Abstract
Endometriosis (EMs) is a common gynecological disorder characterized by abnormal growth of the endometrial stroma and glands outside the uterus. Tanshinone IIA, the active component of Chinese medicine Danshen (Salvia miltiorrhiza Bge.), has a number of pharmacological effects such as anti-inflammation and anti-oxidation and serves a significant role in the treatment of EMs. In the present study, network pharmacology and experimental validation were used to elucidate the potential mechanism of tanshinone IIA for treating EMs. Several databases were used to collect information on EMs and tanshinone IIA and cross-targets for tanshinone IIA and EMs finally obtained. A total of 64 common targets were found between tanshinone IIA and EMs. Subsequently, a protein-protein interaction network was constructed, a total of 14 core targets were screened for enrichment analysis. Furthermore, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis were performed. The network pharmacology showed that intercellular adhesion molecule (ICAM)-1, MMP-9 and VEGF are the core targets while PI3K/AKT pathway and mTOR pathway are the main signaling pathways through which tanshinone IIA regulates relevant biological processes to intervene in EMs. Finally, the therapeutic role and mechanism of tanshinone IIA on EMs was verified in vivo. Female Sprague-Dawley rats were treated by autologous transplantation to establish EMs.
Serum inflammatory factors were detected by enzyme-linked immunosorbent assay (ELISA). The expression of ICAM-1, MMP-9 and VEGF in ectopic endometrial tissues of rats was determined by immunohistochemical. The expression of PI3K/Akt/mTOR pathway-related proteins and genes was detected by western blotting and quantitative PCR. It was found that tanshinone IIA treatment significantly decreased the formation of ectopic endometrium by reducing serum levels of TNF-α and IL-1β, and down regulating the levels of ICAM-1, MMP-9 and VEGF in ectopic uterine tissue. In addition, tanshinone IIA can also block the activation of PI3K/Akt/mTOR signaling pathway by reducing the expression of related proteins and genes. In conclusion, tanshinone IIA can regulate adhesion, invasion and angiogenesis, thereby improving the pathological morphology of ectopic endometrium and inhibiting the formation of ectopic lesions. The PI3K/Akt/mTOR signaling pathway may play a key role in controlling this process.
Keywords: endometriosis, tanshinone IIA, adhesion, invasion, PI3K/Akt/mTOR signaling pathway, Network pharmacology
Introduction
Endometriosis (EMs) is a chronic inflammatory sickness marked by the presence of endometrial glands and matrices outside the uterine cavity (1). The common clinical symptoms are progressive dysmenorrhea, chronic pelvic pain and difficulty in sexual intercourse in women of childbearing age. In addition, it can also cause menstrual disorders, infertility and other adverse consequences, which seriously affect the living standards and lifestyle of patients with EMs. However, the pathogenesis and pathophysiology of EMs are currently unknown. In recent years, research has concentrated on the theories of inflammatory stimulation (2), cellular immune monitoring deficiency (3), retrograde menstruation and coelomic metaplasia (4). According to the classical implantation theory, ectopic endometrial adhesion and implantation, angiogenesis and proliferation are the most prominent pathomorphological features of EMs (5). Increasing evidence shows that cell adhesion and invasive molecules involved in inflammatory and immune processes play an important role in endometrial pathophysiology. Researchers have also proposed the ‘3A model’, which hypothesizes that the progression of EMs is inevitably accompanied by pathological processes of adhesion, invasion and angiogenesis (6), thereby promoting the implantation and growth of counter-current endometrial cells in the pelvic and abdominal cavities and then infiltrating to form lesions under the influence of the immune response and hormones.
In recent decades, the onset age of EMs has gradually become younger with the change of social life style and the increase of uterine surgical procedures such as abortion and caesarean section (7). EMs is challenging to treat at present due to its unique pathogenesis and estrogen-dependent characteristics (8). Pharmacological treatment is often the first choice for patients with EMs due to its features such as ease of use, safety, quick effect and minimal trauma, among which hormonal agents such as combined oral contraceptives, danazol, gonadotropin-releasing hormone agonists (GnRH-a) and non-steroidal anti-inflammatory drugs are often used as first-line treatment for EMs (9). However, a number of patients often have to discontinue treatment due to inevitable side effects such as breakthrough bleeding, peptic ulcers and osteoporosis as well as short-term curative effect and frequent relapses (10). Therefore, surgery is frequently used as the standard treatment for EMs, with complete removal of the ectopic lesion, relief of pelvic pain and improvement in quality of life as the clinical outcomes (11). However, postoperative complications and a high recurrence rate are also significant issues that trouble clinicians today (12). Consequently, it is crucial to seek an EMs treatment strategy with low recurrence rate, few side effects and broad adaptability.
Traditional Chinese medicine (TCM) has a long history of clinical application in China and is able to treat a number of complex diseases through multi-target action. Tanshinone IIA is a lipid-soluble component of the Chinese medicine Danshen (Salvia miltiorrhiza Bge.), which has anti-inflammatory, antioxidant, antibacterial, anti-tumour, anti-fibrosis and neuroprotective effects (13). It is mainly used for the treatment of cardiovascular and cerebrovascular diseases, cancer, bone and joint disease (14–16). Studies have found that tanshinone IIA is also effective in treating polycystic ovary syndrome (17), adenomyosis (18), EMs (19) and other diseases. For the formation of EMs ectopic lesions, tanshinone IIA can significantly inhibit the levels of HAS2, CA-125, IL-8 and TNF-α, and impede the ectopic endometrial epithelial-mesenchymal transformation (EMT) process, thereby inducing apoptosis to achieve antifibrosis, and reducing endothelial cell proliferation and lesion infiltration (20,21). In addition, our previous study demonstrated that tanshinone IIA can reduce mechanical hyperalgesia in EMs rats by lowering the level of E2 and inhibiting the endogenous expression of dorsal root ganglion renin-angiotensin system, while preventing the invasion and growth of ectopic lesions (22). However, the potential therapeutic effect of tanshinone IIA on EMs and its detailed pharmacological mechanisms still need to be further investigated.
Network pharmacology is a new subject based on the theory of systems biology, which analyzes biological systems and selects specific signal nodes for multi-target drug molecular design (23). Network pharmacology explains the occurrence and development of diseases from the perspective of system biology and biological network balance, understands the interaction between drugs and the body from the perspective of improving or restoring the balance of biological network, and guides the discovery of new drugs (24). The research strategy of network pharmacology is holistic and systematic, which conforms to the principle of holistic view of TCM, so it is widely used to elucidate the mechanism of TCM in the treatment of diseases (25). The core targets and pathways obtained through network pharmacological screening can provide an improved basis for follow-up experiment. The present study combined network pharmacology with experimental validation to clarify the main targets and potential mechanisms of tanshinone IIA in treatment of EMs.
Materials and methods
Acquisition of target genes related to tanshinone IIA and EMs
The related targets of tanshinone IIA were predicted by using the TCM systematic pharmacology database (old.tcmsp-e.com/tcmsp.php) (26). In order to collect targets of tanshinone IIA in a more comprehensive way, target prediction was further performed through the PharmMapper database (https://lilabecust.cn/pharmmapper/). Human protein targets were reset only in the PharmMapper database and the default settings kept for other parameters. The PharmMapper server automatically matches small ligand molecules with pharmacophore models in the database and ranks them based on their match scores. Normalized fit score >0.8 was used as the filtering criterion. In addition, the targets of tanshinone IIA were predicted by Swiss Target Prediction database (https://www.swisstargetprediction.ch/) and probability value ≥0.10 was employed as criteria to filter for targets. The selected targets from the three databases were combined for deduplication, and then the targets were converted into standardized gene symbols using the UniProt database (https://www.uniprot.org/).
All gene targets related to EMs were collected from Genecards database (genecards.org/), OMIM database (https://www.omim.org/) and DrugBank database (https://www.ncbi.nlm.nih.gov/gene). Key word ‘endometriosis’ was entered into the search column to obtain target information. The targets obtained from the three databases were combined and deduplicated, sorted by score, and those with higher than the median score were considered as endometriosis targets. The targets of tanshinone IIA and endometriosis were uploaded to the online analysis tool of Venny2.1 (https://bioinfogp.cnb.csic.es/tools/venny/) to screen out the common targets.
Construction of the protein-protein interaction (PPI) network and selection of core genes
The intersection targets of tanshinone IIA and EMs were submitted to the STRING database (https://string-db.org/) with the species set to Homo sapiens. Then, the highest confidence level was set to the minimum necessary interaction score (0.9) for the filter, hiding the discrete points in the network and leaving the remaining parameters as defaults. The PPI network download was saved in tsv format and imported into Cytoscape 3.7.2 software (cytoscape.org) for visualization. Cytohubba can assign a score to each gene in the PPI network through its 12 algorithms, and then select the top gene as the key gene based on the gene score. Therefore, cytohubba network analysis plug-in was used to calculate the degree of connectivity between proteins. The greater the degree value, the more interacting protein nodes and the greater influence on the network, Finally, target nodes with degree centrality, betweenness centrality and closeness centrality values higher relative to the corresponding median values in the PPI network were chosen for subsequent analysis.
Enrichment analysis of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
Pathway enrichment analysis for GO and the KEGG were achieved by the cluster profiler program in R software (27). The screening conditions were set to P-value cutoff=0.05 and q-value cutoff=0.05 and the items with the highest P-values selected for data visualization analysis. GO analysis was used to screen the biological process (BP), cell components (CC) and molecular function (MF). KEGG enrichment primarily revealed pathway related content.
Animals
A total of 36 mature female Sprague Dawley rats (age, 6–7 weeks) at SPF level weighing between 180–200 g were donated by Beijing Vital River Laboratory Animal Technology Co., Ltd. (license no. SCXK 2016–0006). All rats were housed in standard cages at 22–24 °C and relative humidity (40–60%), with a regular 12 h light/dark cycle and ad libitum access to food and water in accordance with rat care protocols. The Animal Care and Welfare Committee of the Capital Medical University (approval no. AEEI-2018-031) reviewed and approved all experimental procedures.
Experimental design and treatment
According to our previous study, the EMs rats model was successfully prepared by autologous transplantation (22). Rats were randomly designated to five groups: Sham, model, positive, tanshinone IIA low dose and tanshinone IIA high dose (n=6 in each group). Rats in both sham group and model group were administered solvent. Positive group rats were intragastrically given 2 mg/kg/d medroxyprogesterone acetate (Zhejiang Xianju Pharmaceutical Co., Ltd.). Rats in the tanshinone IIA low dose and high dose groups received 3 and 12 mg/kg/day tanshinone IIA (Shanghai No.1 biochemical pharmaceutical Co., Ltd.) respectively via intraperitoneal injection. At 21 days after medication, blood samples were taken from the abdominal aorta under anesthesia by intraperitoneal administration of 40 mg/kg pentobarbital sodium and serum was extracted and kept at −20°C. After blood collection, the rats were deeply anesthetized by intraperitoneal injection of pentobarbital sodium 150 mg/kg and subsequently sacrificed by cervical dislocation. Following sacrifice, the animal's death was confirmed from loss of pain response, failure to respond to toe pressure with hands or tweezers and observation of cardiac and respiratory arrest. The ectopic lesion was resected and part of the tissue was fixed in 4% paraformaldehyde for 24 h at room temperature, while the remainder was frozen at −80°C.
Hematoxylin and eosin (H&E) staining
The tissue that had been fixed was withdrawn from the paraformaldehyde solution, dehydrated by gradient ethanol, transparent by xylene, embedded in paraffin and then cut into 4-µm pieces. After xylene deparaffinization and gradient alcohol dehydration, the sections were stained with H&E staining at room temperature for 3 min and evaluated under a BX53 light microscope (Olympus Corporation) to investigate pathological morphological alterations.
Enzyme-linked immunosorbent assay
After centrifuging the blood for 10 min at 1,200 × g at −4°C, the supernatant was gathered and stored at −20°C for further examination. The concentrations of TNF-α and IL-1β in the serum were determined using ELISA (CSB-E11987r, CSB-E08055r) as the protocol provided by the manufacturer (Wuhan Huamei Biological Engineering Co., LTD, China). The luminescent signal produced was measured at 450 nm using a microplate reader (Thermo Fisher Scientific, Inc.).
Immunohistochemical staining
Following xylene deparaffinization, the sections were rehydrated and treated with a buffer containing 0.01 mol/l sodium citrate. Next, the sections were treated with 3% hydrogen peroxide for 20 min to block the activity of endogenous peroxidase. Incubation with 2% bovine serum albumin (Beijing Dingguo Changsheng Biotechnology Co., LTD) inhibited non-specific binding. After the non-specific antibodies were blocked, the primary antibody anti-MMP9 (diluted 1:400; Bioss, bs-4593R), anti-ICAM-1 (diluted 1:400; Bioss, bs-0608R) and anti-VEGF (diluted 1:400; Abcam, ab46154) was incubated with the tissues overnight at 4°C. The antibody labeled with horseradish peroxidase HRP (1:200; Beijing Dingguo Changsheng Biotechnology Co., LTD, IH-0061) was treated for 60 min at 37°C. Following that, the sections were rinsed with PBS and stained with 0.01% DAB. Finally, the optical microscope image was captured under a BX53 light microscope (Olympus) by randomly selecting five fields from each section. The average integral optical density was measured using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Western blotting analysis
The eutopic and ectopic endometria were lysed in RIPA) lysis buffer with protease inhibitors (Beijing Dingguo Changsheng Biotechnology Co., LTD, Beijing, China). Then, lysates were centrifuged at 12,000 × g for 10 min and the protein concentration of the supernatants were measured by BCA assay kit (Beijing Dingguo Changsheng Biotechnology Co., LTD, Beijing, China). Each lysate supernatant was loaded onto 10% SDS-polyacrylamide gels with 80 µg total protein. After the protein samples had been transferred to PVDF membranes, they were blocked for 1 h at room temperature with 5% nonfat milk. The principal antibodies used overnight at 4°C were: β-actin (diluted 1:5000; Immunoway, YM3028), PI3K (diluted 1:500; Bioss, bs-0128R), p-PI3K (diluted 1:1,000; Bioss, bs-3332R), Akt (diluted 1:1,000; Bioss, bs-6951R), phosphorylated (p-)Akt (diluted 1:1,000; Bioss, bs-5182R), mTOR (1:1,000; Bioss, bs-1992R) and p-mTOR (diluted 1:1,000; Bioss, bs-3495R). The internal reference was β-actin. After 1 h incubation at room temperature with a HRP-labeled goat anti-rabbit IgG secondary antibody, the bands were detected using superenhanced chemiluminescence detection tools. Images were captured immediately after color development using a gel imaging system (Bio-Rad Laboratories, Inc.). ImageJ was used to analyze the band intensities (v1.46; National Institutes of Health).
Reverse transcription-quantitative (RT-q) PCR
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract total RNA from the tissues of the eutopic and ectopic endometria according to the manufacturer's instructions., and the microultraviolet spectrophotometer (Quawell Q5000) was utilized to assess the RNA content. cDNA synthesis was conducted with M-MLVReverseTranscriptase reagent kit (Invitrogen, AM2044) in a reaction volume of 20 µl. Following the guidelines provided by the manufacturer, RT-qPCR was conducted utilizing an Applied Biosystems 7500 system (Applied Biosystems) and SYBR Green Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.). The conditions were as follows: 35 cycles of pre-denaturation at 95°C for 2 min, denaturation at 95°C for 15 sec, annealing at 62°C for 30 sec and extension at 72°C for 30 sec. GAPDH served as the internal standard. Each sample was run in triplicate and GAPDH was used as the reference. Foldchange of gene expression was calculated using 2−ΔΔCq method (28). Table I lists the RT-qPCR primers.
Table I.
Reverse transcription-quantitative PCR primer sequences.
| Gene | Primer sequences (5′→3′) | Product size (bp) |
|---|---|---|
| GAPDH | F: CCTGGGCTACACTGAGGA | 164 |
| R: TGAGGTCCACCACCCTGT | ||
| PI3K | F: GTAGGCCCGAGTAAGCTGAA | 206 |
| R: CCGTAGGTGAGACCCCAAGT | ||
| mTOR | F: ATTCGCATTCAGTCCATAGCC | 179 |
| R: AAACAAACTCGTGCCCATTGC |
F, forward; R, reverse.
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used for data analysis and the measurements were presented as the mean ± standard deviation. One-way analysis of variance (ANOVA) and the Bonferroni post hoc test were used to observe the difference between multiple groups. The Mann-Whitney rank sum test was used to perform a nonparametric analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Common targets for tanshinone IIA and EMs
The retrieval process yielded information on 172 drug targets and 1,090 disease targets. A Venn diagram showed that there were 64 overlapping genes between EMs targets and tanshinone IIA action targets (Fig. 1).
Figure 1.
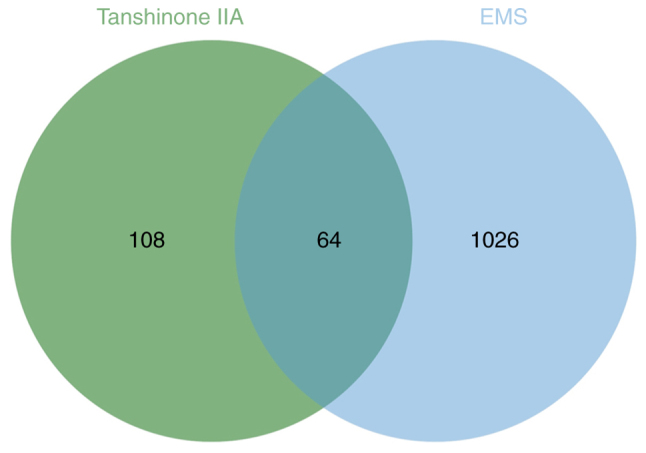
Venn diagram of the target of tanshinone IIA and the target of endometriosis. EMS, endometriosis.
PPI network construction and analysis
Based on the species Homo sapiens, a PPI network with 64 nodes and 454 edges was created in the STRING database, and the analysis results were loaded into Cytoscape 3.7.2 for visualization. As shown in Table II, the cytohubba plugin was used to analyze the nodes in the network diagram and finally selected 14 core genes; VEGFA, MMP9, EGFR, SRC, MMP2, ESR1, HRAS, ICAM1, PPARG, ANXA5, MAPK8, IL2, NOS3 and MAPK1. Degree value of the node and the number of biological functions in the network increase with the color of the node and the greener the color, the more important it is in the network (Fig. 2).
Table II.
Core targets of tanshinone IIA in the treatment of endometriosis.
| Gene | Degree | BetweennessCentrality | ClosenessCentrality | ClusteringCoefficient |
|---|---|---|---|---|
| VEGFA | 47 | 0.14 | 0.80 | 0.32 |
| MMP9 | 41 | 0.08 | 0.72 | 0.37 |
| EGFR | 36 | 0.06 | 0.70 | 0.40 |
| SRC | 33 | 0.04 | 0.67 | 0.46 |
| MMP2 | 32 | 0.03 | 0.66 | 0.46 |
| ESR1 | 31 | 0.11 | 0.65 | 0.37 |
| HRAS | 30 | 0.03 | 0.65 | 0.49 |
| ICAM1 | 29 | 0.03 | 0.62 | 0.46 |
| PPARG | 28 | 0.09 | 0.64 | 0.40 |
| ANXA5 | 26 | 0.01 | 0.62 | 0.60 |
| MAPK8 | 22 | 0.01 | 0.59 | 0.65 |
| IL2 | 22 | 0.01 | 0.57 | 0.57 |
| NOS3 | 22 | 0.02 | 0.59 | 0.53 |
| MAPK1 | 21 | 0.01 | 0.59 | 0.62 |
Figure 2.
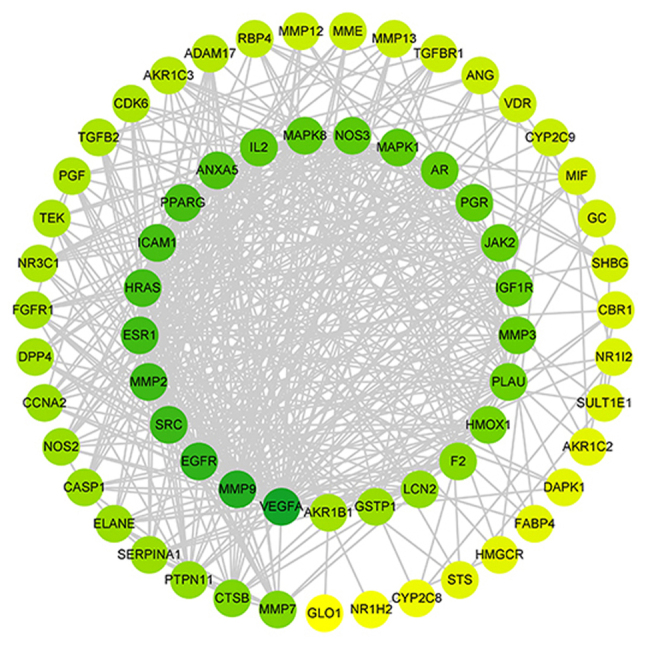
PPI network analysis. The nodes represent the target protein, and the color of the nodes is adjusted according to the degree value, the larger the degree value, the greener the color. PPI, protein-protein interaction.
GO enrichment and KEGG pathway analysis
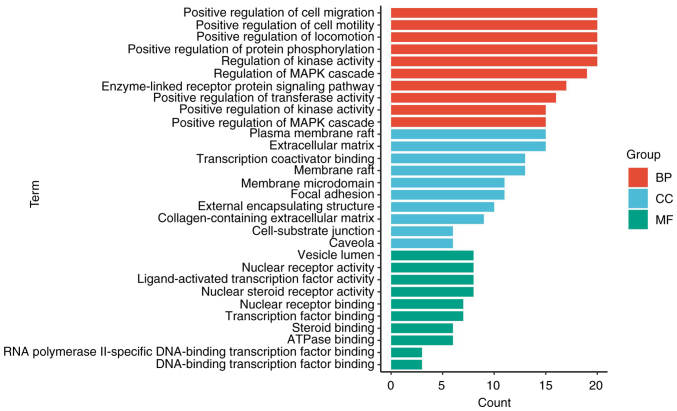
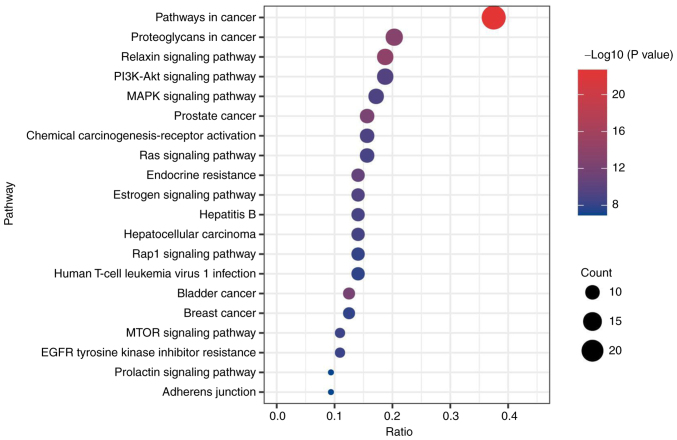
The GO functions of common targets were enriched in 182 BP, 109 CC and 64 MF (P<0.05). The top 10 items of the BP, CC, and MF gene ratio were selected to make a stick chart (Fig. 3). As shown in Fig. 3, BP mainly involved ‘positive regulation of cell migration’, ‘positive regulation of cell motility’, ‘positive regulation of locomotion’, ‘positive regulation of protein phosphorylation’ and ‘regulation of kinase activity’. CC mainly involved ‘plasma membrane raft’, ‘extracellular matrix’, ‘transcription coactivator binding’, ‘membrane raft’ and ‘membrane microdomain’. MF mainly involved ‘vesicle lumen’, ‘nuclear receptor activity’, ‘ligand-activated transcription factor activity’, ‘nuclear steroid receptor activity’ and ‘nuclear receptor binding’. In addition, a total of 198 items were enriched by KEGG analysis (P<0.05). Fig. 4 depicts the initial 20 signaling pathways, mainly including ‘relaxin signaling pathway’, ‘PI3K-Akt signaling pathway’, ‘MAPK signaling pathway’, ‘Ras signaling pathway’ and ‘mTOR signaling pathway’.
Figure 3.
Gene Ontology enrichment analysis. Biological function, cellular function and molecular function were selected and sorted according to the importance of-log10 (P-value). BP, biological process; CC, cell components; MF, molecular function.
Figure 4.
Kyoto Encyclopedia of Genes and Genomes pathways. Abscissa represents gene proportion, ordinate represents pathway name, size of the bubble represents the number of targets in the pathway and the color represents P-value.
Construction of compound-target-pathway network
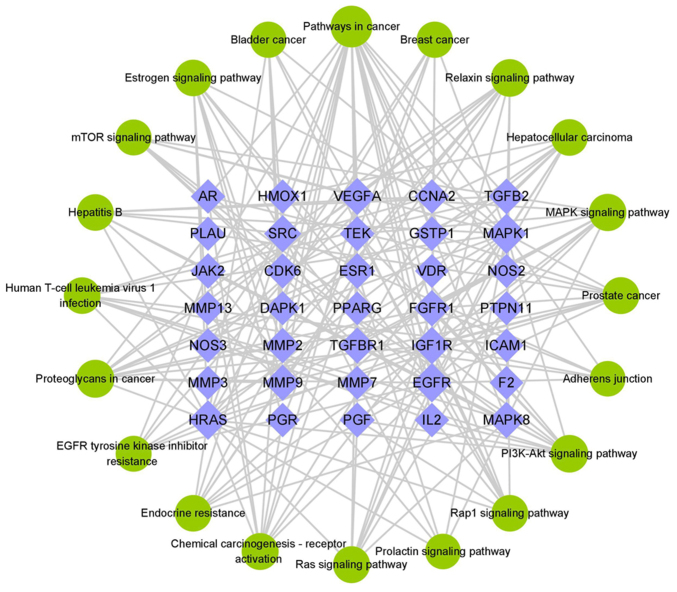
Traditional Chinese medicine compounds are characterized by multiple components and multiple targets, and the relationship among the components, core targets and pathways can be more clearly demonstrated by constructing the network of compound-target-pathway network (Fig. 5). A total of 35 tanshinone IIA targets were implicated in network regulation and the effect of tanshinone IIA on EMs may be mediated by the PI3K-Akt, MAPK, Ras and mTOR signaling pathway. Based on the above results, it was hypothesized that tanshinone IIA participates in the treatment of EMs by regulating adhesion, invasion, angiogenesis and inhibition of PI3K/Akt/mTOR signaling pathway. This hypothesis was further tested with animal experiments.
Figure 5.
Compound-target-pathway network. Purple represents the target, green represents the pathway, and the larger the node area, the larger the connectivity value.
Histologic analysis
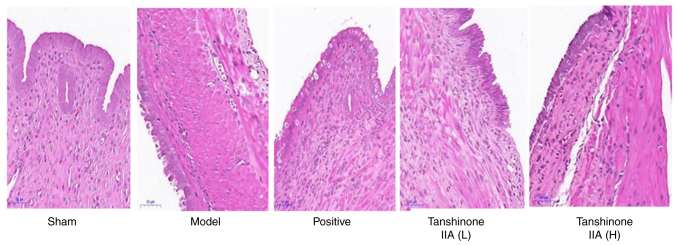
As depicted in Fig. 6, the endometrial epithelial cells in the sham group were organized in an ordered fashion, with recognizable glandular structure and noticeable glandular lumen. Interstitial changes such as edema, hemorrhage and inflammatory cell infiltration were not observed. In the model group, the epithelial cells were disordered and appeared lamellar or pseudolamellar. In addition, subnuclear vacuoles could be observed in some epithelial cells. In the positive group, endothelial epithelial cells showed more necrosis and apoptosis, but the glandular structure was clearer than in the model group. The epithelial cells in the tanshinone IIA (L) and tanshinone IIA (H) groups were arranged as a single layer, the number of glands was diminished, and the ectopic endothelium was atrophied and thinning. The effect of tanshinone IIA (H) group was more prominent than that of tanshinone IIA (L) group.
Figure 6.
Results of hematoxylin and eosin staining in each group. Magnification, ×200.
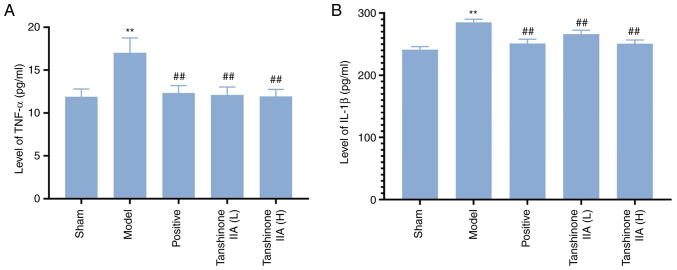
Effect of tanshinone IIA on the expression of TNF-α and IL-1β
The levels of TNF-α and IL-1β in the model group were greater than that in the sham group (P<0.01). Compared with the model group, serum levels of TNF-α and IL-1β in the positive group and tanshinone IIA (L, H) groups were significantly decreased (all P<0.01; Fig. 7).
Figure 7.
Changes of serum inflammatory factors in rats (n=6). (A) Effect of tanshinone IIA on the level of TNF-α in serum of rats. (B) Effect of tanshinone IIA on the level of IL-1β in serum of rats. Data are presented as mean ± standard deviation. **P<0.01 vs. sham; ##P<0.01 vs. model group.
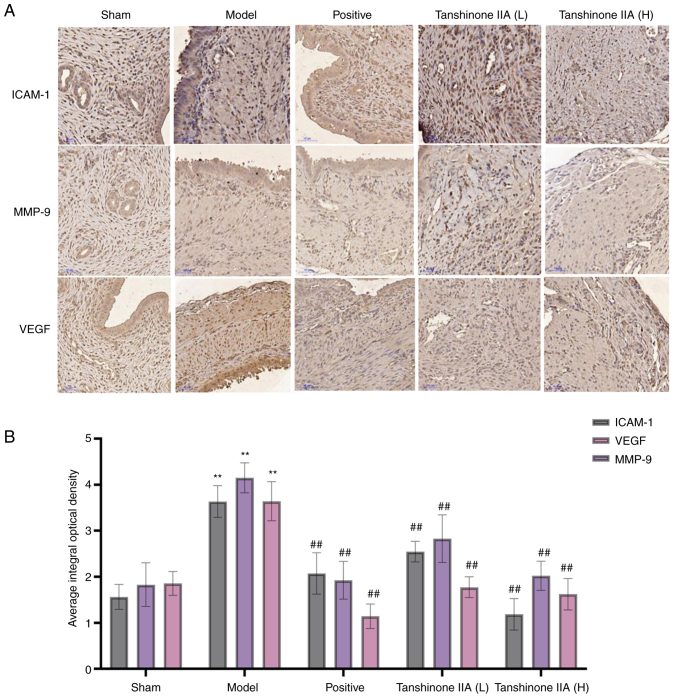
Effect of tanshinone IIA on the expression of MMP-9, ICAM-1 and VEGF by immunohistochemistry
When compared with the sham group, the expression levels of MMP-9, ICAM-1 and VEGF were considerably greater in the model group (P<0.01). When compared with the model group, the expression levels of MMP-9, ICAM-1 and VEGF were considered to be particularly lower in the positive group (P<0.01). It was also discovered that tanshinone IIA (L and H) therapy resulted in a considerable decrease in the expression levels of MMP-9, ICAM-1 and VEGF compared with the model group. Moreover, the tanshinone IIA (H) group experienced a higher reduction than the tanshinone IIA (L) group (Fig. 8).
Figure 8.
Effect of tanshinone IIA on MMP-9, ICAM-1 and VEGF measured by immunohistochemistry (n=6). (A) Positive staining was visualized in brown color; magnification, ×200. (B) Quantification of MMP-9, ICAM-1 and VEGF expression. Data are presented as mean ± standard deviation. **P<0.01 vs. sham group; ##P<0.01 vs. model group. ICAM, intercellular adhesion molecule.
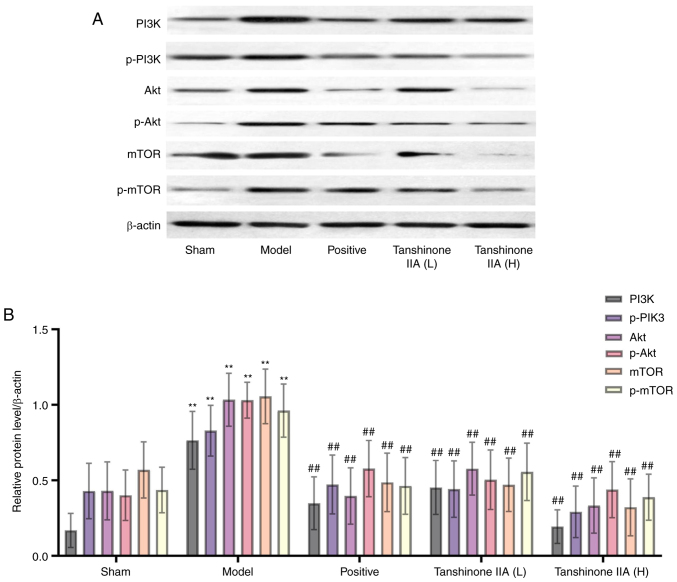
Effect of tanshinone IIA on the PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR expression assessed by western blotting
The results showed that the expression levels of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR were considerably greater in the model group than those in the sham group. Positive and tanshinone IIA (L and H) groups had substantially lower relative expression levels of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR compared with the model group (Fig. 9).
Figure 9.
Protein levels of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR in rats (n=6). (A) Representing western blotting analysis of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR. (B) Quantification of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR expression. β-actin was used as a loading control. Data are presented as mean ± standard deviation. **P<0.01 vs. sham group; ##P<0.01 vs. model group. p-, phosphorylated.
Effect of tanshinone IIA on gene expression of PI3K and mTOR
RT-qPCR was used to investigate PI3K and mTOR mRNA levels. The PI3K and mTOR mRNA levels in the model group were notably greater than those in the sham group. Treatment with medroxyprogesterone acetate or tanshinone IIA (L and H) substantially lowered PI3K and mTOR gene expression levels when compared with the model group (Fig. 10).
Figure 10.
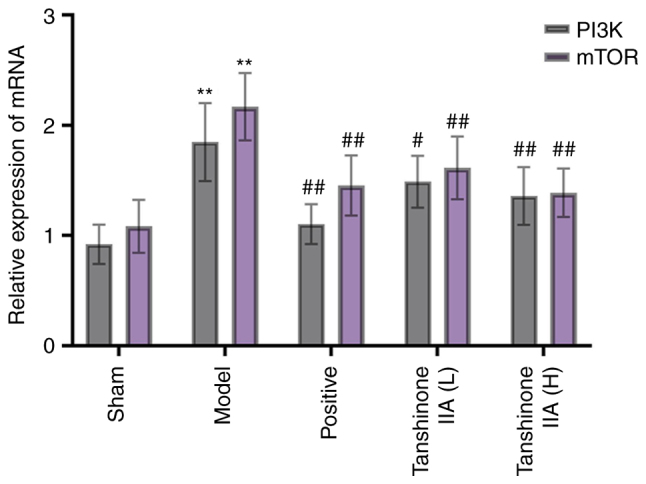
Effect of tanshinone IIA on gene expression levels of PI3K and mTOR (n=6). Data are presented as mean ± standard deviation. **P<0.01 vs. sham; #P<0.05, ##P<0.01 vs. model group.
Discussion
EMs is a multifactorial benign gynaecological disease with a high incidence and complex pathogenesis for which there is no well-established clinical treatment. The traditional surgical treatment is usually to remove the ectopic lesion as the ultimate goal, although it is easy to relapse after surgery (29). Commonly used medications include oral contraceptives, danazol, GnRH-a and aromatase inhibitors. However, long-term use of these drugs can cause a variety of adverse reactions, such as bleeding, pelvic pain, hirsutism, acne and osteoporosis (30,31). As a result, researchers are also exploring new strategies for treating EMs. The present study revealed the regulatory network in EMs and identified tanshinone IIA as a candidate drug for EMs. The network pharmacology analysis revealed the tanshinone IIA related molecular functions and pharmacological targets for treating EMs and the therapeutic effect and mechanism of tanshinone IIA were verified in vivo.
Tanshinone IIA is a lipid-soluble component extracted from the Chinese herb Danshen (Salvia miltiorrhiza Bge.), which can effectively inhibit cell invasion, proliferation and apoptosis. Previous research by our team demonstrated that tanshinone IIA could significantly inhibit the growth of EMs lesions, inhibit the expression of proteins including angiotensinogen and Ang II in the dorsal root ganglion by reducing E2 levels, and alleviate the general hyperalgesia of EMs by regulating dorsal root ganglion sprouting via renin-angiotensin system (22). Network pharmacology combined with bioinformatics technology is a popular tool for studying the pharmacological mechanism of drugs in recent years. Through the construction of drug-target-disease interaction network, it provides valuable predictive guidance for further elucidation of the mechanism of drugs for disease treatment (32,33). The present study analyzed the relevant targets and mechanisms of tanshinone IIA in the management of EMs using network pharmacology.
The present study first used the network pharmacology approach to screen the target genes related to tanshinone IIA and EMs. After screening and matching, 64 common targets were obtained and used for further study. Through PPI network construction, it was predicted that the core biological targets of tanshinone IIA for EMs treatment such as VEGFA, MMP-9, ESR1, ICAM-1 and IL-2, involving the pathological effects of adhesion, invasion, angiogenesis, inflammatory response and cellular immune response. To illustrate the functional and signaling pathways of the 14 protein targets associated with tanshinone IIA for EMs, GO and KEGG analyses were performed. The results showed that the target genes were mainly enriched in the positive regulation of cell migration, positive regulation of cell motility, positive regulation of protein phosphorylation, regulation of kinase activity and other biological pathways. KEGG analysis showed that 14 target genes were enriched in 198 signaling pathways, indicating that tanshinone IIA acts as a treatment for EMs by regulating multiple signaling pathways. Among them, the PI3K-AKT pathway and mTOR pathway showed high enrichment. Therefore, it was hypothesized that tanshinone IIA might regulate the adhesion, invasion and angiogenesis of EMs via PI3K/Akt/mTOR signaling pathway. Based on the above results, the therapeutic role of tanshinone IIA on EMs was detected.
EMs is known to be an inflammatory disease. Studies have found that the presence of a large number of macrophages in the peritoneal fluid of patients with EMs can release more pro-inflammatory cytokines such as IL and TNF-α to participate in the intercellular communication modification, promote fibroblast mitosis, adhesion and maintain the adhesion of ectopic endometrial tissue (2,34). In the present study, the serum levels of TNF-α and IL-1β were upregulated in the model group. After intervention with tanshinone IIA (3 or 12 mg/kg), the serum levels of TNF-α and IL-1β were downregulated, suggesting that tanshinone IIA may play an anti-inflammatory role in EMs.
ICAM-1, MMP-9 and VEGF are essential cytokines for the adhesion, invasion and angiogenesis of ectopic endometrial tissue in the pelvic and abdominal cavities of EMs. As the most potent cell adhesion molecule in the immunoglobulin superfamily, ICAM-1 plays a crucial role in regulating cell-to-cell and cell-extracellular matrix adhesion (35). In EMs pathogenesis, the concentration of ICAM-1 increases significantly and reduces the toxicity of natural killer cells, which mediate a defective immune surveillance response mechanism and promote the adhesion, implantation and proliferation of ectopic tissue (36). MMP-9, an inflammatory mediator associated with inflammation and angiogenic remodeling, can be significantly upregulated in EMs progression by TNF-α. MMP-9 promotes ectopic tissue invasion and ectopic lesion formation by degrading extracellular matrix components (37). It has been found that macrophages in EMs peritoneal fluid are the primary source of VEGF (38). Macrophages release pro-inflammatory cytokines TNF-α and IL-1β, which induce upregulation of VEGF expression through the COX-2 signaling pathway, thereby stimulating peripheral endometrial angiogenesis and proliferation of ectopic endometrium (39,40). Immunohistochemistry was employed in the present study to detect alterations in the expression of ICAM-1, MMP-9 and VEGF. Results showed that the expression levels of ICAM-1, MMP-9 and VEGF were significantly elevated in the EMs lesions of rats in the model group, whereas they were downregulated in the tanshinone IIA (3 or 12 mg/kg) treatment groups. There were no statistically significant changes between the positive group (medroxyprogesterone acetate) and the tanshinone IIA group. This suggested that tanshinone IIA can limit the infiltration of ectopic lesions in EMs by modulating adhesion, invasion and angiogenesis.
Angiogenesis plays a crucial role in the formation of EMs. As the most important angiogenic factor, VEGF and its receptors can upregulate CD62E and CD105 levels by inducing the PI3K/Akt signaling pathway, thus leading to a large number of neovascularization of vascular endothelial cells in an EMs environment, which is conducive to the occurrence and development of EMs (41,42). Other studies have shown that blocking the mTOR signaling pathway can significantly inhibit the formation and progression of ectopic lesions in EMs rats (43,44). As is well known, EMs is also an estrogen-dependent disease, and studies have proven that the estrogen signaling pathway is implicated in the formation of EMs (10,45). By participating in EMs estrogen-signal transduction pathway, the PI3K/Akt/mTOR signaling pathway affects endometrial homeostasis and increases vascular permeability, participates in cell adhesion, invasion and angiogenesis in ectopic endometrium, and ultimately leads to the formation of EMs (46,47). The present study found that the expression of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR proteins in the ectopic endometrium of rats in the model group was significantly increased, suggesting that the PI3K/Akt/mTOR signaling pathway may be involved in the progression of EMs. Following treatment with tanshinone IIA, the protein expressions of PI3K, p-PI3K, Akt, p-Akt, mTOR and p-mTOR were significantly downregulated. RT-qPCR also showed that the mRNA expressions of PI3K and mTOR in the ectopic endometrium of rats treated with tanshinone IIA was downregulated. Therefore, it was hypothesized that tanshinone IIA may prevent the excitation of PI3K/Akt/mTOR signaling pathway, thus further restricting the progression of EMs.
At present, the pathogenesis of EMs is not fully understood and its treatment options are still limited. TCM is effective in treating EMs and deserves further study. There are several suggestions for future work. First, make good use of network pharmacology tools to predict the core targets of TCM and combine experimental verification to promote the development of new TCM drugs. Second, combined with the latest progress in the pathological mechanism of EMs, the principle of multi-pathway and multi-target action of TCM should be expanded as far as possible. Finally, combined with clinical trials to further confirm the relevant efficacy of TCM, to form expert consensus, to provide reference and basis for the formulation of disease guidelines.
In conclusion, the present study used a combination of network pharmacology and animal experiments to explore the mechanism of tanshinone IIA in treating EMs. It found that tanshinone IIA can inhibit secretion of inflammatory factors TNF-α and IL-1β, inhibit the expression of ICAM-1, MMP-9 and VEGF and regulate the adhesion, invasion and angiogenesis of ectopic endometrial tissue, thereby preventing the formation of ectopic lesions. In addition, the PI3K/Akt/mTOR signaling pathway plays an important role in regulating this effect. The present study provided a new therapeutic strategy for EMs and provided a reference for further study of the pharmacological mechanism of tanshinone IIA.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the Capital Health Development Scientific Research Special Project (grant no. Shou-fa 2018-4-4204) and the Beijing TCM Science and Technology Development Funding Project (grant no. QN-2020-18).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
XG and ZC designed and conceived the study. XZ, SL, WL, SP and FG conducted the experiments. ZJ and ZG analyzed the experimental data. XZ and SL drafted the manuscript, which was reviewed and edited by XG and ZC. XG and ZC confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved (approval no. AEEI-2018-031) by and followed the guidelines of the Ethics Committee for Experimental Animals of Capital Medical University (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen LH, Lo WC, Huang HY, Wu HM. A Lifelong impact on endometriosis: Pathophysiology and pharmacological treatment. Int J Mol Sci. 2023;24:7503. doi: 10.3390/ijms24087503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnez J, Cacciottola L. Endometriosis: An inflammatory disease that requires new therapeutic options. Int J Mol Sci. 2022;23:1518. doi: 10.3390/ijms23031518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramiuk M, Grywalska E, Małkowska P, Sierawska O, Hrynkiewicz R, Niedźwiedzka-Rystwej P. The role of the immune system in the development of endometriosis. Cells. 2022;11:2028. doi: 10.3390/cells11132028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: Pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 5.Amro B, Ramirez Aristondo ME, Alsuwaidi S, Almaamari B, Hakim Z, Tahlak M, Wattiez A, Koninckx PR. New understanding of diagnosis, treatment and prevention of endometriosis. Int J Environ Res Public Health. 2022;19:6725. doi: 10.3390/ijerph19116725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallée A, Vallée JN, Le Blanche A, Lecarpentier Y. PPARγ Agonists: Emergent Therapy in Endometriosis. Pharmaceuticals (Basel) 2021;14:543. doi: 10.3390/ph14060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nominato NS, Prates LF, Lauar I, Morais J, Maia L, Geber S. Caesarean section greatly increases risk of scar endometriosis. Eur J Obstet Gynecol Reprod Biol. 2010;152:83–85. doi: 10.1016/j.ejogrb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Horne AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. Bmj. 2022;14:2022–070750. doi: 10.1136/bmj-2022-070750. [DOI] [PubMed] [Google Scholar]
- 9.Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666–682. doi: 10.1038/s41574-019-0245-z. [DOI] [PubMed] [Google Scholar]
- 10.Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet. 2021;397:839–852. doi: 10.1016/S0140-6736(21)00389-5. [DOI] [PubMed] [Google Scholar]
- 11.Leonardi M, Gibbons T, Armour M, Wang R, Glanville E, Hodgson R, Cave AE, Ong J, Tong YYF, Jacobson TZ, et al. When to Do Surgery and When Not to Do Surgery for Endometriosis: A Systematic Review and Meta-analysis. J Minim Invasive Gynecol. 2020;27:390–407. doi: 10.1016/j.jmig.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Yela DA, Vitale SG, Vizotto MP, Benetti-Pinto CL. Risk factors for recurrence of deep infiltrating endometriosis after surgical treatment. J Obstet Gynaecol Res. 2021;47:2713–2719. doi: 10.1111/jog.14837. [DOI] [PubMed] [Google Scholar]
- 13.Guo R, Li L, Su J, Li S, Duncan SE, Liu Z, Fan G. Pharmacological activity and mechanism of Tanshinone IIA in Related Diseases. Drug Des Devel Ther. 2020;14:4735–4748. doi: 10.2147/DDDT.S266911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Wang Q, Wang X, Chen X, Shao M, Zhang Q, Guo D, Wu Y, Li C, Wang W, Wang Y. Tanshinone IIA protects against heart failure post-myocardial infarction via AMPKs/mTOR-dependent autophagy pathway. Biomed Pharmacother. 2019;112:108599. doi: 10.1016/j.biopha.2019.108599. [DOI] [PubMed] [Google Scholar]
- 15.Miao Q, Wang R, Sun X, Du S, Liu L. Combination of puerarin and tanshinone IIA alleviates ischaemic stroke injury in rats via activating the Nrf2/ARE signalling pathway. Pharm Biol. 2022;60:1022–1031. doi: 10.1080/13880209.2022.2070221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni H, Ruan G, Sun C, Yang X, Miao Z, Li J, Chen Y, Qin H, Liu Y, Zheng L, et al. Tanshinone IIA inhibits gastric cancer cell stemness through inducing ferroptosis. Environ Toxicol. 2022;37:192–200. doi: 10.1002/tox.23388. [DOI] [PubMed] [Google Scholar]
- 17.Jin J, Hu QY, Xu WW, Zhu WJ, Liu B, Liu J, Wang W, Zhou HF. Tanshinone IIA attenuates estradiol-induced polycystic ovarian syndrome in mice by ameliorating FSHR expression in the ovary. Exp Ther Med. 2019;17:3501–3508. doi: 10.3892/etm.2019.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y, Li ZM, Li LP, Zou Y, Xu XY, Zhang ZY, Liu FY, Xiong Y, Wan L. ITRAQ-based proteomics analysis of tanshinone IIA on human ectopic endometrial stromal cells of adenomyosis. Arch Gynecol Obstet. 2021;303:1501–1511. doi: 10.1007/s00404-020-05936-1. [DOI] [PubMed] [Google Scholar]
- 19.Luo M, Cai X, Yan D, Liu X, Guo SW. Sodium tanshinone IIA sulfonate restrains fibrogenesis through induction of senescence in mice with induced deep endometriosis. Reprod Biomed Online. 2020;41:373–384. doi: 10.1016/j.rbmo.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Liu X, Guo SW. Progressive development of endometriosis and its hindrance by anti-platelet treatment in mice with induced endometriosis. Reprod Biomed Online. 2017;34:124–136. doi: 10.1016/j.rbmo.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Zhou ZH, Weng Q, Zhou JH, Zhou J. Extracts of Salvia miltiorrhiza Bunge on the cytokines of rat endometriosis models. Afr J Tradit Complement Altern Med. 2012;9:303–314. doi: 10.4314/ajtcam.v9i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ZZ, Gong X. Tanshinone IIA contributes to the pathogenesis of endometriosis via renin angiotensin system by regulating the dorsal root ganglion axon sprouting. Life Sci. 2020;240:117085. doi: 10.1016/j.lfs.2019.117085. [DOI] [PubMed] [Google Scholar]
- 23.Jiashuo WU, Fangqing Z, Zhuangzhuang LI, Weiyi J, Yue S. Integration strategy of network pharmacology in traditional Chinese medicine: A narrative review. J Tradit Chin Med. 2022;42:479–486. doi: 10.19852/j.cnki.jtcm.20220408.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Wei S, Niu S, Ma X, Li H, Jing M, Zhao Y. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput Biol Med. 2022;144:105389. doi: 10.1016/j.compbiomed.2022.105389. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R, Zhu X, Bai H, Ning K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front Pharmacol. 2019;10:123. doi: 10.3389/fphar.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:1758–2946. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding J, Tan X, Song K, Ma W, Xiao J, Song Y, Zhang M. Bushen huoxue recipe alleviates implantation loss in mice by enhancing estrogen-progesterone signals and promoting decidual angiogenesis through FGF2 during early pregnancy. Front Pharmacol. 2018;9:437. doi: 10.3389/fphar.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders PTK, Horne AW. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell. 2021;184:2807–2824. doi: 10.1016/j.cell.2021.04.041. [DOI] [PubMed] [Google Scholar]
- 30.Casper RF. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril. 2017;107:533–536. doi: 10.1016/j.fertnstert.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Qin Z, Dong Z, Liu J, Zhong A, Bao M, Wang H, Yu H, Zhang S, Zhang W, Shen L, et al. A Preliminary study on the effects of black cohosh preparations on bone metabolism of rat models With GnRH-a-Induced Peri-Menopausal symptoms. Front Endocrinol. 2022;13:854345. doi: 10.3389/fendo.2022.854345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L, Zhang H, Li N, Chen J, Xu H, Wang Y, Liang Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. doi: 10.1016/j.jep.2023.116306. [DOI] [PubMed] [Google Scholar]
- 33.Han Y, Xiao Y, Yu L, Chen J, Yang X, Cui H, Liang J. Advances in the mechanism of luteolin against hepatocellular carcinoma based on bioinformatics and network pharmacology. J Cancer. 2023;14:966–980. doi: 10.7150/jca.80456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Dragovic RA, Greaves E, Becker CM, Southcombe JH. Macrophages and small extracellular vesicle mediated-intracellular communication in the peritoneal microenvironment: Impact on endometriosis development. Front Reprod Health. 2023;5:1130849. doi: 10.3389/frph.2023.1130849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KH, Lee EN, Park JK, Lee JR, Kim JH, Choi HJ, Kim BS, Lee HW, Lee KS, Yoon S. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother Res. 2012;26:1037–1047. doi: 10.1002/ptr.3694. [DOI] [PubMed] [Google Scholar]
- 36.Chopyak VV, Koval HD, Havrylyuk AM, Lishchuk-Yakymovych KA, Potomkina HA, Kurpisz MK. Immunopathogenesis of endometriosis-a novel look at an old problem. Cent Eur J Immunol. 2022;47:109–116. doi: 10.5114/ceji.2022.113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng J, Li C, Ying Y, Lv J, Qu X, McGowan E, Lin Y, Zhu X. Metformin alleviates endometriosis and potentiates endometrial receptivity via decreasing VEGF and MMP9 and increasing leukemia inhibitor factor and HOXA10. Front Pharmacol. 2022;13:750208. doi: 10.3389/fphar.2022.750208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F, Liu XL, Wang W, Dong HL, Xia YF, Ruan LP, Liu LP. Expression of MMIF, HIF-1α and VEGF in Serum and Endometrial Tissues of Patients with Endometriosis. Curr Med Sci. 2018;38:499–504. doi: 10.1007/s11596-018-1906-1. [DOI] [PubMed] [Google Scholar]
- 39.Ansariniya H, Hadinedoushan H, Javaheri A, Zare F. Vitamin C and E supplementation effects on secretory and molecular aspects of vascular endothelial growth factor derived from peritoneal fluids of patients with endometriosis. J Obstet Gynaecol. 2019;39:1137–1142. doi: 10.1080/01443615.2019.1601167. [DOI] [PubMed] [Google Scholar]
- 40.Hattori K, Ito Y, Honda M, Sekiguchi K, Hosono K, Shibuya M, Unno N, Majima M. Lymphangiogenesis induced by vascular endothelial growth factor receptor 1 signaling contributes to the progression of endometriosis in mice. J Pharmacol Sci. 2020;143:255–263. doi: 10.1016/j.jphs.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Chang KK, Liu LB, Jin LP, Meng YH, Shao J, Wang Y, Mei J, Li MQ, Li DJ. NME1 suppression of endometrial stromal cells promotes angiogenesis in the endometriotic milieu via stimulating the secretion of IL-8 and VEGF. Int J Clin Exp Pathol. 2013;6:2030–2038. [PMC free article] [PubMed] [Google Scholar]
- 42.Samimi M, Pourhanifeh MH, Mehdizadehkashi A, Eftekhar T, Asemi Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J Cell Physiol. 2019;234:19384–19392. doi: 10.1002/jcp.28666. [DOI] [PubMed] [Google Scholar]
- 43.Madanes D, Bilotas MA, Bastón JI, Singla JJ, Meresman GF, Barañao RI, Ricci AG. PI3K/AKT pathway is altered in the endometriosis patient's endometrium and presents differences according to severity stage. Gynecol Endocrinol. 2020;36:436–440. doi: 10.1080/09513590.2019.1680627. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Qin X, Lu X, Jiang J. Effects of inhibiting the PI3K/Akt/mTOR signaling pathway on the pain of sciatic endometriosis in a rat model. Can J Physiol Pharmacol. 2019;97:963–970. doi: 10.1139/cjpp-2019-0156. [DOI] [PubMed] [Google Scholar]
- 45.Peiris AN, Chaljub E, Medlock D. Endometriosis. JAMA. 2018;320:2608. doi: 10.1001/jama.2018.17953. [DOI] [PubMed] [Google Scholar]
- 46.Jin X, Feng J, Cheng X. LncRNA IGF2-AS promotes endometriosis progression through targeting miR-370-3p/IGF2 axis and activating PI3K/AKT/mTOR signaling pathway. J Assist Reprod Genet. 2022;39:2699–2710. doi: 10.1007/s10815-022-02638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin R, Zheng F, Qin W, Wang J, Ma N, Tian W, Li J, Liao M, Qin A. Progranulin promotes proliferation, migration and invasion via the PI3K/Akt signalling pathway in a model of endometriosis. Reprod Biomed Online. 2023;46:425–435. doi: 10.1016/j.rbmo.2022.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.