Abstract
Non-conventional yeast species, or non-Saccharomyces yeasts, are increasingly recognized for their involvement in fermented foods. Many of them exhibit probiotic characteristics that are mainly due to direct contacts with other cell types through various molecular components of their cell wall. The biochemical composition and/or the molecular structure of the cell wall components are currently considered the primary determinant of their probiotic properties. Here we first present the techniques that are used to extract and analyze the cell wall components of food industry-related non-Saccharomyces yeasts. We then review the current understanding of the cell wall composition and structure of each polysaccharide from these yeasts. Finally, the data exploring the potential beneficial role of their cell wall components, which could be a source of innovative functional ingredients, are discussed. Such research would allow the development of high value-added products and provide the food industry with novel inputs beyond the well-established S. cerevisiae.
Keywords: Yeast, Non-conventional, Cell wall, β-glucan, Mannan, Prebiotic, Probiotic
Graphical abstract
Highlights
-
•
Non-conventional yeasts exhibit potential probiotic attributes with various food applications.
-
•
Their cell wall is crucial in the functional properties but not well-characterized.
-
•
There is evidence for the bioactivity on health of their mannans and β-glucans.
-
•
Non-Saccharomyces species can be envisaged as a new source of food additives.
1. Introduction
Although yeasts have long been recognized for their role in fermented food and beverages, the diversity of genera and species involved has only been deciphered in the last 15 years (Tamang and Lama, 2022). In addition to Saccharomyces species, non-conventional yeasts have attracted much interest for their technological properties (in oenology for instance) and their potential probiotic attributes. Thus, the specific functional and biotechnological properties of probiotic Saccharomyces and non-Saccharomyces species have been explored in many recent works (Fernández-Pacheco et al., 2021). Apart from live yeast cells (Shruthi et al., 2022), their cell wall components can be valorized as value-added products in the production of functional foods such as nutraceuticals. According to the Food and Agriculture Organization of the United Nations and the World Health Organization (FAO/WHO), probiotics are living microorganisms that can be used to improve the health and well-being of the host, while prebiotics are microbial components with beneficial properties to the host. The best-known and best-characterized probiotic yeast is S. cerevisiae var. boulardii (S. boulardii). This yeast species is widely used as a preventive and curative probiotic for the treatment of various gastrointestinal diseases in humans and animals. It has also been shown to be effective in the treatment of inflammatory bowel disease and diarrhea in humans induced by antibiotic treatment (Szajewska and Kołodziej, 2015) or colonization with enterotoxigenic Escherichia coli (Gresse et al., 2021). Recently, various screenings of potential probiotic yeasts other than S. cerevisiae and S. boulardii have been performed (Staniszewski and Kordowska-Wiater, 2021). Yeasts species from oenological and dairy origin were reported to have potential probiotic properties including Kluyveromyces marxianus (Smith et al., 2016; Galinari et al., 2018), Kluyveromyces lactis (Kumura et al., 2004), Debaryomyces hansenii (Ochangco et al., 2016; Angulo et al., 2020), Torulaspora delbrueckii (Andrade et al., 2021), Yarrowia lypolytica, Pichia pastoris (renamed as Komagataella spp.) (Birmann et al., 2021), Wickerhamomyces anomalus (Helmy et al., 2019) and Pichia kudriavzevii (Saber et al., 2017). Phenotypes ranging from in vitro adhesion to enterocytes to immunomodulation, anti-pathogen or anti-oxidative properties (Fortin et al., 2018a; Galinari et al., 2018; Smith et al., 2014) have been explored and open new avenues for future use as food additives. However, despite several reports on their potential functionality, only a few studies explored the molecular basis of these properties. Such studies would be very helpful for the development of prebiotics for the food and feed industry.
Most of these non-Saccharomyces yeasts possess a wide range of hydrolytic activities on polysaccharides (Escribano et al., 2017) and can survive under extreme conditions (high pH, high temperature or high salt concentration) (Karim et al., 2020; Prista et al., 2016). Their cell wall, whose thickness is thought to be variable depending on the species, allows them to cope with many stresses encountered during biotechnological processes such as temperature and osmotic shock. Also, yeasts are in direct contact with other cell types such as pathogenic bacteria and epithelial cells, mostly through their cell wall components. Since these interactions involve the cell wall at first, its components are very likely the first determinants of the probiotic attributes of these yeasts. Despite the crucial role of the cell wall in the functional properties of these yeasts, only a few works have attempted to find out whether the cell wall composition (mainly β-glucans, mannans and chitin), the degree of connection and branching between these components or their accessibility, are fundamental in determining the probiotic properties of these yeasts. While the cell wall of the model organism S. cerevisiae and the pathogenic yeast Candida albicans are well-characterized, the current knowledge on the cell wall of other yeast species is more limited. The objective of this review is to present the extraction methods and techniques that are used to study the structure and composition of the cell wall, the key characteristics of the cell wall of non-conventional yeasts and their potential beneficial properties to the host. Thus, we highlight connections between the composition and architecture of the cell wall and the properties as possible functional ingredients of non-conventional yeasts.
2. Investigation methods for the study of the cell wall
2.1. Visualization by transmission electron microscopy
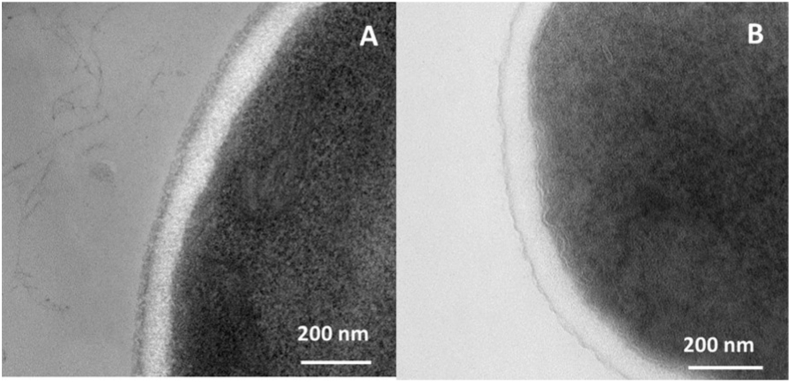
Transmission electron microscopy has revealed that many ascomycetous yeasts such as S. cerevisiae, K. lactis, W. anomalus, Blastobotrys (Arxula) adeninivorans, Cyberlindnera jadinii or K. marxianus have a cell wall formed by two layers (see Fig. 1; K. marxianus and W. anomalus, as examples) (Agboola et al., 2021; Backhaus et al., 2011). In these species, the cell wall is composed of a network of chitin, β-glucans and mannoproteins that are all cross-linked to form a complex and flexible structure. Chitin, β-1,3 and β-1,6-glucans form the electron-transparent inner layer and give it its rigidity (Schiavone et al., 2017). The electron-dense outer layer is composed of mannoproteins. This layer is less permeable to macromolecules than the inner layer (Zlotnik et al., 1984). More specifically, the cell wall of the fission yeast Schizosaccharomyces pombe has a particular three-layer structure. The inner and outer layer are electron-dense and composed of galactomannans and the electron-transparent middle layer is composed of β-1,3/β-1,6-glucans and α-1,3-glucans (Pérez et al., 2018) (Table 1).
Fig. 1.
Transmission electron micrographs (TEM) of Kluyveromyces marxianus and Wickerhamomyces anomalus (A,B) (unpublished data).
Table 1.
Cell wall components extracted from different non-Saccharomyces yeasts: methods of extraction, yield, and molecular weight. (Mw, molecular weight; nd, not determined).
| Cell wall component | Yeast species | Starting material | Extraction method | Yield (%) (purity) | Mw (kDa) | Reference |
|---|---|---|---|---|---|---|
| β-glucans | S. boulardii | Autolyzed cells | 1M NaOH – 90 °C | nd (40.5%) a nd (24.0%) b |
1921 0.73–160 |
(Fortin et al., 2018a) |
| D. hansenii | Cells | 3% NaOH - 100 °C | 6.6–11 (nd)a | nd | (Medina-Córdova et al., 2018) | |
| Cells | 3% NaOH - 100 °C | 14.8 (63.1%) a | nd | (Angulo et al., 2018) | ||
| Cells | 3% NaOH - 100 °C Acetic acid 0.5N |
19.8 (79.3%) a | 0.69 | (Reyes-Becerril et al., 2020) | ||
| Cells | 3% NaOH - 90 °C | 12.0–12.4 (98%) a | nd | (Sukumaran et al., 2010) | ||
| Cell walls | 1M KOH - 4 °C | 20.9–48.4 (83–87%) a 14.5–37.4 (92–93%) b |
nd | (Nguyen et al., 1998) | ||
| C. tropicalis | Cells | 3% NaOH - 90 °C | 8.1 (98%) a | nd | (Sukumaran et al., 2010) | |
| K. marxianus | Autolyzed cells | 1M NaOH – 90 °C | nd (49.2%) a nd (30.4%) b |
2086 0.74–165 |
(Fortin et al., 2018a) | |
| Cell walls | 1M KOH - 4 °C | 18.5–40.6 (89–93%) a 10.0–48 (95–98%) b |
nd | (Nguyen et al., 1998) | ||
| P. pastoris | Autolyzed cells | High-pressure hot-water treatment, ultrasonication, isopropanol extraction, and protease treatment | 11.7 (85.3%) a | nd | (Xing et al., 2018) | |
| K. apiculata, | Cell walls | 1M KOH - 4 °C | (94–97%) a (90–92%) b |
nd | (Nguyen et al., 1998) | |
| Z. bailii, | Cell walls | 1M KOH - 4 °C | 20.8–23.8 (96%) a 33.9–37.3 (92–94%) b |
nd | (Nguyen et al., 1998) | |
| Y. lypolitica | Cells | 3% NaOH – 100 °C | 9.52 (61.07%) a | 3.30 | (Angulo et al., 2021) | |
| Mannan | S. boulardii | Autolyzed cells | 1M NaOH - 90 °C, precipitation with ethanol | nd (3.2%) | 0.72–87 | (Fortin et al., 2018a) |
| K. marxianus | Cells | 3% NaOH - 80 °C, precipitation with methanol and filtration (cutoff of 100 kDa) | 13.3 (90%) | 203 | (Galinari et al., 2017) | |
| Autolyzed cells | 1M NaOH - 90 °C, precipitation with ethanol | nd (9.1%) | 0.48–77 | (Fortin et al., 2018a) | ||
| Cells | 2% NaOH - 80 °C, precipitation with ethanol, purification with DEAE anion exchange column | 71.1–84.7 (91.1–97.1%) | 646–698 | (Tang et al., 2022) | ||
| Cell walls | 1M KOH - 4 °C | 25.6–34.1 (93–95%) | nd | (Nguyen et al., 1998) | ||
| W. anomalus | Cells | Autoclave, ethanol precipitation | nd | 1.9–84 | (Bzducha-Wróbel et al., 2022) | |
| M. reukaufii | Cells | Autoclave, ethanol precipitation | nd | 1.9–150 | (Bzducha-Wróbel et al., 2022) |
for insoluble glucans.
for soluble glucans.
2.2. Extraction of polysaccharides
Many approaches for the extraction of β-glucans and α-mannans from yeast biomass are performed under alkaline conditions (see Table 1). Alkaline extraction can be performed directly on yeast cells or on yeast cell walls obtained after autolysis (Sukumaran et al., 2010; Medina-Córdova et al., 2018; Xing et al., 2018) or cell disruption by bead-milling (Nguyen et al., 1998). Subsequent extractions are then carried out by hot alkali treatment using 3% NaOH or 1M NaOH, which is the condition often preferred to cold alkali to solubilize β-glucans (Table 1). Glucans fractions are therefore defined according to their solubility under alkaline conditions after extraction. Alkali-insoluble glucans have higher molecular weight than alkali-soluble glucans (Fortin et al., 2018a). In non-Saccharomyces yeasts, alkali-insoluble glucans range from 6.6 to 20% of the whole cell biomass (Medina-Córdova et al., 2018; Angulo et al., 2018, 2021; Sukumaran et al., 2010; Reyes-Becerril et al., 2020) and from 14.5 to 37.4% of the cell walls (Nguyen et al., 1998). This higher yield can be explained by the fact that cell wall isolation process leads to the removal of intracellular proteins and thus to an increase in the proportion of cell wall's polysaccharides in the starting material. To obtain a mannoproteins-rich fraction (i.e. mannan-protein complexes), the alkali-soluble fraction is further treated with cold organic solvents such as ethanol (Tang et al., 2022; Bzducha-Wróbel et al., 2022; Fortin et al., 2018a) or methanol (Galinari et al., 2017) to precipitate mannoproteins. This yields a low purity mannan fraction (3.2–9.1%) with a molecular weight below 200 kDa (Fortin et al., 2018a; Bzducha-Wróbel et al., 2022). Further purification steps are required to obtain a higher yield and purity of mannoproteins and thus mannans. This could be achieved by taking advantage of the structure of mannoproteins, using an anion exchange chromatography technique with DEAE columns or by ultrafiltration techniques (Galinari et al., 2017; Tang et al., 2022). To summarize, the extraction and quantification of yeast cell wall polysaccharides are highly cumbersome. Notably, there are few information regarding the chitin content, which otherwise is a vital component for S. cerevisiae (Shaw et al., 1991). Standard procedures that enable to determine glucans, mannans and chitin contents as well as their isolated monosaccharides remain to be established for non-Saccharomyces yeasts. They should enable to compare conditions and strains for changes in cell wall polysaccharides.
2.3. Techniques for the investigation of cell wall architecture from non-conventional yeasts
To quantify the polysaccharides extracted from the cell wall, different common chemical methods are used. The phenol-sulfuric acid method (DuBois et al., 1956) is a well-established method to determine the total polysaccharide content, while the monosaccharide content is determined after their release by acid hydrolysis using strong acids such as sulfuric acid or trifluoroacetic acid (TFA) (Dallies et al., 1998). Structural features of polysaccharides extracted from yeast cell walls are usually analyzed by Fourier Transformed Infrared (FTIR) or Nuclear Magnetic Resonance (NMR) techniques. The functional chemical groups and hence the purity of the polysaccharides extracted are detected by FTIR (Vaithanomsat et al., 2022; Bacha et al., 2017; Zhao et al., 2022). To unravel the type of linkage and the degree of branching of each polysaccharide, fine chemical analysis can be performed by liquid NMR (Galinari et al., 2017; Vaithanomsat et al., 2022). The structure and molecular weight of polysaccharides extracted from a same yeast species may vary due to divergent yeast strains and culture conditions but also to extraction procedures using different chemical reagents that may alter the native structure of polysaccharides and destroy covalent bonds.
Parietal proteins are studied by proteomic approaches using mass spectrometry. According to the extraction method, cell wall mannoproteins were divided into three groups: non-covalently attached proteins that are extractable by SDS or reducing agents, glycosylphosphatidylinositol (GPI)-anchored proteins extractable by β-1,3-glucanase, and Pir proteins that are extractable with mild alkaline conditions (Lozančić et al., 2021). These proteomic techniques should be applied in the future to investigate the cell wall proteomes of these non-Saccharomyces yeasts, which may likely vary under different growth and stress conditions.
In addition to classical extraction methods in which the structure of polysaccharides, other strategies are now being used to study the physical properties of the cell wall at atomic resolution, such as solid-state NMR (ssNMR) and atomic force microscopy (AFM). Recently, ssNMR spectroscopy combined with glycosylated bond analysis has been successfully used to develop a high-resolution model of the cell wall architecture of the filamentous fungus Aspergillus fumigatus (Kang et al., 2018). This non-destructive method provides insight into the structure of polysaccharides and the organization of the cell wall (Loquet et al., 2013; Zhao et al., 2020), although it requires 13C/15N labelling of the sample. AFM provides access to the mechanical properties of the yeast cell wall through the value of the elasticity modulus or Young's modulus (Francois et al., 2013). Screening of mutants involved in cell wall biosynthesis and assembly has revealed that cross-linking of cell wall components is essential for cell wall strength (Dague et al., 2010). AFM can also be used in single-molecule force spectroscopy mode to map and unfold the polysaccharides at specific sites on the cell wall (Schiavone et al., 2019; Francius et al., 2009). In addition, techniques such as AFM and ss-NMR can evaluate the dynamics of the cell wall, which is a fundamental property as the structure and composition of the cell wall is constantly changing during the life cycle of yeast and under industrial culture conditions (often stressful and challenging for microorganisms).
3. Cell wall composition and architecture of non-conventional yeasts
3.1. Cell wall composition
Schweigkofler (2002) studied the monosaccharide composition of purified cell walls of 114 ascomycetes fungi. Although the method used did not allow the measurement of the chitin content, this screening allowed determining the sugar content released upon hydrolysis of cell wall polysaccharides by trifluoro acetic acid (TFA). Three types of sugar patterns were found: glucose/mannose, glucose/mannose/galactose and glucose/mannose/galactose/rhamnose. The glucose/mannose pattern was found in 51 out of 114 ascomycetes belonging to the Hemiascomycetes clade with different proportions of glucose (25–75%) and mannose (22–75%). S. cerevisiae, C. albicans and K. lactis belong to this glucose/mannose group because their cell wall contains glucans, mannans and chitin. The cell wall of S. cerevisiae accounts for 20–30% of the dry mass of the cell and it contains 80–90% of polysaccharides (Lesage and Bussey, 2006; Klis, Koster and Brul, 2014). Nguyen et al., 1998 also studied the sugar composition of the cell wall polysaccharides of different non-Saccharomyces yeasts, including D. hansenii, K. marxianus, Zygosaccharomyces bailii and Kloeckera apiculate. These yeasts species belong to the glucose/mannose type and the cell wall represents 26–32% of the dry weight and is composed of 84–89% carbohydrates depending on the species, strain and growth conditions. The cell wall of K. marxianus accounts for 33% of the dry mass of the cell (Nguyen et al., 1998) and contains about 90% polysaccharides (Fortin et al., 2018a) (Table 1). Cell walls containing glucose, mannose and galactose were found in 26 species that covered the Hemiascomycetes clade, the Euascomycetes clade and Schizosaccharomycetales fungi from the “Protomyces-clade” that notably included the fission yeast S. pombe and Y. lypolitica. The percentage of glucose ranged from 28 to 65%, that of mannose from 18 to 56% and the proportion of galactose varied from 2 to 27% (Schweigkofler 2002). The third type of monosaccharide, which contains rhamnose in its cell wall, was found in Euascomycetes clade as well as in Taphrina spp. and Protomyces spp. of the Promycetes clade.
These global studies provide information on the type of polysaccharides that can be found in the cell wall of different fungi (Table 2). Nevertheless, further studies are needed to determine the nature of the linkages within the parietal polysaccharides of ascomycetes yeast species (β-1,3; β-1,6; β-1,4 or α-1,3). The composition of the cell wall is dynamic and can vary according to growth conditions and carbon sources. Indeed, the cell wall is the direct target of various external physical, osmotic, and mechanical stresses, which requires permanent remodeling such as increasing its polysaccharide or protein content or forming new crosslinks between cell wall components (Aguilar-Uscanga and François, 2003).
Table 2.
Cell wall polysaccharides in different yeasts species.
| Cell wall polysaccharide | S. cerevisiae | K. lactis | S. pombe | D. hansenii | K. marxianus |
|---|---|---|---|---|---|
| β-1,3 and β-1,6-glucans | 50–65% | 50–55% | 54–60% | 50–60% | 50% |
| α-1,3-glucan | none | none | 28–32% | nd | nd |
| Chitin | 1–5% | 1–3% | in conidia only | 2–7% | 1–4% |
| Mannoproteins | 35–40% | 30–40% | None | 29–35% | 20–25% |
| Galactomannan | None | None | 9–14% | None | None |
The cell wall components listed were identified from the following sources: S. cerevisiae (Klis et al., 2002), K. lactis (Backhaus et al., 2010; Bahmed et al., 2002), S. pombe (Pérez et al., 2018), D. hansenii and K. marxianus (Nguyen et al., 1998). Results are the means of each component expressed as % of cell wall dry weight. nd = not determined.
3.2. Cell wall architecture
3.2.1. β-glucans
β-glucans represent the common architectural signature of the inner layer of yeast cell walls studied to date (Table 2). In the fission yeast S. pombe, the middle layer is composed of β-1,3/β-1,6-glucans (54–60% of the cell wall) and α-1,3-glucans (Pérez et al., 2018). In S. cerevisiae, β-glucans (50–60% of the yeast cell wall dry mass) consist of glucose chains that are mainly connected by β-1,3-linkages and include 10–15% of glucose branched by β-1,6-linkages. The β-1,3/β-1,6-glucans vary according to the degree of branching, degree of polymerization and molecular weight. The S. cerevisiae β-1,3-glucans have a degree of polymerization of about 1500, while β-1,6-glucans consist of an average of 140 glucose units (Klis et al., 2002). Highly branched β-1,3-glucans are thus found to be alkali-insoluble due to strong hydrogen bonds formed by the hydroxyl groups of their glucans chains and due to their covalent binding with chitin (Hartland et al., 1994). Depending on the strain, alkali-insoluble β-glucans derived from D. hansenii can reach up to 12% of the yeast biomass (Medina-Córdova et al., 2018; Sukumaran et al., 2010) (Table 1), but this amount can vary considerably within the same yeast species. NMR analyses showed that the alkali-insoluble fraction of the marine yeast D. hansenii consists of low molecular weight β-1,3-glucans with β-1,6 side branches (0.69 kDa) (Reyes-Becerril et al., 2020). In contrast, higher molecular weight alkali-insoluble glucans were extracted from marine strain of Y. lypolytica (3.3 103 Da) (Angulo et al., 2021), K. marxianus and S. boulardii (1.9 and 2.106 Da, respectively) (Fortin et al., 2018a). In addition, alkali-insoluble particulate β-glucans from S. uvarum, K. marxianus and S. boulardii accounted for 51%, 49% and 41% of the cell wall dry mass, respectively, indicating a difference in cell wall composition (Fortin et al., 2018b; Suphantharika et al., 2003). The cell wall of K. lactis is similar to that of S. cerevisiae, with 50% of the cell wall dry weight composed of β-1,3-glucans (Backhaus et al., 2010). W. anomalus has a higher content of β-glucans than C. jadinii (20.4% and 11.1% of the cell dry weight, respectively), resulting in greater stiffness of its cell wall (Agboola et al., 2021). The methylotrophic yeast Komagataella spp. has recently been used to produce a β-glucan rich fraction by autolysis and hot water treatment, representing 11.7% of the dry weight of the cell (Xing et al., 2018). As with S. cerevisiae, these works support that cell wall composition varies between strains. Finally, these studies showed that the proportion and structure of β-1,3- and β-1,6-glucans can vary depending on the extraction method used. These variations of the cell wall architecture can lead to different in vivo properties, with high molecular weight β-glucans and high degree of branching reported to possess important biological activities (see below) (Murphy et al., 2020; Chen et Seviour, 2007).
3.2.2. Mannoproteins
The chemical structure of the mannoproteins from some yeast species has been characterized (Klis 1994). With the exception of the cell wall of S. pombe, which contains galactomannans (9–14% of the cell wall) in its inner and outer cell wall, the mannoproteins of most yeasts, including S. cerevisiae, present in the outer layer of the cell wall and are highly glycosylated proteins with a carbohydrate fraction (85–90%) composed of α-linked mannose units (Table 2). Cell wall mannoproteins carry numerous N-linked mannans and clustered O-linked mannans. N-linked glycans have a hypermannosylated outer chain composed of a long backbone of about 50 α-1,6 mannose residues with short side chains of mannose units linked to the backbone by α-1,2 and α-1,3-linkages, whereas O-linked mannans have a smaller core structure of α-1,6-linked mannose (Munro 2001). Golgi glycosyltransferases involved in N-glycan modifications were first identified by genetic analysis of mannans synthesis mutants (mnn) in S. cerevisiae (Rayner and Munro, 1998). Other screens affecting Golgi N-glycosylation (och1, mnn9) have been performed in other yeasts species such as Y. lypolitica (Barnay-Verdier et al., 2008), confirming that the mechanisms of mannosylation synthesis are conserved. Different amounts of phosphate can be esterified to N- and O-linked mannans in S. cerevisiae, with phosphate being esterified to the hydroxyl groups of α-1,2 linked mannans chains (Jigami and Odani, 1999). Kocourek and Ballou observed a proportion of phosphates in the mannan fraction ranging from 0.04% (K. lactis) to 4.4% (Candida atmospherica), testifying to a difference in the structure of the side branches decorating the α-1,6-mannans chain backbone (Kocourek and Ballou, 1969). The mannans of K. marxianus appear to be similar to those of S. cerevisiae, with a structure consisting of an α-1,6-mannan backbone substituted with α-1,3 and α-1,2 branches (Galinari et al., 2017). Tang et al. reported the isolation of α-1,6-mannans from two different K. marxianus strains LZ-JM1 and GY3 with molecular weights ranging from 650 to 700 kDa (Tang et al., 2022), while others have isolated water soluble α-mannans with low molecular weights ranging from 0.5 to 77 kDa from the same yeast species (Galinari et al., 2018; Fortin et al., 2018a). These variations in molecular weight could be related to the yeast strain, the composition of the culture medium and/or the extraction method used (Table 1). Nevertheless, the α-mannans extracted from K. marxianus seem to have a higher structure than those of S. boulardii, for which an isolation of mannans from S. cerevisiae var. boulardii ATCC MYA-796 with molecular weights ranging from 0.1 to 10 kDa has been reported (Fortin et al., 2018a).
Cell wall proteins are mainly divided into two main classes: cell wall proteins that can be retained covalently through a glycosylphosphatidylinositol (GPI) anchor to β-1,6-glucan (GPI-CWPs) and Pir family cell wall proteins covalently bound by an alkali-sensitive linkage to β-1,3 glucans (Pir-CWPs). In the S. cerevisiae genome, 60 putative GPI-CWPs have been identified (Caro et al., 1997). These GPI-glycosylated proteins have many roles, some serving as enzymes for cell wall biosynthesis and maintenance or as structural components, while others are adhesive proteins involved in flocculation, biofilm formation or invasive growth (Pittet and Conzelmann, 2007). Like the polysaccharide content, the cell wall proteome can vary according to the growth conditions (Groot et al., 2005). Recently, an in silico analysis of cell wall proteins from 92 yeast species showed that they are largely conserved, but some are highly species-specific (Lozančić et al., 2021). Yeasts that are taxonomically close to S. cerevisiae show a similar protein profile. For example, S. boulardii and S. cerevisiae have the same GPI-bound proteins, non-covalently attached proteins and alkaline-extractable proteins. In contrast, the genera Kluyveromyces, Lachancea, Wickerhamii show different profiles (Lozančić et al., 2021). Pir proteins are present in many budding yeasts and S. cerevisiae PIR homologous genes have been found in several yeasts including K. lactis (Backhaus et al., 2010) and Y. lypolytica (Jaafar et al., 2003) but not in S. pombe (Sharifmoghadam et al., 2006). This suggests a unique role for these genes in the budding yeast. In K. lactis, two Pir proteins have been identified so far by proteomics (KlPir1a, KlPir1b) (Backhaus et al., 2010), which is in contradiction with a recent work (Lozančić et al., 2021) that did not detect any Pir protein in K. lactis and other Kluyveromyces species using specific antibodies to streptavidin/biotin. Also, Pir proteins were not found in D. hansenii, B. adenivorans and S. pombe neither in silico nor by streptavidin/biotin blot (Lozančić et al., 2021). In Y. lypolitica only YlPir1 has been identified so far (Jaafar et al., 2003). Further studies are therefore needed to confirm these findings and to determine whether their potential absence could have a physiological role in cell wall architecture. The cell wall proteome of K. lactis has similar characteristics to that of S. cerevisiae and mass spectrometry analyses revealed that many K. lactis cell wall proteins were homologous to those of S. cerevisiae. However some species-specific proteins still have unknown functions (Backhaus et al., 2010). Among the non-conventional yeasts, Komagataella spp. and Y. lypolitica are two well-established yeasts used in biotechnology for the production and secretion of heterologous proteins. However, little is known about their cell wall structure. In Y. lypolitica, only Ylcwp1, a GPI-CWP (Jaafar and Zueco, 2004), and Ylywp1, a cell wall protein bound covalently bound to mycelial-specific β-1,3-glucans (Ramon et al., 1999), have been identified, but the total number of cell wall proteins is not known. Analysis of the secretome of Komagataaella spp grown on methanol, glycerol or glucose in fed-batch bioreactor identified a core of cell wall proteins (Pir1, Scw10) or cell wall-associated proteins (flocculins, chitinase and glucanase) that are present independently of the carbon sources used, but whose abundance changes during fermentation (Burgard et al., 2020). Despite minor differences, a high degree of similarity in the secretome was observed for all carbon sources in Komagataella spp, which contrasts with the effects of carbon sources on the secretome of S. cerevisiae, K. lactis and C. utilis (Madinger et al., 2009; Giardina et al., 2014; Buerth et al., 2011). These differences could be explained by a difference in the regulatory mechanisms underlying cell wall biogenesis between these different yeasts, which have been investigated so far only in the yeast S. cerevisiae (Levin 2011).
3.2.3. Chitin
Chitin is a polymer of β-1,4-linked N-acetylglucosamine units, which accounts for 1–5% of the cell wall of S. cerevisiae. As shown in Table 2, the chitin content can vary from 1 to 7% in non-Saccharomyces species D. hansenii, K. lactis, K. marxianus and Z. baili (Nguyen et al., 1998). Although a minor component of the yeast cell wall, chitin is essential for yeast survival since simultaneous deletion of the three genes encoding chitin synthases (CHS1 to -3) is lethal in S. cerevisiae (Shaw et al., 1991). This property is probably shared by other yeast species, but this has not yet been directly demonstrated. Indeed, yeast species such as C. albicans or filamentous fungi such as A. fumigatus have several genes encoding chitin synthases (see review (Rogg et al., 2012)), which may reflect a functional redundancy representing a survival mechanism. The exception is S. pombe, which contains only two chitin synthases, one of which is apparently inactive (Martín-García et al., 2003). In K. lactis, there are three chitin synthases and KlChs2 has an essential role in cytokinesis. The deletion of this gene results in a defect in spore germination (Rippert and Heinisch, 2016). In conclusion, chitin synthases are conserved among yeast species, but it remains to be investigated whether the synthesis and distribution of chitin in these non-Saccharomyces yeast species is comparable or different from what has been described in S. cerevisiae (Orlean and Funai, 2019; Sánchez and Roncero, 2022) and whether the lack of chitin is lethal in these non-Saccharomyces species as in the case of S. cerevisiae.
4. Relationship between cell wall biochemical composition and structure and probiotic properties
Live yeasts and their derivatives in the form of inactive cells, total extracts and cell wall extracts have various applications with health-promoting properties in the food industry. A recent review summarized the probiotic attributes of yeasts other than S. boulardii and showed that these other probiotic yeasts could have various applications in food biotechnology (Shruthi et al., 2022). Here we report data exploring the role of cell wall components in the probiotic effects of live yeasts or the use of cell wall components as prebiotics.
4.1. β-glucans
The cell wall of S. cerevisiae is rich in β-glucans, which have been shown to have numerous nutritional and functional properties in human and animal health. Indeed, fungal β-glucans composed of a mix of β-1,3 and β-1,6-linkages have various biological activities, including immunostimulation (Batbayar et al., 2012). For example, S. cerevisiae β-glucans are able to stimulate TNF-α production and induce IL-6 production when lipopolysaccharides are used as co-stimulators (Seong and Kim, 2010). A more detailed study then showed that yeast cell wall extracts containing β-glucans, from S. boulardii and K. marxianus, are potent inducers of IL-1β, IL-6, and IL-10, but not IL-12, in dendritic cells (Smith et al., 2016). Interestingly, stronger stimulatory effects on mouse macrophages were detected with β-1,3-glucans with a higher molecular weight or a greater degree of β-1,6-branching (Cleary et al., 1999). Anti-inflammatory and antioxidant effects conferred by S. cerevisiae β-glucans have also been observed (Bacha et al., 2017). In a mouse model, S. cerevisiae β-1,3-glucans significantly increased the expression levels of IL-2, IL-6, and TNF-α (Mo et al., 2017). This stimulation of host immune function by β-1,3-glucans was associated with anti-tumor effects without toxicity to normal mouse cells. Thus, β-1,3-glucans are known potentiators of innate immunity.
The action of β-glucans on the immune system has been studied for antitumor targeting (Qi et al., 2011; Geller et al., 2019). In addition, insoluble extracts of β-glucans and mannoproteins from S. boulardii showed chemopreventive properties in a rat model of colorectal cancer in vivo (Fortin et al., 2018b). Numerous other studies have also demonstrated anticancer effects of S. cerevisiae β-glucan (Sambrani et al., 2021) including prevention of DNA damage in CHO cells (Oliveira et al., 2007), induced proliferation and activation of peripheral blood monocytes in patients with advanced breast cancer (Demir et al., 2007) and a variety of other immunostimulatory effects on various cell lines (Yoon et al., 2008) and in vivo (Mo et al., 2017). S. cerevisiae β-glucans have also been reported as antidiabetic agents through their effects on blood glucose levels and insulin resistance (Cao et al., 2017), as potential prebiotic compounds by altering gut microbiome and metabolite profiles (Zhen et al., 2021; Wang et al., 2020), and finally as capable of reducing blood cholesterol levels and promoting wound healing (Borchani et al., 2016).
In addition to β-glucans from S. cerevisiae and S. boulardii, β-glucans extracted from different non-Saccharomyces yeasts also showed prebiotic effects on animals or cell lines (Table 3). Cancer chemopreventive activities as well as antiproliferative and antioxidant effects of the polysaccharides (glucan, chitin, and mannan) from the K. marxianus cell wall were observed on HT-29 cells in vitro (Fortin et al., 2018a). Few other studies have analyzed the effects of non-Saccharomyces β-glucans together with their molecular architecture. For example, proton NMR studies revealed structures containing β-1,3-glucans branched with β-1,6 in different strains of D. hansenii of marine origin, which are probiotic and immunostimulatory in fish. These β-glucans increase cellular immune parameters (phagocytic capacity, reactive oxygen species production (respiratory burst), peroxidase activity and nitric oxide production) in goat peripheral blood leukocytes (Medina-Córdova et al., 2018). Similarly, Reyes-Becerril et al. (2020) observed by NMR that one of these marine strains of D. hansenii (BCS004) contains β-1,3-glucans branched with β-1,6 of low molecular weight (Reyes-Becerril et al., 2020). These β-glucans can upregulate macrophage receptor genes in the gut of the Pacific red snapper Lutjanus peru, and exhibit significant free radical scavenging capacity. Another study also showed that, upon challenge with E. coli, β-glucans from a strain of D. hansenii isolated from the rainbow trout gut increased leukocyte viability, phagocytic capacity and nitric oxide production (Angulo et al., 2018). Thus, yeast β-glucans are immunomodulators for fish (see for review Machuca et al., 2022). In addition, β-glucans from K. marxianus also induce cytokine secretion by dendritic cells that is dependent on the Dectin-1 receptor (Smith et al., 2016). The β-glucans recovered from K. marxianus have recently been analyzed by NMR for their purity, functional groups, linkages and tested for their functional properties such as glucose adsorption capacity among others (Vaithanomsat et al., 2022). Finally, Y. lipolytica–derived glucans have also been tested on goat leucocytes which showed increased phagocytic ability and nitric oxide production (Angulo et al., 2021). Several immune-related signaling pathways were also stimulated by these Y. lipolytica β-glucans, leading to the conclusion that they are immunostimulants in animals.
Table 3.
Prebiotic effects on animals or cell lines of cell wall components extracted from different non-Saccharomyces yeasts.
| Cell wall component | Yeast species | Animal or cell line | Beneficial properties | Reference |
|---|---|---|---|---|
| Beta-glucans | K. marxianus | Human monocyte-derived dendritic cells | Induction of IL-1β, IL-6, and IL-10 | (Smith et al., 2016) |
| K. marxianus | Human HT-29 cells | Improved superoxide anion scavenging (antiradical capacity), NAD(P)H: quinone reductase induction and antiproliferative properties | (Fortin et al., 2018a) | |
| D. hansenii | Goat peripheral blood leukocytes | Increased phagocytic capacity, reactive oxygen species production, peroxidase activity and nitric oxide production | (Medina-Córdova et al., 2018) | |
| D. hansenii | Pacific red snapper (Lutjanus peru) | Upregulated macrophage receptor genes in the gut, improved free radical scavenging capacity | (Reyes-Becerril et al., 2020) | |
| Y. lipolytica | Goat leukocytes | Increased leukocyte viability, phagocytic ability, nitric oxide production and immune-related signalling pathways | (Angulo et al., 2021) | |
| D. hansenii | Goat peripheral blood leukocytes | Increased leukocyte viability, phagocytic capacity and nitric oxide production upon challenge with E. coli | (Angulo et al., 2018) | |
| Mannans | Combined fractions of S. cerevisiae and C. jadinii | Atlantic salmon (Salmo salar) | Enhanced gut and skin mucosal barriers | (Rawling et al., 2019) |
| K. marxianus | Murine macrophages (RAW 264.7) | Mitogenic activity and induction of nitric oxide production | (Galinari et al., 2018) | |
| K. marxianus | Human Hep-G2 tumor cells | Antiproliferative activity | (Galinari et al., 2018) | |
| K. marxianus | Wistar rats | Hypocholesterolemic activity | (Yoshida et al., 2009) |
4.2. Mannans
Mannans from S. cerevisiae have potential beneficial effects on human and animal health (Faustino et al., 2021), including anti-inflammatory (Lew et al., 2017) and immunomodulatory effects (Jin et al., 2019), and wound repair (Michael et al., 2017). Mannans also have various other beneficial effects, for example as a feed supplement in aquaculture. Dietary supplementation with mannans can improve the resistance of fish to bacterial infections (Torrecillas et al., 2007; Liu et al., 2013) and it has recently been shown that mannans also have beneficial effects on the antiviral immune response of fish (Liang et al., 2023). A multi-strain yeast fraction combining selected fractions of S. cerevisiae and C. jadinii and having a high level of long chains of α-mannans (Rawling et al., 2019) produce positive effects on the immune balance and gut health of different aquatic species (Leclercq et al., 2020; Xie et al., 2022). This indicates that the use of non-conventional yeast components alone or in combination with S. cerevisiae components could be of interest for the improvement of host health.
S. cerevisiae mannans also possess in vitro antioxidant activities against several types of free radicals depending on their concentration and molecular weight (Zhao et al., 2022). This property depends on the composition of the mannans and suggests interesting applications in the food and medical industries. In addition, yeast mannans can be utilized by intestinal bacteria, in particular Bacteroides thetaiotaomicron (Cuskin et al., 2015), and may have an impact on the gut microbiota ecosystem as they increase the abundance of B. thetaiotaomicron in in vitro fermentation of rat feces (Oba et al., 2020a, Oba et al., 2020b) and of B. thetaiotaomicron and Bacteroides ovatus in a human colonic microbiota model (Tanihiro et al., 2020; Oba et al., 2020a, Oba et al., 2020ba). Thus, there is increasing evidence for the bioactivity of S. cerevisiae mannans, suggesting that they represent a sustainable source of functional ingredients and paving the way for their use in food, feed and pharmaceutical industries (Faustino et al., 2021). The cell wall of S. boulardii has a different oligosaccharide composition from that of S. cerevisiae, including a higher mannan content (Bzducha-Wróbel et al., 2013). Recent metabolic engineering strategies have attempted to modify the mannan content of the S. boulardii cell wall with success and increasing the mannan content of the S. boulardii cell wall improves its ability to adhere to Salmonella enterica Typhimurium (Kwak et al., 2022).
In recent years, among non-Saccharomyces species (Table 3), several α-mannans from K. marxianus have attracted interest because of their copper and iron chelating abilities (Galinari et al. 2017, 2018), their mitogenic activity in macrophages, their antiproliferative activity on Hep-G2 tumor cells (Galinari et al., 2018) or their ability to regulate the composition of the gut microbiota (Tang et al., 2022). Interestingly, some K. marxianus also show a more potent hypocholesterolemic activity than S. cerevisiae and this functional activity depends on the side chain length and phosphate content of the mannans (Yoshida et al., 2009). Another recent study also tested the effects of mannoproteins or oligosaccharide fractions from S. cerevisiae and two non-Saccharomyces yeasts, Metschnikowia reukaufii and Wickerhamomyces anomalus, on the growth of a variety of bacteria and showed a positive effect on the growth of beneficial lactic acid bacteria while decreasing the abundance of pathogenic bacteria (Bzducha-Wróbel et al., 2022). Interestingly, the degree of stimulation or inhibition of bacterial growth is dependent on the composition and dose of mannoproteins and the bacterial strain, but it remains to be determined how the structure and/or composition of mannoproteins can impact bacterial growth.
4.3. Chitin
Chitin can be obtained from non-plant sources, including fungi, and has a particular impact on the human gut microbiota (Lopez-Santamarina et al., 2020). Although their chitin content is lower than that of crustaceans, fungi are an alternative source of chitin that is of increasing interest to scientists and the food industry. However, very few studies have examined the health and/or nutritional benefits of yeast chitin. Chitin is known to stimulate immunogenic activity during fungal infection (Bueter et al., 2013) and is a conserved Microbe-Associated Molecular Pattern (Lee et al., 2008). As such, it can induce immunity in monocytes and it is interesting to note that the different chitin contents of different S. cerevisiae strains could explain the differences between strains in terms of induced-driven immunity (differences in cytokine production) and antimicrobial activity in vitro and in vivo (Rizzetto et al., 2016). Thus, although studies on the effects of yeast chitin on immunity are still in their infancy, these results suggest that chitin content may be an important factor in the immunomodulatory properties of various Saccharomyces and non-Saccharomyces species.
5. Conclusion
For decades, yeast have played a vital role in the production of fermented food and beverage. Recently, however, the potential health benefits of non-Saccharomyces yeast species have come to light, sparking growing interest in their use that may be promising in many applications, including health and welfare. For now, S. boulardii is the only probiotic yeast that shows well-characterized effects on the prevention and treatment of intestinal disorders, but there are increasing evidence that some non-conventional yeasts such as Debaryomyces, Kluyveromyces, Komagataella have such probiotic potential. Beneficial effects on the health of the host with these new yeasts are strain-specific and mediated through the recognition of their cell wall. For instance, their mannans and β-glucans have important biological activities including antioxidant properties, promotion of the host immunity by modulation of cytokines secretion and enhancement of the phagocytic efficiency as well as limitation of the bacterial invasion. Therefore, their unique cell wall components have been identified as a promising source of functional ingredients, particularly as prebiotics.
However, to harness their full potential, the composition, structure, and 3D architecture of these components need to be investigated. While research on the cell wall components of S. cerevisiae and C. albicans (Orlean 2012; Gow and Lenardon, 2023; Klis et al., 2002) has been extensive, integrated molecular, biochemical, and biophysical approaches need to be applied to study the cell wall of non-conventional yeasts. In addition to these studies on the cell wall, more mechanistic and systemic approaches are required to fully understand how these compounds exert their beneficial properties. The rapid development of new in vitro and in vivo models such as the Zebrafish (Danio rerio) could serve as interesting platforms for the study of the relationships between cell wall structure and beneficial effects on health. The evaluation of the safety of use of these new yeasts cell wall as prebiotics must be also further evaluated.
Ultimately, these new microbial products may require a pre-market approval by the authorities in some jurisdictions for their use as food/feed ingredients. In Europe, the European Food Safety Agency (EFSA) developed in 2007 a Qualified Presumption Safety (QPS) procedure. When sufficient scientific studies on a species are available to assess its safety, it can be included in the QPS list. In 2023, the re-evaluated QPS list included 17 yeast species (Koutsoumanis et al., 2023). This list is not exhaustive and does not preclude a non-QPS microorganism for a pre-market assessment for EFSA. In US, there are three possibilities to approve a new ingredient in the food and feed industry: the certification of the ingredient as Generally Recognized As Safe (GRAS) by the Food and Drug Administration (FDA), the recognition of the ingredient by the Association of American Feed Control Officials (AAFCO) or by the FDA through the food additive petition (FAP). The annual official publication of the AAFCO lists all the legal ingredients and is recognized by all states but only few non-Saccharomyces species are included. As already reviewed for the beer industry, these regulatory processes for non-Saccharomyces are limited (Miguel et al., 2022) and may represent a limitation for the rapid development of new products from yeast species that would not yet be on these lists.
Despite the vast potential of non-Saccharomyces yeasts in promoting health and well-being, only few studies have investigated the functional properties conferred by their cell wall components. With numerous non-Saccharomyces yeast species of interest for such applications, this research field is poised to advance rapidly in the coming years. This will pave the way for the future use of non-Saccharomyces cell walls as additives in the food and feed industry.
Funding
This work was in part supported in part by the project Lallwall, grant INSA-SAIC2021/139 to JMF & JPC.
CRediT authorship contribution statement
Marion Schiavone: compiled the data, wrote the manuscript and created tables and figures. Jean M. François: reviewed and edited the original draft. Didier Zerbib: reviewed and edited the original draft. Jean-Pascal Capp: compiled the data, wrote the manuscript and created tables and figures.
Declaration of competing interest
MS is an employee of Lallemand SAS.
Acknowledgements
We are grateful to Dr Mathieu Castex and Vanessa Demey from Lallemand Inc. for their support to this work and advice about non-conventional yeasts.
Data availability
No data was used for the research described in the article.
References
- Agboola Jeleel Opeyemi, Schiavone Marion, Øverland Margareth, Morales-Lange Byron, Lagos Leidy, Arntzen Magnus Øverlie, et al. Impact of down-stream processing on functional properties of yeasts and the implications on gut health of Atlantic salmon (Salmo salar) Sci. Rep. 2021;11(1):4496. doi: 10.1038/s41598-021-83764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Uscanga B., François J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003;37(3):268–274. doi: 10.1046/j.1472-765x.2003.01394.x. [DOI] [PubMed] [Google Scholar]
- Andrade Gabrielly Carvalho, Andrade Rafaela Pereira, Oliveira Daelen Resende, Quintanilha Mônica F., Martins Flaviano S., Duarte Whasley Ferreira. Kluyveromyces lactis and Torulaspora delbrueckii: probiotic characterization, anti-Salmonella effect, and impact on cheese quality. LWT. 2021;151 doi: 10.1016/j.lwt.2021.112240. [DOI] [Google Scholar]
- Angulo Miriam, Reyes-Becerril Martha, Angulo Carlos. Yarrowia lipolytica N6-glucan protects goat leukocytes against Escherichia coli by enhancing phagocytosis and immune signaling pathway genes. Microb. Pathog. 2021;150 doi: 10.1016/j.micpath.2021.104735. [DOI] [PubMed] [Google Scholar]
- Angulo Miriam, Reyes-Becerril Martha, Medina-Córdova Noe, Tovar-Ramírez Dariel, Angulo Carlos. Probiotic and nutritional effects of Debaryomyces hansenii on animals. Appl. Microbiol. Biotechnol. 2020;104(18):7689–7699. doi: 10.1007/s00253-020-10780-z. [DOI] [PubMed] [Google Scholar]
- Angulo Miriam, Reyes-Becerril Martha, Tovar-Ramírez Dariel, Ascencio Felipe, Angulo Carlos. Debaryomyces hansenii CBS 8339 β-glucan enhances immune responses and down-stream gene signaling pathways in goat peripheral blood leukocytes. Dev. Comp. Immunol. 2018;88:173–182. doi: 10.1016/j.dci.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Bacha Umar, Nasir Muhammad, Iqbal Sanaullah, Anjum Aftab Ahmad. Nutraceutical, anti-inflammatory, and immune modulatory effects of β-glucan isolated from yeast. BioMed Res. Int. 2017 doi: 10.1155/2017/8972678. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus Katja, Buchwald Ulf, Heppeler Nele, Schmitz Hans-Peter, Rodicio Rosaura, Heinisch Jürgen J. Milk and sugar: regulation of cell wall synthesis in the milk yeast Kluyveromyces lactis. Eur. J. Cell Biol. 2011;90(9):745–750. doi: 10.1016/j.ejcb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Backhaus Katja, Heilmann Clemens J., Sorgo Alice G., Purschke Günter, Koster Chris G. de, Klis Frans M., Heinisch Jürgen J. A systematic study of the cell wall composition of Kluyveromyces lactis. Yeast. 2010;27(8):647–660. doi: 10.1002/yea.1781. [DOI] [PubMed] [Google Scholar]
- Bahmed Karim, Bonaly Roger, Wathier Michel, Pucci Bernard, Coulon Joël. Change of cell wall chitin content in amphotericin B resistant Kluyveromyces strains. FEMS Microbiol. Lett. 2002;216(1):99–103. doi: 10.1111/j.1574-6968.2002.tb11421.x. [DOI] [PubMed] [Google Scholar]
- Barnay-Verdier Stéphanie, Beckerich Jean-Marie, Boisramé Anita. New components of Yarrowia lipolytica Golgi multi-protein complexes containing the alpha-1,6-mannosyltransferases YlMnn9p and YlAnl1p. Curr. Genet. 2008;54(6):313–323. doi: 10.1007/s00294-008-0219-5. [DOI] [PubMed] [Google Scholar]
- Batbayar Sainkhuu, Lee Dong Hee, Kim Ha Won. Immunomodulation of fungal β-glucan in host defense signaling by dectin-1. Biomolecules & therapeutics. 2012;20(5):433–445. doi: 10.4062/biomolther.2012.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmann Paloma T., Casaril Angela M., Pesarico Ana Paula, Caballero Pamela S., Smaniotto Thiago Â., Rodrigues Rafael R., et al. Komagataella pastoris KM71H modulates neuroimmune and oxidative stress parameters in animal models of depression: a proposal for a new probiotic with antidepressant-like effect. Pharmacol. Res. 2021;171 doi: 10.1016/j.phrs.2021.105740. [DOI] [PubMed] [Google Scholar]
- Borchani Chema, Fonteyn Fabienne, Jamin Guilhem, Destain Jacqueline, Willems Luc, Paquot Michel, et al. Structural characterization, technological functionality, and physiological aspects of fungal β-D-glucans: a review. Crit. Rev. Food Sci. Nutr. 2016;56(10):1746–1752. doi: 10.1080/10408398.2013.854733. [DOI] [PubMed] [Google Scholar]
- Buerth Christoph, Heilmann Clemens J., Klis Frans M., Koster Chris G. de, Ernst Joachim F., Tielker Denis. Growth-dependent secretome of Candida utilis. Microbiology (Reading, England) 2011;157(Pt 9):2493–2503. doi: 10.1099/mic.0.049320-0. [DOI] [PubMed] [Google Scholar]
- Bueter Chelsea L., Specht Charles A., Levitz Stuart M. Vol. 9. 2013. Innate sensing of chitin and chitosan. (PLoS Pathogens). 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard Jonas, Grünwald-Gruber Clemens, Altmann Friedrich, Zanghellini Jürgen, Valli Minoska, Mattanovich Diethard, Gasser Brigitte. The secretome of Pichia pastoris in fed-batch cultivations is largely independent of the carbon source but changes quantitatively over cultivation time. Microb. Biotechnol. 2020;13(2):479–494. doi: 10.1111/1751-7915.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzducha-Wróbel Anna, Farkaš Pavol, Chraniuk Paulina, Popielarz Dominika, Synowiec Alicja, Pobiega Katarzyna, Janowicz Monika. Antimicrobial and prebiotic activity of mannoproteins isolated from conventional and nonconventional yeast species-the study on selected microorganisms. World J. Microbiol. Biotechnol. 2022;38(12):256. doi: 10.1007/s11274-022-03448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzducha-Wróbel Anna, Kieliszek Marek, Błażejak Stanisław. Chemical composition of the cell wall of probiotic and brewer's yeast in response to cultivation medium with glycerol as a carbon source. Eur. Food Res. Technol. 2013;237(4):489–499. doi: 10.1007/s00217-013-2016-8. [DOI] [Google Scholar]
- Cao Yan, Sun Ying, Zou Siwei, Li Mengxia, Xu Xiaojuan. Orally administered baker's yeast β-glucan promotes glucose and lipid homeostasis in the livers of obesity and diabetes model mice. J. Agric. Food Chem. 2017;65(44):9665–9674. doi: 10.1021/acs.jafc.7b03782. [DOI] [PubMed] [Google Scholar]
- Caro L., Heleen P., Tettelin Hervé, Vossen Jack H., Ram Arthur F.J., van den Ende Herman, Klis Frans M. In silicio identification of glycosyl-phosphatidylinositol-anchored plasma-membrane and cell wall proteins ofSaccharomyces cerevisiae. Yeast. 1997;13(15):1477–1489. doi: 10.1002/(SICI)1097-0061(199712)13:15<1477::AID-YEA184>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chen Jiezhong, Seviour Robert. Medicinal importance of fungal beta-(1--3), (1--6)-glucans. Mycol. Res. 2007;111:635–652. doi: 10.1016/j.mycres.2007.02.011. Pt 6. [DOI] [PubMed] [Google Scholar]
- Cleary J.A., Kelly G.E., Husband A.J. The effect of molecular weight and beta-1,6-linkages on priming of macrophage function in mice by (1,3)-beta-D-glucan. Immunol. Cell Biol. 1999;77(5):395–403. doi: 10.1046/j.1440-1711.1999.00848.x. [DOI] [PubMed] [Google Scholar]
- Cuskin Fiona, Lowe Elisabeth C., Temple Max J., Zhu Yanping, Cameron Elizabeth, Pudlo Nicholas A., et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature. 2015;517(7533):165–169. doi: 10.1038/nature13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dague Etienne, Bitar Rajaa, Ranchon Hubert, Durand Fabien, Yken Hélène Martin, François Jean M. An atomic force microscopy analysis of yeast mutants defective in cell wall architecture. Yeast. 2010;27(8):673–684. doi: 10.1002/yea.1801. [DOI] [PubMed] [Google Scholar]
- Dallies Nathalie, François Jean, Paquet Veronique. A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants ofSaccharomyces cerevisiae. Yeast. 1998;14(14):1297–1306. doi: 10.1002/(SICI)1097-0061(1998100)14:14<1297::AID-YEA310>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Demir Gokhan, Klein H.O., Mandel-Molinas Nil, Tuzuner N. Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Int. Immunopharm. 2007;7(1):113–116. doi: 10.1016/j.intimp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- DuBois Michel, Gilles K.A., Hamilton J.K., Rebers P.A., Smith Fred. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Escribano Rocío, González-Arenzana Lucía, Garijo Patrocinio, Berlanas Carmen, López-Alfaro Isabel, López Rosa, et al. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017;54(6):1555–1564. doi: 10.1007/s13197-017-2587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino Margarida, Durão Joana, Pereira Carla F., Pintado Manuela E., Carvalho Ana P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae - a sustainable source of functional ingredients. Carbohydr. Polym. 2021;272 doi: 10.1016/j.carbpol.2021.118467. [DOI] [PubMed] [Google Scholar]
- Fernández-Pacheco Pilar, Pintado Cristina, Briones Pérez Ana, Arévalo-Villena María. Potential probiotic strains of Saccharomyces and non-Saccharomyces: functional and biotechnological characteristics. Journal of fungi (Basel, Switzerland) 2021;7(3) doi: 10.3390/jof7030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin Olivier, Aguilar-Uscanga Blanca, Vu Khanh Dang, Salmieri Stephane, Lacroix Monique. Cancer chemopreventive, antiproliferative, and superoxide anion scavenging properties of Kluyveromyces marxianus and Saccharomyces cerevisiae var. boulardii cell wall components. Nutr. Cancer. 2018;70(1):83–96. doi: 10.1080/01635581.2018.1380204. [DOI] [PubMed] [Google Scholar]
- Fortin Olivier, Aguilar-Uscanga Blanca R., Vu Khanh D., Salmieri Stephane, Lacroix Monique. Effect of Saccharomyces boulardii cell wall extracts on colon cancer prevention in male F344 rats treated with 1,2-dimethylhydrazine. Nutr. Cancer. 2018;70(4):632–642. doi: 10.1080/01635581.2018.1460672. [DOI] [PubMed] [Google Scholar]
- Francius Grégory, Alsteens David, Dupres Vincent, Lebeer Sarah, Keersmaecker Sigrid de, Vanderleyden Jos, et al. Stretching polysaccharides on live cells using single molecule force spectroscopy. Nat. Protoc. 2009;4(6):939–946. doi: 10.1038/nprot.2009.65. [DOI] [PubMed] [Google Scholar]
- Francois Jean Marie, Formosa Cécile, Schiavone Marion, Pillet Flavien, Martin-Yken Hélène, Dague Etienne. Use of atomic force microscopy (AFM) to explore cell wall properties and response to stress in the yeast Saccharomyces cerevisiae. Curr. Genet. 2013;59(4):187–196. doi: 10.1007/s00294-013-0411-0. [DOI] [PubMed] [Google Scholar]
- Galinari Éder, Almeida-Lima Jailma, Macedo Gorete Ribeiro, Mantovani Hilário Cuquetto, Rocha Hugo Alexandre Oliveira. Antioxidant, antiproliferative, and immunostimulatory effects of cell wall α-d-mannan fractions from Kluyveromyces marxianus. Int. J. Biol. Macromol. 2018;109:837–846. doi: 10.1016/j.ijbiomac.2017.11.053. [DOI] [PubMed] [Google Scholar]
- Galinari Éder, Sabry Diego Araújo, Sassaki Guilherme Lanzi, Macedo Gorete Ribeiro, Passos Flávia Maria Lopes, Mantovani Hilário Cuquetto, Rocha Hugo Alexandre Oliveira. Chemical structure, antiproliferative and antioxidant activities of a cell wall α-d-mannan from yeast Kluyveromyces marxianus. Carbohydr. Polym. 2017;157:1298–1305. doi: 10.1016/j.carbpol.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Geller Anne, Shrestha Rejeena, Yan Jun. Yeast-derived β-glucan in cancer: novel uses of a traditional therapeutic. Int. J. Mol. Sci. 2019;20(15) doi: 10.3390/ijms20153618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina Bennett J., Stanley Bruce A., Chiang Hui-Ling. Glucose induces rapid changes in the secretome of Saccharomyces cerevisiae. Proteome Sci. 2014;12(1):9. doi: 10.1186/1477-5956-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow Neil A.R., Lenardon Megan D. Architecture of the dynamic fungal cell wall. Nat. Rev. Microbiol. 2023;21(4):248–259. doi: 10.1038/s41579-022-00796-9. [DOI] [PubMed] [Google Scholar]
- Gresse Raphaële, Garrido Juan J., Jiménez-Marín Angeles, Denis Sylvain, van de Wiele Tom, Forano Evelyne, et al. Saccharomyces cerevisiae var boulardii CNCM I-1079 reduces expression of genes involved in inflammatory response in porcine cells challenged by enterotoxigenic E. Coli and influences bacterial communities in an in vitro model of the weaning piglet colon. Antibiotics (Basel, Switzerland) 2021;10(9) doi: 10.3390/antibiotics10091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot Piet W. J. de, Ram Arthur F., Klis Frans M. Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet. Biol. 2005;42(8):657–675. doi: 10.1016/j.fgb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hartland R.P., Vermeulen C.A., Klis F.M., Sietsma J.H., Wessels J.G. The linkage of (1-3)-beta-glucan to chitin during cell wall assembly in Saccharomyces cerevisiae. Yeast. 1994;10(12):1591–1599. doi: 10.1002/yea.320101208. [DOI] [PubMed] [Google Scholar]
- Helmy E.A., Soliman S.A., Abdel-Ghany Tarek M., Ganash Magdah. Evaluation of potentially probiotic attributes of certain dairy yeast isolated from buffalo sweetened Karish cheese. Heliyon. 2019;5(5) doi: 10.1016/j.heliyon.2019.e01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaafar Lahcen, Moukadiri Ismaïl, Zueco Jesús. Characterization of a disulphide-bound Pir-cell wall protein (Pir-CWP) of Yarrowia lipolytica. Yeast. 2003;20(5):417–426. doi: 10.1002/yea.973. [DOI] [PubMed] [Google Scholar]
- Jaafar Lahcen, Zueco Jesús. Characterization of a glycosylphosphatidylinositol-bound cell-wall protein (GPI-CWP) in Yarrowia lipolytica. Microbiology (Reading, England) 2004;150(Pt 1):53–60. doi: 10.1099/mic.0.26430-0. [DOI] [PubMed] [Google Scholar]
- Jigami Y., Odani T. Mannosylphosphate transfer to yeast mannan. Biochim. Biophys. Acta. 1999;1426(2):335–345. doi: 10.1016/s0304-4165(98)00134-2. [DOI] [PubMed] [Google Scholar]
- Jin Xin, Zhang Man, Cao Gui-Fang, Yang Yin-Feng. Saccharomyces cerevisiae mannan induces sheep beta-defensin-1 expression via Dectin-2-Syk-p38 pathways in ovine ruminal epithelial cells. Vet. Res. 2019;50(1):8. doi: 10.1186/s13567-019-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Xue, Kirui Alex, Muszyński Artur, Widanage Malitha C. Dickwella, Chen Adrian, Azadi Parastoo, et al. Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat. Commun. 2018;9(1):2747. doi: 10.1038/s41467-018-05199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim Ahasanul, Gerliani Natela, Aïder Mohammed. Kluyveromyces marxianus: an emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020;333 doi: 10.1016/j.ijfoodmicro.2020.108818. [DOI] [PubMed] [Google Scholar]
- Klis F.M. Review: cell wall assembly in yeast. Yeast. 1994;10(7):851–869. doi: 10.1002/yea.320100702. [DOI] [PubMed] [Google Scholar]
- Klis Frans M., Koster Chris G. de, Brul Stanley. Cell wall-related bionumbers and bioestimates of Saccharomyces cerevisiae and Candida albicans. Eukaryot. Cell. 2014;13(1):2–9. doi: 10.1128/EC.00250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis Frans M., Mol Pieternella, Hellingwerf Klaas, Brul Stanley. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002;26(3):239–256. doi: 10.1111/j.1574-6976.2002.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Kocourek J., Ballou C.E. Method for fingerprinting yeast cell wall mannans. J. Bacteriol. 1969;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoumanis Konstantinos, Allende Ana, Álvarez‐Ordóñez Avelino, Bolton Declan, Bover‐Cid Sara, Chemaly Marianne, et al. Update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA J. 2023;21(1) doi: 10.2903/j.efsa.2023.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumura H., Tanoue Y., Tsukahara M., Tanaka T., Shimazaki K. Screening of dairy yeast strains for probiotic applications. J. Dairy Sci. 2004;87(12):4050–4056. doi: 10.3168/jds.S0022-0302(04)73546-8. [DOI] [PubMed] [Google Scholar]
- Kwak Suryang, Robinson Scott J., Lee Jae Won, Lim Hayoon, Wallace Catherine L., Jin Yong-Su. Dissection and enhancement of prebiotic properties of yeast cell wall oligosaccharides through metabolic engineering. Biomaterials. 2022;282 doi: 10.1016/j.biomaterials.2022.121379. [DOI] [PubMed] [Google Scholar]
- Leclercq Eric, Pontefract Nicola, Rawling Mark, Valdenegro Victoria, Aasum Elisabeth, Andujar Luisa Vera, et al. Dietary supplementation with a specific mannan-rich yeast parietal fraction enhances the gut and skin mucosal barriers of Atlantic salmon (Salmo salar) and reduces its susceptibility to sea lice (Lepeophtheirus salmonis) Aquaculture. 2020;529 doi: 10.1016/j.aquaculture.2020.735701. [DOI] [Google Scholar]
- Lee Chun Geun, Da Silva Carla A., Lee Jae-Young, Hartl Dominik, Elias Jack A. Chitin regulation of immune responses: an old molecule with new roles. Curr. Opin. Immunol. 2008;20(6):684–689. doi: 10.1016/j.coi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage Guillaume, Bussey Howard. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. : MMBR (Microbiol. Mol. Biol. Rev.) 2006;70(2):317–343. doi: 10.1128/MMBR.00038-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin David E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics. 2011;189(4):1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. Betty, Michael Christie F., Overbeck Tracie, Robinson W. Scout, Rohman Erin L., Lehman Jeffrey M., et al. Beneficial effects of prebiotic Saccharomyces cerevisiae mannan on allergic asthma mouse models. Journal of immunology research. 2017 doi: 10.1155/2017/3432701. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Hui, Xie Yadong, Li Yu, Xie Mingxu, Li Ming, Zhou Wei, et al. Dietary supplementation of yeast mannan enhances antiviral immunity of zebrafish (Danio rerio) Aquaculture. 2023;563 doi: 10.1016/j.aquaculture.2022.739003. [DOI] [Google Scholar]
- Liu Bo, Xu Lei, Ge Xianping, Xie Jun, Xu Pao, Zhou Qunlan, et al. Effects of mannan oligosaccharide on the physiological responses, HSP70 gene expression and disease resistance of Allogynogenetic crucian carp (Carassius auratus gibelio) under Aeromonas hydrophila infection. Fish Shellfish Immunol. 2013;34(6):1395–1403. doi: 10.1016/j.fsi.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Lopez-Santamarina Aroa, Del Mondragon Alicia Carmen, Lamas Alexandre, Miranda Jose Manuel, Franco Carlos Manuel, Cepeda Alberto. Animal-origin prebiotics based on chitin: an alternative for the future? A critical review. Foods. 2020;9(6) doi: 10.3390/foods9060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loquet Antoine, Habenstein Birgit, Lange Adam. Structural investigations of molecular machines by solid-state NMR. Accounts Chem. Res. 2013;46(9):2070–2079. doi: 10.1021/ar300320p. [DOI] [PubMed] [Google Scholar]
- Lozančić Mateja, Žunar Bojan, Hrestak Dora, Lopandić Ksenija, Teparić Renata, Mrša Vladimir. Systematic comparison of cell wall-related proteins of different yeasts. Journal of fungi (Basel, Switzerland) 2021;7(2) doi: 10.3390/jof7020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machuca Cristian, Méndez-Martínez Yuniel, Reyes-Becerril Martha, Angulo Carlos. Yeast β-glucans as fish immunomodulators: a review. Animals : an open access journal from MDPI. 2022;12(16) doi: 10.3390/ani12162154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madinger Catherine L., Sharma Shamik S., Anton Brian P., Fields Lauren G., Cushing Michelle L., Canovas Julie, et al. The effect of carbon source on the secretome of Kluyveromyces lactis. Proteomics. 2009;9(20):4744–4754. doi: 10.1002/pmic.200800915. [DOI] [PubMed] [Google Scholar]
- Martín-García Rebeca, Durán Angel, Valdivieso M-Henar. In Schizosaccharomyces pombe chs2p has no chitin synthase activity but is related to septum formation. FEBS Lett. 2003;549(1–3):176–180. doi: 10.1016/s0014-5793(03)00812-3. [DOI] [PubMed] [Google Scholar]
- Medina-Córdova Noé, Reyes-Becerril Martha, Ascencio Felipe, Castellanos Thelma, Campa-Córdova Angel I., Angulo Carlos. Immunostimulant effects and potential application of β-glucans derived from marine yeast Debaryomyces hansenii in goat peripheral blood leucocytes. Int. J. Biol. Macromol. 2018;116:599–606. doi: 10.1016/j.ijbiomac.2018.05.061. [DOI] [PubMed] [Google Scholar]
- Michael Christie F., Waters Christopher M., LeMessurier Kim S., Samarasinghe Amali E., Song Chi Y., Malik Kafait U., Lew D. Betty. Airway epithelial repair by a prebiotic mannan derived from Saccharomyces cerevisiae. Journal of immunology research. 2017 doi: 10.1155/2017/8903982. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel Gabriela A., Carlsen Simon, Arneborg Nils, Saerens Sofie M.G., Laulund Svend, Knudsen Gitte M. Non-Saccharomyces yeasts for beer production: insights into safety aspects and considerations. Int. J. Food Microbiol. 2022;383 doi: 10.1016/j.ijfoodmicro.2022.109951. [DOI] [PubMed] [Google Scholar]
- Mo Li, Chen Yafei, Li Wenjian, Guo Shuai, Wang Xuzhao, An Hailong, Zhan Yong. Anti-tumor effects of (1→3)-β-d-glucan from Saccharomyces cerevisiae in S180 tumor-bearing mice. Int. J. Biol. Macromol. 2017;95:385–392. doi: 10.1016/j.ijbiomac.2016.10.106. [DOI] [PubMed] [Google Scholar]
- Munro S. What can yeast tell us about N-linked glycosylation in the Golgi apparatus? FEBS Lett. 2001;498(2–3):223–227. doi: 10.1016/S0014-5793(01)02488-7. [DOI] [PubMed] [Google Scholar]
- Murphy Emma J., Rezoagli Emanuele, Major Ian, Rowan Neil J., Laffey John G. β-Glucan metabolic and immunomodulatory properties and potential for clinical application. Journal of fungi (Basel, Switzerland) 2020;6(4):356. doi: 10.3390/jof6040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.H., Fleet G.H., Rogers P.L. Composition of the cell walls of several yeast species. Appl. Microbiol. Biotechnol. 1998;50(2):206–212. doi: 10.1007/s002530051278. [DOI] [PubMed] [Google Scholar]
- Oba Shunsuke, Sunagawa Tadahiro, Tanihiro Reiko, Awashima Kyoko, Sugiyama Hiroshi, Odani Tetsuji, et al. Prebiotic effects of yeast mannan, which selectively promotes Bacteroides thetaiotaomicron and Bacteroides ovatus in a human colonic microbiota model. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-74379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba Shunsuke, Washida Kazuto, Shimada Yu, Sunagawa Tadahiro, Tanihiro Reiko, Sugiyama Hiroshi, Nakamura Yasunori. Yeast mannan increases Bacteroides thetaiotaomicron abundance and suppresses putrefactive compound production in in vitro fecal microbiota fermentation. Biosc. Biotech. Biochem. 2020;84(10):2174–2178. doi: 10.1080/09168451.2020.1784704. [DOI] [PubMed] [Google Scholar]
- Ochangco Honeylet Sabas, Gamero Amparo, Smith Ida M., Christensen Jeffrey E., Jespersen Lene, Arneborg Nils. In vitro investigation of Debaryomyces hansenii strains for potential probiotic properties. World J. Microbiol. Biotechnol. 2016;32(9):141. doi: 10.1007/s11274-016-2109-1. [DOI] [PubMed] [Google Scholar]
- Oliveira Rodrigo Juliano, Matuo Renata, Da Silva Ariane Fernanda, Matiazi Hevenilton José, Mantovani Mário Sérgio, Ribeiro Lúcia Regina. Protective effect of beta-glucan extracted from Saccharomyces cerevisiae, against DNA damage and cytotoxicity in wild-type (k1) and repair-deficient (xrs5) CHO cells. Toxicol. Vitro : an international journal published in association with BIBRA. 2007;21(1):41–52. doi: 10.1016/j.tiv.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Orlean Peter. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics. 2012;192(3):775–818. doi: 10.1534/genetics.112.144485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlean Peter, Funai Danielle. Priming and elongation of chitin chains: implications for chitin synthase mechanism. Cell surface (Amsterdam, Netherlands) 2019;5 doi: 10.1016/j.tcsw.2018.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez Pilar, Cortés Juan C.G., Cansado Jose, Ribas Juan C. Fission yeast cell wall biosynthesis and cell integrity signalling. The Cell Surface. 2018;4:1–9. doi: 10.1016/j.tcsw.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet Martine, Conzelmann Andreas. Biosynthesis and function of GPI proteins in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2007;1771(3):405–420. doi: 10.1016/j.bbalip.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Prista Catarina, Michán Carmen, Miranda Isabel M., Ramos José. The halotolerant Debaryomyces hansenii, the Cinderella of non-conventional yeasts. Yeast. 2016;33(10):523–533. doi: 10.1002/yea.3177. [DOI] [PubMed] [Google Scholar]
- Qi Chunjian, Cai Yihua, Gunn Lacey, Ding Chuanlin, Li Bing, Kloecker Goetz, et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood. 2011;117(25):6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon A.M., Montero M., Sentandreu R., Valentin E. Yarrowia lipolytica cell wall architecture: interaction of Ywp1, a mycelial protein, with other wall components and the effect of its depletion. Res. Microbiol. 1999;150(2):95–103. doi: 10.1016/s0923-2508(99)80027-8. [DOI] [PubMed] [Google Scholar]
- Rawling Mark D., Pontefract Nicola, Rodiles Ana, Anagnostara Ilektra, Leclercq Eric, Schiavone Marion, et al. The effect of feeding a novel multistrain yeast fraction on European seabass (Dicentrachus labrax) intestinal health and growth performance. J. World Aquacult. Soc. 2019;50(6):1108–1122. doi: 10.1111/jwas.12591. [DOI] [Google Scholar]
- Rayner J.C., Munro S. Identification of the MNN2 and MNN5 mannosyltransferases required for forming and extending the mannose branches of the outer chain mannans of Saccharomyces cerevisiae. J. Biol. Chem. 1998;273(41):26836–26843. doi: 10.1074/jbc.273.41.26836. [DOI] [PubMed] [Google Scholar]
- Reyes-Becerril Martha, Angulo Miriam, Sanchez Veronica, Guluarte Crystal, Angulo Carlos. β-D-glucan from marine yeast Debaryomyces hansenii BCS004 enhanced intestinal health and glucan-expressed receptor genes in Pacific red snapper Lutjanus Peru. Microb. Pathog. 2020;143 doi: 10.1016/j.micpath.2020.104141. [DOI] [PubMed] [Google Scholar]
- Rippert Dorthe, Heinisch Jürgen J. Investigation of the role of four mitotic septins and chitin synthase 2 for cytokinesis in Kluyveromyces lactis. Fungal Genet. Biol. : FG & B. 2016;94:69–78. doi: 10.1016/j.fgb.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Rizzetto Lisa, Ifrim Daniela C., Moretti Silvia, Tocci Noemi, Cheng Shih-Chin, Quintin Jessica, et al. Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with Saccharomyces cerevisiae. J. Biol. Chem. 2016;291(15):7961–7972. doi: 10.1074/jbc.M115.699645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg Luise E., Fortwendel Jarrod R., Juvvadi Praveen R., Steinbach William J. Regulation of expression, activity and localization of fungal chitin synthases. Med. Mycol. 2012;50(1):2–17. doi: 10.3109/13693786.2011.577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber Amir, Alipour Beitollah, Faghfoori Zeinab, Mousavi Jam Ali, Yari Khosroushahi Ahmad. Secretion metabolites of probiotic yeast, Pichia kudriavzevii AS-12, induces apoptosis pathways in human colorectal cancer cell lines. Nutr. Res. (N.Y.) 2017;41:36–46. doi: 10.1016/j.nutres.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Sambrani Roshanak, Abdolalizadeh Jalal, Kohan Leila, Jafari Behboud. Recent advances in the application of probiotic yeasts, particularly Saccharomyces, as an adjuvant therapy in the management of cancer with focus on colorectal cancer. Mol. Biol. Rep. 2021;48(1):951–960. doi: 10.1007/s11033-020-06110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Noelia, Roncero César. Chitin synthesis in yeast: a matter of trafficking. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232012251. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone Marion, Déjean Sébastien, Sieczkowski Nathalie, Castex Mathieu, Dague Etienne, François Jean M. Integration of biochemical, biophysical and transcriptomics data for investigating the structural and nanomechanical properties of the yeast cell wall. Front. Microbiol. 2017;8:1806. doi: 10.3389/fmicb.2017.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone Marion, Sieczkowski Nathalie, Castex Mathieu, Trevisiol Emmanuelle, Dague Etienne, François Jean Marie. AFM dendritips functionalized with molecular probes specific to cell wall polysaccharides as a tool to investigate cell surface structure and organization. Cell surface (Amsterdam, Netherlands) 2019;5 doi: 10.1016/j.tcsw.2019.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigkofler W. Analysis of phylogenetic relationships among Ascomycota with yeast phases using ribosomal DNA sequences and cell wall sugars. Org. Divers. Evol. 2002;2(1):1–17. doi: 10.1078/1439-6092-00029. [DOI] [Google Scholar]
- Seong Su Kyoung, Kim Ha Won. Potentiation of innate immunity by β-glucans. MYCOBIOLOGY. 2010;38(2):144–148. doi: 10.4489/MYCO.2010.38.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifmoghadam Mohammad Reza, Bustos-Sanmamed Pilar, Valdivieso Maria-Henar. The fission yeast Map4 protein is a novel adhesin required for mating. FEBS Lett. 2006;580(18):4457–4462. doi: 10.1016/j.febslet.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Shaw J.A., Mol P.C., Bowers B., Silverman S.J., Valdivieso M.H., Durán A., Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 1991;114(1):111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruthi B., Deepa N., Somashekaraiah Rakesh, Adithi G., Divyashree S., Sreenivasa M.Y. Exploring biotechnological and functional characteristics of probiotic yeasts: a review. Biotechnology reports (Amsterdam, Netherlands) 2022;34 doi: 10.1016/j.btre.2022.e00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Ida M., Baker Adam, Christensen Jeffrey E., Boekhout Teun, Frøkiær Hanne, Arneborg Nils, Jespersen Lene. Kluyveromyces marxianus and Saccharomyces boulardii induce distinct levels of dendritic cell cytokine secretion and significantly different T cell responses in vitro. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167410. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Ida M., Christensen Jeffrey E., Arneborg Nils, Jespersen Lene. Yeast modulation of human dendritic cell cytokine secretion: an in vitro study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096595. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniszewski Adam, Kordowska-Wiater Monika. Probiotic and potentially probiotic yeasts-characteristics and food application. Foods. 2021;10 doi: 10.3390/foods10061306. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran Vrinda, Lowman Douglas W., Sajeevan Thavarool P., Philip Rosamma. Marine yeast glucans confer better protection than that of baker's yeast in Penaeus monodon against white spot syndrome virus infection. Aquacult. Res. 2010;41(12):1799–1805. doi: 10.1111/j.1365-2109.2010.02520.x. [DOI] [Google Scholar]
- Suphantharika M., Khunrae P., Thanardkit P., Verduyn C. Preparation of spent brewer's yeast beta-glucans with a potential application as an immunostimulant for black tiger shrimp, Penaeus monodon. Bioresour. Technol. 2003;88(1):55–60. doi: 10.1016/S0960-8524(02)00257-2. [DOI] [PubMed] [Google Scholar]
- Szajewska H., Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2015;42(7):793–801. doi: 10.1111/apt.13344. [DOI] [PubMed] [Google Scholar]
- Tamang Jyoti Prakash, Lama Sonam. Probiotic properties of yeasts in traditional fermented foods and beverages. J. Appl. Microbiol. 2022;132(5):3533–3542. doi: 10.1111/jam.15467. [DOI] [PubMed] [Google Scholar]
- Tang Nanyu, Wang Xiaomeng, Yang Rui, Liu Zaimei, Liu Yuxiao, Tian Juanjuan, et al. Extraction, isolation, structural characterization and prebiotic activity of cell wall polysaccharide from Kluyveromyces marxianus. Carbohydr. Polym. 2022;289 doi: 10.1016/j.carbpol.2022.119457. [DOI] [PubMed] [Google Scholar]
- Tanihiro Reiko, Sakano Katsuhisa, Oba Shunsuke, Nakamura Chikako, Ohki Kohji, Hirota Tatsuhiko, et al. Effects of yeast mannan which promotes beneficial Bacteroides on the intestinal environment and skin condition: a randomized, double-blind, placebo-controlled study. Nutrients. 2020;12 doi: 10.3390/nu12123673. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecillas S., Makol A., Caballero M.J., Montero D., Robaina L., Real F., et al. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 2007;23(5):969–981. doi: 10.1016/j.fsi.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Vaithanomsat Pilanee, Boonlum Nutthamon, Trakunjae Chanaporn, Apiwatanapiwat Waraporn, Janchai Phornphimon, Boondaeng Antika, et al. Functionality of yeast β-glucan recovered from Kluyveromyces marxianus by alkaline and enzymatic processes. Polymers. 2022;14:8. doi: 10.3390/polym14081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Huali, Chen Guijie, Li Xiang, Zheng Fuping, Zeng Xiaoxiong. Yeast β-glucan, a potential prebiotic, showed a similar probiotic activity to inulin. Food Funct. 2020;11(12):10386–10396. doi: 10.1039/D0FO02224A. [DOI] [PubMed] [Google Scholar]
- Xie Xiaoze, Wang Jie, Guan Ying, Xing Shujuan, Liang Xiaofang, Xue Min, et al. Cottonseed protein concentrate as fishmeal alternative for largemouth bass (Micropterus salmoides) supplemented a yeast-based paraprobiotic: effects on growth performance, gut health and microbiome. Aquaculture. 2022;551 doi: 10.1016/j.aquaculture.2022.737898. [DOI] [Google Scholar]
- Xing Yan, Chen Chaonan, Sun Wenlong, Zhang Bowei, Sang Yuanbin, Xiu Zhilong, Dong Yuesheng. An environment-friendly approach to isolate and purify glucan from spent cells of recombinant Pichia pastoris and the bioactivity characterization of the purified glucan. Eng. Life Sci. 2018;18(4):227–235. doi: 10.1002/elsc.201700125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Taek Joon, Kim Tack Joong, Lee Hwa, Shin Kwang Soon, Yun Yeo Pyo, Moon Won Kook, et al. Anti-tumor metastatic activity of beta-glucan purified from mutated Saccharomyces cerevisiae. Int. Immunopharm. 2008;8(1):36–42. doi: 10.1016/j.intimp.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Yoshida Yasuto, Naito Eiichiro, Mizukoshi Harumi, Watanabe Yoko, Kimura Kazumasa, Yokoi Wakae, et al. Side-chain structure of cell surface polysaccharide, mannan, affects hypocholesterolemic activity of yeast. J. Agric. Food Chem. 2009;57(17):8003–8009. doi: 10.1021/jf900347q. [DOI] [PubMed] [Google Scholar]
- Zhao Wancheng, Fernando Liyanage D., Kirui Alex, Deligey Fabien, Wang Tuo. Solid-state NMR of plant and fungal cell walls: a critical review. Solid State Nucl. Magn. Reson. 2020;107 doi: 10.1016/j.ssnmr.2020.101660. [DOI] [PubMed] [Google Scholar]
- Zhao Yingyuan, Wang Jiaqi, Fu Qianzhen, Zhang Huiru, Liang Jin, Xue Wenjie, et al. Characterization and antioxidant activity of mannans from Saccharomyces cerevisiae with different molecular weight. Molecules. 2022;27(14):4439. doi: 10.3390/molecules27144439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Wenrui, Liu Yuchen, Shao Yujing, Ma Yanbo, Wu Yuanyuan, Guo Fangshen, et al. Yeast β-glucan altered intestinal microbiome and metabolome in older hens. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.766878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik H., Fernandez M.P., Bowers B., Cabib E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J. Bacteriol. 1984;159(3):1018–1026. doi: 10.1128/jb.159.3.1018-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.