol 2012;23:1665–71. 10.1093/annonc/mdr603 [DOI] [PubMed] [Google Scholar]
- 13. Jackson MD, Tulloch-Reid MK, McCaw-Binns AM, et al. Central adiposity at diagnosis may reduce prostate cancer-specific mortality in African-Caribbean men with prostate cancer: 10-year follow-up of participants in a case-control study. Cancer Causes Control 2020;31:651–62. 10.1007/s10552-020-01306-z [DOI] [PubMed] [Google Scholar]
- 14. Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4:486–501. 10.1158/1940-6207.CAPR-10-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhong S, Yan X, Wu Y, et al. Body mass index and mortality in prostate cancer patients: a dose-response meta-analysis. Prostate Cancer Prostatic Dis 2016;19:122–31. 10.1038/pcan.2015.64 [DOI] [PubMed] [Google Scholar]
- 16. Rivera-Izquierdo M, Pérez de Rojas J, Martínez-Ruiz V, et al. Obesity as a risk factor for prostate cancer mortality: a systematic review and dose-response meta-analysis of 280,199 patients. Cancers (Basel) 2021;13:4169. 10.3390/cancers13164169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stangl-Kremser J, Suarez-Ibarrola R, Andrea DD, et al. Assessment of body composition in the advanced stage of castration-resistant prostate cancer: special focus on sarcopenia. Prostate Cancer Prostatic Dis 2020;23:309–15. 10.1038/s41391-019-0186-6 [DOI] [PubMed] [Google Scholar]
- 18. Cheng E, Kirley J, Cespedes Feliciano EM, et al. Adiposity and cancer survival: a systematic review and meta-analysis. Cancer Causes Control 2022;33:1219–46. 10.1007/s10552-022-01613-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopez P, Newton RU, Taaffe DR, et al. Associations of fat and muscle mass with overall survival in men with prostate cancer: a systematic review with meta-analysis. Prostate Cancer Prostatic Dis 2022;25:615–26. 10.1038/s41391-021-00442-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Cancer Research Fund International - global cancer update programme (CUP global). Available: https://www.wcrf.org/diet-activity-and-cancer/global-cancer-update-programme/about-the-global-cancer-update-programme/ [Accessed 1 Aug 2022].
- 23. Jackson D, White IR, Thompson SG. Extending dersimonian and laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282–97. 10.1002/sim.3602 [DOI] [PubMed] [Google Scholar]
- 24. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata Journal 2006;6:40–57. 10.1177/1536867X0600600103 [DOI] [Google Scholar]
- 25. Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008;27:954–70. 10.1002/sim.3013 [DOI] [PubMed] [Google Scholar]
- 26. Shim SR, Lee J. Dose-response meta-analysis: application and practice using the R software. Epidemiol Health 2019;41:e2019006. 10.4178/epih.e2019006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 28. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 29. Bekkering GE, Harris RJ, Thomas S, et al. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? Am J Epidemiol 2008;167:1017–26. 10.1093/aje/kwn005 [DOI] [PubMed] [Google Scholar]
- 30. Crippa A, Orsini N. Multivariate dose-response meta-analysis: the dosresmeta R package. J Stat Softw 2016;72:1–15. 10.18637/jss.v072.c01 [DOI] [Google Scholar]
- 31. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ 2007;335:914–6. 10.1136/bmj.39343.408449.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rücker G, Schwarzer G, Carpenter JR, et al. Undue reliance on I (2) in assessing heterogeneity may mislead. BMC Med Res Methodol 2008;8:79. 10.1186/1471-2288-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 35. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137–59. 10.1111/j.1467-985X.2008.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 38. Debray TPA, Moons KGM, Riley RD. Detecting small-study effects and funnel plot asymmetry in meta-analysis of survival data: a comparison of new and existing tests. Res Synth Methods 2018;9:41–50. 10.1002/jrsm.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010;1:112–25. 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 40. Haidich AB. Meta-analysis in medical research. Hippokratia 2010;14(Suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 41. Chubak J, Boudreau DM, Wirtz HS, et al. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst 2013;105:1456–62. 10.1093/jnci/djt211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Savitz DA, Wellenius GA, Trikalinos TA. The problem with mechanistic risk of bias assessments in evidence synthesis of observational studies and a practical alternative: assessing the impact of specific sources of potential bias. Am J Epidemiol 2019;188:1581–5. 10.1093/aje/kwz131 [DOI] [PubMed] [Google Scholar]
- 43. Troeschel AN, Hartman TJ, Jacobs EJ, et al. Postdiagnosis body mass index, weight change, and mortality from prostate cancer, cardiovascular disease, and all causes among survivors of nonmetastatic prostate cancer. J Clin Oncol 2020;38:2018–27. 10.1200/JCO.19.02185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vidal AC, Howard LE, de Hoedt A, et al. Obese patients with castration-resistant prostate cancer may be at a lower risk of all-cause mortality: results from the shared equal access regional cancer hospital (search) database. BJU Int 2018;122:76–82. 10.1111/bju.14193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farris MS, Courneya KS, Kopciuk KA, et al. Anthropometric measurements and survival after a prostate cancer diagnosis. Br J Cancer 2018;118:607–10. 10.1038/bjc.2017.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taborelli M, Polesel J, Parpinel M, et al. Fruit and vegetables consumption is directly associated to survival after prostate cancer. Mol Nutr Food Res 2017;61:1600816. 10.1002/mnfr.201600816 [DOI] [PubMed] [Google Scholar]
- 47. Cantarutti A, Bonn SE, Adami H-O, et al. Body mass index and mortality in men with prostate cancer. Prostate 2015;75:1129–36. 10.1002/pros.23001 [DOI] [PubMed] [Google Scholar]
- 48. Efstathiou JA, Bae K, Shipley WU, et al. Obesity and mortality in men with locally advanced prostate cancer. Cancer 2007;110:2691–9. 10.1002/cncr.23093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Armstrong AJ, Halabi S, de Wit R, et al. The relationship of body mass index and serum testosterone with disease outcomes in men with castration-resistant metastatic prostate cancer. Prostate Cancer Prostatic Dis 2009;12:88–93. 10.1038/pcan.2008.36 [DOI] [PubMed] [Google Scholar]
- 50. Froehner M, Kellner A-E, Koch R, et al. A combined index to classify prognostic comorbidity in candidates for radical prostatectomy. BMC Urol 2014;14:28. 10.1186/1471-2490-14-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davies BJ, Smaldone MC, Sadetsky N, et al. The impact of obesity on overall and cancer specific survival in men with prostate cancer. J Urol 2009;182:112–7; 10.1016/j.juro.2009.02.118 [DOI] [PubMed] [Google Scholar]
- 52. Darcey E, Pereira G, Salter A, et al. The impact of lifestyle-related factors on survival after a prostate cancer diagnosis. Eur Urol 2019;75:884–5. 10.1016/j.eururo.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 53. Bonn SE, Wiklund F, Sjölander A, et al. Body mass index and weight change in men with prostate cancer: progression and mortality. Cancer Causes Control 2014;25:933–43. 10.1007/s10552-014-0393-3 [DOI] [PubMed] [Google Scholar]
- 54. Smith MR, Cook R, Lee K-A, et al. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer 2011;117:2077–85. 10.1002/cncr.25762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Roermund JGH, Hinnen KA, Battermann JJ, et al. Body mass index is not a prognostic marker for prostate-specific antigen failure and survival in Dutch men treated with brachytherapy. BJU Int 2010;105:42–8. 10.1111/j.1464-410X.2009.08687.x [DOI] [PubMed] [Google Scholar]
- 56. Pfitzenmaier J, Pritsch M, Haferkamp A, et al. Is the body mass index a predictor of adverse outcome in prostate cancer after radical prostatectomy in a mid-European study population? BJU Int 2009;103:877–82. 10.1111/j.1464-410X.2008.08149.x [DOI] [PubMed] [Google Scholar]
- 57. Verma S, Arora S, Sahoo RK, et al. Differential effect of body mass index (BMI) on outcomes of patients treated with docetaxel in prostate cancer-an exploratory analysis. Cancer Treat Res Commun 2022;31:100520. 10.1016/j.ctarc.2022.100520 [DOI] [PubMed] [Google Scholar]
- 58. Martini A, Shah QN, Waingankar N, et al. The obesity paradox in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2022;25:472–8. 10.1038/s41391-021-00418-0 [DOI] [PubMed] [Google Scholar]
- 59. Xu MC, Huelster HL, Hatcher JB, et al. Obesity is associated with longer survival independent of sarcopenia and myosteatosis in metastatic and/or castrate-resistant prostate cancer. J Urol 2021;205:800–5. 10.1097/JU.0000000000001428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ikeda T, Ishihara H, Iizuka J, et al. Prognostic impact of sarcopenia in patients with metastatic hormone-sensitive prostate cancer. Jpn J Clin Oncol 2020;50:933–9. 10.1093/jjco/hyaa045 [DOI] [PubMed] [Google Scholar]
- 61. Wang LS, Murphy CT, Ruth K, et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer 2015;121:3010–7. 10.1002/cncr.29472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Siddiqui SA, Inman BA, Sengupta S, et al. Obesity and survival after radical prostatectomy: a 10-year prospective cohort study. Cancer 2006;107:521–9. 10.1002/cncr.22030 [DOI] [PubMed] [Google Scholar]
- 63. Abdel-Rahman O. Impact of diabetes on the outcomes of patients with castration-resistant prostate cancer treated with docetaxel: a pooled analysis of three phase III studies. Clin Genitourin Cancer 2019;17:e104–12. 10.1016/j.clgc.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 64. Kashiwagi E, Shiota M, Masaoka H, et al. Relationship between body composition and hormone sensitivity for androgen deprivation therapy in patients with metastatic prostate cancer. Prostate Int 2020;8:22–6. 10.1016/j.prnil.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jeong BC, Chalfin HJ, Lee SB, et al. The relationship between the extent of extraprostatic extension and survival following radical prostatectomy. Eur Urol 2015;67:342–6. 10.1016/j.eururo.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 66. Wu W, Liu X, Chaftari P, et al. Association of body composition with outcome of docetaxel chemotherapy in metastatic prostate cancer: a retrospective review. PLoS One 2015;10:e0122047. 10.1371/journal.pone.0122047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chalfin HJ, Lee SB, Jeong BC, et al. Obesity and long-term survival after radical prostatectomy. J Urol 2014;192:1100–4. 10.1016/j.juro.2014.04.086 [DOI] [PubMed] [Google Scholar]
- 68. Geinitz H, Thamm R, Mueller T, et al. Impact of body mass index on outcomes after conformal radiotherapy in patients with prostate cancer. Int J Radiat Oncol Biol Phys 2011;81:16–22. 10.1016/j.ijrobp.2010.05.059 [DOI] [PubMed] [Google Scholar]
- 69. Halabi S, Ou S-S, Vogelzang NJ, et al. Inverse correlation between body mass index and clinical outcomes in men with advanced castration-recurrent prostate cancer. Cancer 2007;110:1478–84. 10.1002/cncr.22932 [DOI] [PubMed] [Google Scholar]
- 70. Dickerman BA, Ahearn TU, Giovannucci E, et al. Weight change, obesity and risk of prostate cancer progression among men with clinically localized prostate cancer. Int J Cancer 2017;141:933–44. 10.1002/ijc.30803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Keto CJ, Aronson WJ, Terris MK, et al. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the search database. BJU Int 2012;110:492–8. 10.1111/j.1464-410X.2011.10754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Polesel J, Gini A, Dal Maso L, et al. The negative impact of tobacco smoking on survival after prostate cancer diagnosis. Cancer Causes Control 2015;26:1299–305. 10.1007/s10552-015-0624-2 [DOI] [PubMed] [Google Scholar]
- 73. Polesel J, Gini A, Dal Maso L, et al. The impact of diabetes and other metabolic disorders on prostate cancer prognosis. J Diabetes Complications 2016;30:591–6. 10.1016/j.jdiacomp.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 74. Bandini M, Gandaglia G, Briganti A. Obesity and prostate cancer. Curr Opin Urol 2017;27:415–21. 10.1097/MOU.0000000000000424 [DOI] [PubMed] [Google Scholar]
- 75. Bensimon L, Yin H, Suissa S, et al. Type 2 diabetes and the risk of mortality among patients with prostate cancer. Cancer Causes Control 2014;25:329–38. 10.1007/s10552-013-0334-6 [DOI] [PubMed] [Google Scholar]
- 76. Kelkar S, Oyekunle T, Eisenberg A, et al. Diabetes and prostate cancer outcomes in obese and nonobese men after radical prostatectomy. JNCI Cancer Spectr 2021;5:pkab023. 10.1093/jncics/pkab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cho HJ, Kwon GT, Park H, et al. A high-fat diet containing lard accelerates prostate cancer progression and reduces survival rate in mice: possible contribution of adipose tissue-derived cytokines. Nutrients 2015;7:2539–61. 10.3390/nu7042539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Langlais CS, Cowan JE, Neuhaus J, et al. Obesity at diagnosis and prostate cancer prognosis and recurrence risk following primary treatment by radical prostatectomy. Cancer Epidemiol Biomarkers Prev 2019;28:1917–25. 10.1158/1055-9965.EPI-19-0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wilson RL, Taaffe DR, Newton RU, et al. Obesity and prostate cancer: a narrative review. Crit Rev Oncol Hematol 2022;169:103543. 10.1016/j.critrevonc.2021.103543 [DOI] [PubMed] [Google Scholar]
- 80. Schmidt SF, Rohm M, Herzig S, et al. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer 2018;4:849–60. 10.1016/j.trecan.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 81. Kokal WA. The impact of antitumor therapy on nutrition. Cancer 1985;55(1 Suppl):273–8. [DOI] [PubMed] [Google Scholar]
- 82. Di Bella CM, Howard LE, Oyekunle T, et al. Abdominal and pelvic adipose tissue distribution and risk of prostate cancer recurrence after radiation therapy. Prostate 2020;80:1244–52. 10.1002/pros.24054 [DOI] [PubMed] [Google Scholar]
- 83. Hill JH, Solt C, Foster MT. Obesity associated disease risk: the role of inherent differences and location of adipose depots. Horm Mol Biol Clin Investig 2018;33. 10.1515/hmbci-2018-0012 [DOI] [PubMed] [Google Scholar]
- 84. Park Y, Peterson LL, Colditz GA. The plausibility of obesity paradox in cancer-point. Cancer Res 2018;78:1898–903. 10.1158/0008-5472.CAN-17-3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Banack HR, Stokes A. The obesity paradox may not be a paradox at all. Int J Obes (Lond) 2017;41:1162–3. 10.1038/ijo.2017.99 [DOI] [PubMed] [Google Scholar]
- 86. Bero L, Chartres N, Diong J, et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: concerns arising from application to observational studies of exposures. Syst Rev 2018;7:242. 10.1186/s13643-018-0915-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Müller MJ, Braun W, Enderle J, et al. Beyond BMI: conceptual issues related to overweight and obese patients. Obes Facts 2016;9:193–205. 10.1159/000445380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care 2015;18:535–51. 10.1097/MCO.0000000000000216 [DOI] [PubMed] [Google Scholar]
- 89. Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond) 2008;32 Suppl 3:S56–9. 10.1038/ijo.2008.87 [DOI] [PubMed] [Google Scholar]
- 90. Caan BJ, Cespedes Feliciano EM, Kroenke CH. The importance of body composition in explaining the overweight paradox in cancer-counterpoint. Cancer Res 2018;78:1906–12. 10.1158/0008-5472.CAN-17-3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Christakoudi S, Tsilidis KK, Muller DC, et al. A body shape index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci Rep 2020;10:14541. 10.1038/s41598-020-71302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lin L, Shi L, Chu H, et al. The magnitude of small-study effects in the Cochrane database of systematic reviews: an empirical study of nearly 30 000 meta-analyses. BMJ Evid Based Med 2020;25:27–32. 10.1136/bmjebm-2019-111191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1119–34. 10.1016/j.annonc.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 94. Meyerhardt JA, Ma J, Courneya KS. Energetics in colorectal and prostate cancer. J Clin Oncol 2010;28:4066–73. 10.1200/JCO.2009.26.8797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Montgomery RB, Goldman B, Tangen CM, et al. Association of body mass index with response and survival in men with metastatic prostate cancer: Southwest Oncology Group trials 8894 and 9916. J Urol 2007;178:1946–51; 10.1016/j.juro.2007.07.026 [DOI] [PubMed] [Google Scholar]
- 96. Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019;25:141–51. 10.1038/s41591-018-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kitagawa Y, Namiki M. Prostate-specific antigen-based population screening for prostate cancer: current status in Japan and future perspective in Asia. Asian J Androl 2015;17:475–80. 10.4103/1008-682X.143756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Saito T, Komatsubara S, Hara N, et al. Significance of PSA screening in Niigata, Japan: survey of actual status of new cases of prostate cancer. Res Rep Urol 2021;13:859–66. 10.2147/RRU.S341347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fang Z, Song M, Lee DH, et al. The role of Mendelian randomization studies in deciphering the effect of obesity on cancer. J Natl Cancer Inst 2022;114:361–71. 10.1093/jnci/djab102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjmed-2022-000339supp001.pdf (6.3MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


